46, XX Male or Ovotesticular DSD (SRY-negative) without SRY, is it Possible to have Testicular Tissues?
Received: 26-Oct-2017 / Accepted Date: 09-Nov-2017 / Published Date: 20-Nov-2017 DOI: 10.4172/2572-4983.1000139
Abstract
The 46, XX male syndrome, or the 46, XX testicular Disorder of Sexual Differentiation (DSD) is rare. We report a clinical case of a male infant 46, XX, DSD with SRY negative. This observation suggests that genes other than SRY play an important role in determining sex. From this case we will present a review of the literature to take stock of the subject.
Keywords: XX DSD; Karyotype; SRY-negative
Introduction
The foetal sex differentiation depends on a number of cellular and hormonal signals that interact in a specific order but still poorly defined to contribute to the establishment of the genital apparatus and a male or female phenotype [1].
Chronologically, it takes place in four major sequential steps:
• Establishment of the genetic sex at the time of fertilization.
• The combination of a maternal oocyte (23, X) and a paternal spermatozoon, either (23, X) or (23, Y) will give a homogametic egg (46, XX) of female genetic sex or heterogametic (46, XY) of male genetic sex.
• The establishment of the gonadal sex which represents the differentiation pathway of the gonads. It encompasses the determination and subsequent differentiation of the bipotential foetal gonad into testis or ovary. Then, the internal structures will be set up from two parallel channel systems present in the embryo: the Wolff and Müller ducts.
• The hormonal production by the differentiated testis of two capital hormones for the virilisation of the male foetus: the anti-Müllerian hormone (AMH) and the testosterone.
• The AMH will allow the regression of the Müller ducts while the testosterone is responsible for the development of the Wolff ducts.
• The development of phenotypic sex results in differentiation of the external genital organs and the urogenital sinus.
This last stage of sexual differentiation depends on the testosterone and dihydrotestosterone (DHT) action via the androgen receptor [2].
A good knowledge of sexual differentiation makes it possible to understand the classification of "Anomalies of sexual development" or rather disorders of Sex Development (DSD) [2].
Described for the first time by De la Chapelle, in 1964, the syndrome of male 46, XX intrigued the world of genetics [3,4]. It is defined as the presence of a male phenotype with a karyotype 46, XX. It was originally called "XX male syndrome," but in 2005, its nomenclature was revised in 46, XX DSD by a joint committee of the American Society of Paediatric Endocrinology and of the European Society for Paediatric Endocrinology. The incidence is estimated at 1 in 20,000 to 25,000 male live births [5].
Clinically, there is heterogeneity with three phenotypes identified: the traditional male XX, characterized by infertility with normal and male external (EGOs) and internal genital organs; males XX with ambiguous genital organs with hypospadias, micropenis, or clitoral hypertrophy; and the true XX hermaphrodites, which have an ambiguity of external or internal genital organs detected at birth.
At the molecular level, males XX can be classified as 46, XX SRY positive or 46, XX SRY negative, depending on whether or not the SRY gene is present.
We report the case of a male infant, 46, XX, DSD with SRY negative. The SRY gene is crucial for the genetic signal triggering the development of the embryonic testis. [6] Therefore, cases of male 46, XX DSD, where SRY is absent, are of particular interest as they suggest the existence of other genes instrumental to sex determination.
Case Report
This is an infant, the fourth of four siblings (3 living healthy brothers) sent to the genetic reference center for the management of a sexual differentiation anomaly. The child had already been declared a boy by the parents and raised as such.
Anomalies of the external genital organs were observed by the mother about the fifteenth day of life.
The interrogation did not find any consanguinity or any maternal antecedents except for arterial hypertension.
Pregnancy was complicated by pre-eclampsia requiring hospitalization for surveillance. The child was born through spontaneous eutocic vaginal delivery to 35 weeks of amenorrhea and 4 days; with an Apgar score of 7 then 9 respectively at 1 and 5 minutes of life.
The birth weight was 1800 g (-1 SD); the size at 48 cm (+1 SD) and the head circumference was estimated at 33 cm (+1 SD)
The infant was seen at the genetic reference center at the age of 45 days and his examination found:
• Measurements: Weight: 3100 g (-1 SD); Size: 52 cm (-1 SD); HC: 35 cm (-1 SD)
• General condition satisfactory.
• Neurologically: normal, reactive axial and peripheral tone, archaic reflexes present and normal, normal anterior fontanelle; eye tracking was obtained.
• Haemodynamically: very pink; normal time of immediate skin recolouration; heart sounds regular, femoral pulses perceived, symmetrical.
• As regards respiration: Eupnoeic, stable in ambient air, free lung fields.
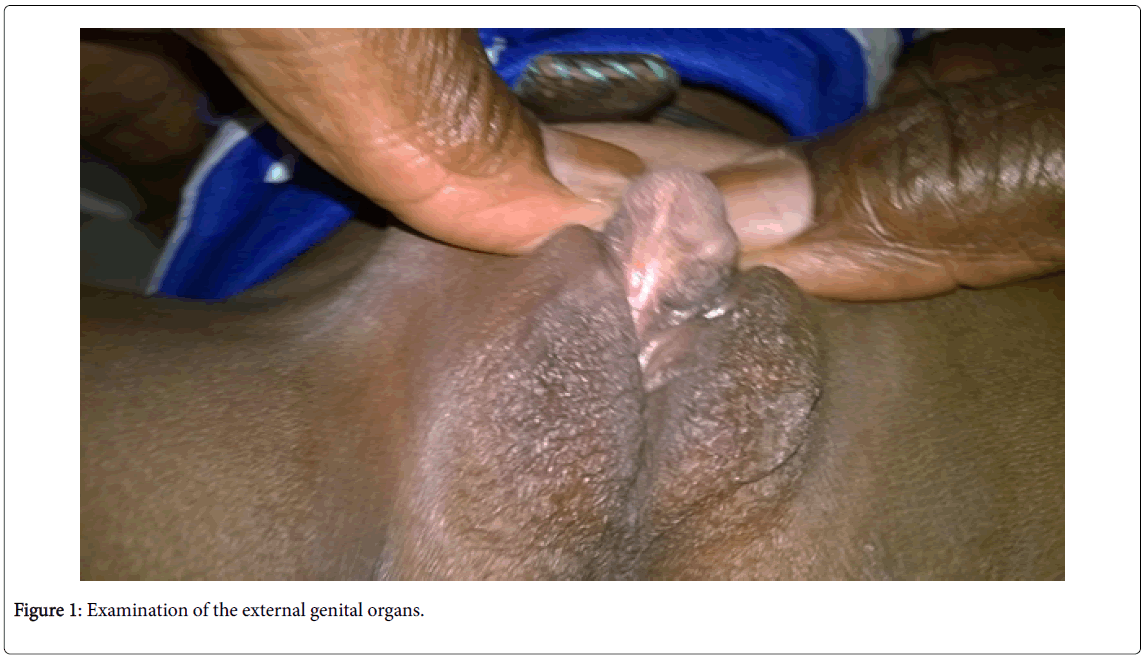
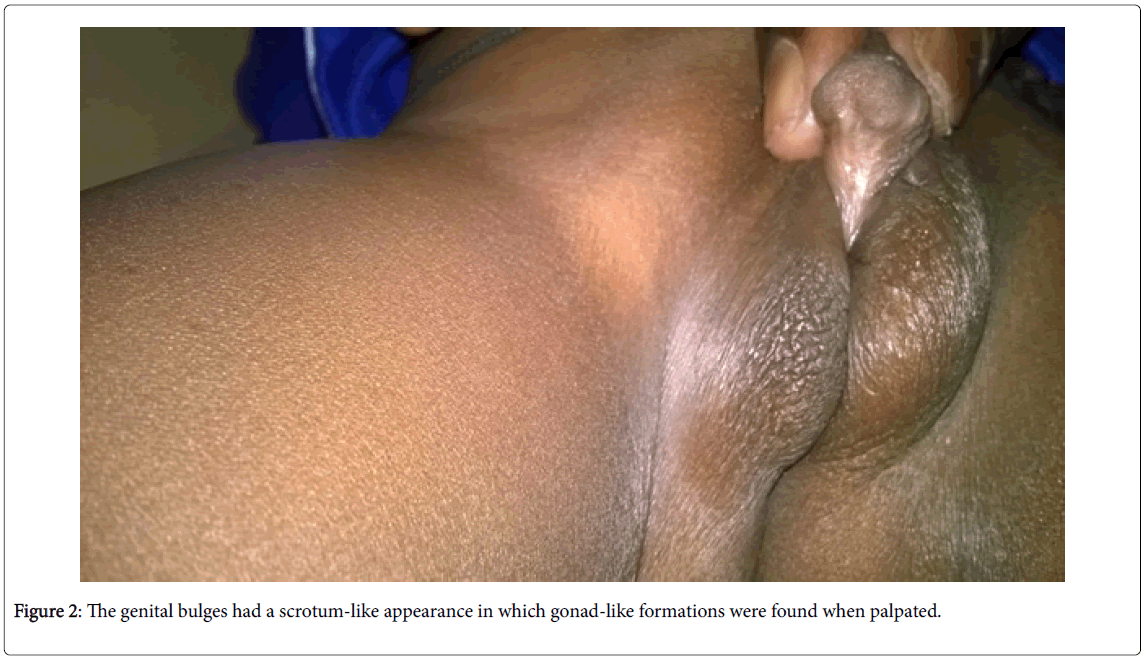
• Examination of the external genital organs (Figures 1 and 2) found a Stage IV of the PRADER classification (aspect of posterior hypospadias male) with a penniform bud, an incomplete prepuce, and a posterior perineal hypospadias. The genital bulges had a scrotum-like appearance in which gonad-like formations were found when palpated.
• There were no other malformations.
The paraclinical assessment found a discrepancy between imaging results and the genetic test conclusions and the dosage of the hormones with:
• A standard karyotype: 46, XX,
• A SRY gene search: negative,
• An abdominal-pelvic ultrasound noted an absence of uterus, testis in place and bilateral hydrocele of medium abundance associated with persistence of the bilateral peritoneal-vaginal canal,
• The biological assessment included normal blood ionograms, blood glucose, renal function and a blood count and formula that found microcytic hypochromic anaemia,
• The hormonal examination showed a rate of 17 alpha hydroxy progesterone at 0.10 ng/ml (normal), a normal (55 ng/ml) cortisol level, a testosterone level of 0.17 nmol/l (normal) and a dihydrotestosterone less than 0.05 ng/ml.
In total, it was an infant with a karyotype 46, XX, DSD and a discrepancy between the karyotype and the gonophoric and anatomical aspect.
The surgical exploration decided at a multidisciplinary meeting found an absence of uterus, a right gonad, with an ovarian aspect (without vas deferens or epididymis) and a left gonad with an undifferentiated aspect without epididymis or deferent whose vessels were joined to a bag.
Gonadal biopsies revealed:
• For the left gonad: a fibrous tissue encompassing seminiferous tubes evolving in an abundant fibrous mesenchyme devoid of Leydig cells.
For the right gonad: an abundant fibrous tissue sheltering tubes of little seminiferous aspect evolving in this abundant mesenchyme deprived of Leydig cells.
As a result, the left and right Gonads had testicular differentiation.
Discussion
XX DSD with normal male phenotype remains a rare diagnosis with an incidence of 1 in 20,000 to 25,000 live births.
In this case, it is a 46, XX testicular or ovotesticular DSD whose testes appear to be very dysgenetic although the macroscopic aspect of the right gonad is that of an ovary.
In most cases reported in the literature, the diagnosis is made much later, in adulthood and most often in the context of the assessment of an azoospermia.
The SRY gene is present only in 80% of men 46, XX. [2] For many years, the presence of testicular tissue in the absence of SRY was not explained.
Most males 46, XX without SRY show a severe hypomasculinization with an asymmetry of EGOs, most often confirmed by the presence of an ovotestis when a gonad study was carried out. More than 70% of ovotestis, formerly ‘’true hermaphrodite’’, have a karyotype 46, XX [7,8]. A duplication of the SOX9 gene leading to virilisation of EGOs in a subject 46, XX was the first molecular evidence in humans that in the absence of SRY, overexpression of SOX9 may allow the formation of testicular tissue [9].
Later, mutations in the RSPO1 gene involved in the formation of the ovary were identified in several males 46, XX also showing severe palmoplantar hyperkeratosis and high susceptibility to skin cancer [10]. Recently, overexpression of SOX9 due to duplication of a specific region upstream of the SOX9 gene promoter explains several cases of males 46, XX with ovotestis [11].
These observations confirm the studies in transgenic mice where the invalidation of a gene responsible for ovarian differentiation or the overexpression of Sox9 virilise female mice by allowing the development of Leydig cells in the ovary [12,13]. These observations underline the fragility of the balance between the factors responsible for testicular and ovarian differentiation. However, overexpression of SOX9 or SOX3 and RSPO1 and WNT4 gene mutations does not explain the pathology of all males 46, XX without SRY and 46, XX ovotestis.
For our patient the inaccessibility of the molecular biology techniques explains that we could not go so far in the investigations. The management of these patients is multidisciplinary and requires coordinated actions between endocrinologists, pediatricians and neonatologists, surgeons, geneticists and biologists who can improve management while insisting on the role of psychological support to families.
From a therapeutic point of view, our patient is expected to have a correction of the hypospad, and long-term monitoring to detect puberty disorders, appearance of gynecomastia at puberty, but also, and above all, impairment of fertility.
Conclusion
Although abnormalities of sexual development with testicles associated with a karyotype 46, XX are rare, but this clinical case illustrates all their interest for the clinician.
From a practical point of view, the approach to etiologic diagnosis must always begin with a thorough clinical analysis of anomalies in the development of internal and external genital organs and the associated signs, a karyotype, a biological assessment based on a few key parameters (AMH, steroids and gonadotropins) and evolution.
The management is multidisciplinary and requires coordinated actions between endocrinologists, paediatricians and neonatologists, surgeons, geneticists and biologists who can improve the management of these patients while insisting on the place of psychological support to families.
References
- Morel Y, Roucher F, Mallet D, Plotton I (2014) Genetic of gonadal determination. Ann Endocrinol 75: 32-39.
- Roucher F, Morel Y, Mallet D, Plotton I, Tardy V (2015) Pathophysiology and Classification of Disorders of Sex Development. Rev Méd Périnat 7: 137-146.
- Chapelle de la A, Hortling H, Niemi M, Wennstrom J (1964) XX sex chromosomes in a human male. First case. Acta Med Scand (Suppl) 412: 25-38.
- Vorona E, Zitzmann M, Gromoll J, Schuring A, Nieschlag E (2007) Clinical, endocrinological, and epigenetic features of the 46, XX male syndrome, compared with 47, XXY Klinefelter patients. J Clin Endocrinol Metab 92: 3458-3465.
- Ryan NAJ, Akbar S (2013) A case report of an incidental finding of a 46,XX, SRY-negative male with masculine phenotype during standard fertility workup with review of the literature and propose immediate and long-term management guidance. Fertil Steril 99: 1273-1276.
- Sinclair AH, Berta P, Palmer MS, Hawkins JR, Griffiths BL, et al. (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346: 240-244.
- Hadjiathanasiou CG, Brauner R, Lortat-Jacob S, Nivot S, Jaubert F, et al. (1994) True hermaphroditism: genetic variants and clinical management. J Pediatr 125: 738-744.
- Huang B, Wang S, Ning Y, Lamb AN, Bartley J (1999) Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet 87: 349-353.
- Parma P, Radi O, Vidal V, Chaboissier MC, Dellambra E, et al. (2006) R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet 38: 1304-1309.
- Benko S, Gordon CT, Mallet D, Sreenivasan R, Thauvin-Robinet C, et al. (2011) Disruption of a long distance regulatory region upstream of SOX9 in isolated disorders of sex development. J Med Genet 48: 825-830.
- Bishop CE, Whitworth DJ, Qin Y, Agoulnik AI, Agoulnik IU, et al. (2000) A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet 26: 490-494.
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397: 405-409.
Citation: Ndour D, Faye PM, Sagna A, Gueye D, Gassama O, et al. (2017) 46, XX Male or Ovotesticular DSD (SRY-negative) without SRY, is it Possible to have Testicular Tissues?. Neonat Pediatr Med 3: 139. DOI: 10.4172/2572-4983.1000139
Copyright: © 2017 Ndour D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7676
- [From(publication date): 0-2017 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 6694
- PDF downloads: 982