Research Article Open Access
One-Stage Model of Experimental Myocardial Infarction in Rats
Chupakhin O.N1, Sarapultsev P.A2, Sarapultsev A.P3*, Rantsev M.?4, Danilova I.G5 and Medvedeva S.Y6
1Scientific Supervisor at the I.J. Postovsky Institute of Organic Synthesis, Ural Branch of the RAS, MD, PhD in chemistry;
2Chief Researcher at the Institute of Immunology and Physiology, Ural Branch of the Russian Academy of Sciences, MD, PhD in medicine
3Associate Researcher at the Institute of Immunology and Physiology, Ural Branch of RAS, PhD in medicine
4Rantsev Maxim, Associate Professor of General Surgery at GOU VPO “Ural State Medical Academy of the Ministry of Health and Social Development”, PhD in medicine
5Chief Researcher at the Institute of Immunology and Physiology, Ural Branch of the Russian Academy of Sciences, MD, PhD in physiology.
6Senior Researcher at GOU VPO “the Institute of Immunology and Physiology, Ural Branch of the RAS”, PhD in medicine
- *Corresponding Author:
- Sarapultsev Alexei, Ph.D
Associate Researcher
Institute of Immunology and Physiology
Ural Branch of RAS, 620049 Ekaterinburg
106 Pervomayskaya Str, Russia
Tel: 343 3740070
E-mail: sarapultsev@sky.ru
Received Date: September 30, 2011; Accepted Date: November 17, 2011; Published Date: November 18, 2011
Citation: Chupakhin ON, Sarapultsev PA, Sarapultsev AP, Rantsev MA, Danilova IG, et al. (2011) One-Stage Model of Experimental Myocardial Infarction in Rats. J Clinic Experiment Pathol 1:101. doi: 10.4172/2161-0681.1000101
Copyright: © 2011 Chupakhin ON, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Clinical & Experimental Pathology
Abstract
Background: In recent years, due to technical and economic considerations much of the experimental work is carried out on small laboratory animals such as mice and rats. In addition, researchers have to use mainly one-stage methods of MI modeling, which rely on vessel ligation. Objective: The aim of the study was to create a simple model of experimental myocardial infarction (MI) to examine the effect of medications on its development and course. Methods: Acute MI in rats was induced by left coronary artery ligation. Animals were taken out of the experiment at days 1, 5 or 7. Myocardium of all heart parts was morphologically studied after embedding. In addition to visual assessment there was a light-optical examination of infraction and peri-infarction myocardial areas, as well as left ventricular areas distant from the damaged tissues. Serum activity of creatine phosphokinase (CPK), aspartate aminotransferase (AST), isoenzymes 1 and 2, lactate dehydrogenase (LDH1-2) before and in the process of MI development was investigated (at days 1, 5 and 7). Results: Based on the study results, in animals with MI model standard level of affecting the coronary artery was determined, as well as similar amount of ischemic myocardial tissues; and histomorphological findings confirmed standard development of transmural MI. Meanwhile, staging of MI development in rodents correlated with other authors’ data. Conclusions: Thus, the presented method of experimental MI modeling is relatively simple for use and provides a high level of reproducibility of results. In addition, it’s not associated with risks of developing cardiac rhythm disturbances, provides 100 % survival rate of experimental animals and can be used for laboratory studies on larger groups of small laboratory animals.
Keywords
Myocardial infarction; Inflammation
Introduction
The increasing prevalence of ischemic heart disease and myocardial infarction (MI) in the world, severity of clinical course, and high mortality rate contribute to the importance of searching for new methods of treatment and prevention of these conditions [1-3]. At the same time, the MI pathogenesis is rather complex and not entirely understood process [1-3]. Use of ischemia and MI experimental models can substantially improve our understanding of pathological and pharmacological mechanisms involved in development and healing of MI [4].
Left anterior descending artery ligation that was proposed at the end of the past century (Porter, 1896) remains the most common method of MI modeling up to this day, even though it has been modified many times. Determination of an optimal ligation level on the vessel is fundamentally important in these techniques, and the least threatening level regarding the occurrence of fibrillation is considered to be ligation of the left descending coronary artery immediately after the divergence of the transverse branch [4].
Two-stage models can be considered as more sophisticated variants of experimental MI creation [4]. The initial stage in these models of experimental MI is placement of the ligature under the appropriate segment of the coronary artery. The second stage, the principal one, is performed a few days later in a closed chest and consists of tightening the ligature. The level and degree of compression and some other factors may vary depending on the study objectives. The disadvantage of these models is significant labor-intensiveness and complexity of surgical technique; therefore, it is not always possible to use them in laboratory studies with relatively large groups of small laboratory animals (mice, rats).
A special place belongs to metabolic methods of MI modeling [4]. These methods may or may not require thoracotomy (Selye 1970; Bruyneel et al., 1972). They include steroid-induced electrolyte imbalance, introduction of “damaging” solutions into myocardium, etc [4]. This group of experimental methods is also rather labor-intensive and requires trained personnel. At the same time, regarding physical methods of myocardial perfusion blockade, at least hypothetical judgments can be made about their probable impact on the lymphatic and neurohumoral components of the modeled condition; however, there are virtually no grounds for such judgments in relation to metabolic methods [4]. Their effects in each particular case might be specific [4].
In recent years, due to technical and economic considerations much of the experimental work is carried out on small laboratory animals such as mice and rats [5,6]. Ischemic heart damage and MI are also modeled predominantly in small laboratory animals [7,8], and these models are becoming more common in exploring the mechanisms of ischemic myocardial damage [9,10].
However, in experimental studies on fairly large groups of small laboratory animals, researchers have to apply mostly one-stage methods of MI moeling, which rely on vessel ligation.
Thermal coagulation of vessels can be considered the most convenient option for researchers, which also provides good reproducibility of results. This method increases the chances for obtaining positive outcomes while minimizing operative time and increasing the time of the experiment. Moreover, this method neither disturbs the cardiac rhythm nor causes arrhythmias associated with the effect of electric current in contrast to electrocoagulation. In addition, this method provides more reproducible results compared to surgical ligation or clamping of the artery.
According to current concepts, healing processes in MI can be divided into three phases: inflammation, proliferation, and maturation [11]. However, duration of these phases in large mammals (including humans) is quite different from rodents [12]. Thus, the inflammation phase in rodents finishes at day 2, whereas in large mammals it finishes at day 4, the proliferative phase in rodents peaks at day 5, whereas in large mammals it lasts 14 days [13-15]. In line with this approach the major points of taking animals out of the experiment were chosen – at days 1, 5, and 7.
It is known that comparison of ECG to biochemistry diagnosis when using post mortem diagnosis as a “gold standard” showed that sensitivity and specificity of biochemistry is superior to ECG [14,15], therefore, biochemistry diagnosis remains a part of the WHO criteria for diagnosing MI [16]. For this reason, biochemical and morphological examinations were used in this work for experimental MI detection.
Objectives
The aim of the study was to create a model of experimental MI to examine the effects of medications on its development and course.
Materials and Methods
Modeling of an acute MI was performed in rats according to the authors’ method (PCT RF Patent ¹ 2407062 of 20.12.2010).
The method is based on surgical MI modeling in rats, characterized by a sequence of the following steps: skin is incised and muscles exposed in the left part of the chest; pectoral muscles are spread with an exposure of costal arches and intercostal muscles; the intercostal muscles are transsected at the level of 4-5 intercostal space for 1 cm; heart is visualized; coagulation is performed within the standard limited area of the branches of the left coronal artery in its middle or lower third. Coagulation is performed with an “L” -shaped instrument with cautering surface area of 2.5 × 3.0 mm, preheated using an alcohol lamp (this instrument shape allows clear heart visualization during coagulation); thoracotomy wound is closed with a running suture using an atraumatic needle; pneumothorax is eliminated using a needle syringe; skin incision is closed. This method increases the chances for a positive outcome while minimizing operative time and increasing time of the experiment. Besides, this method neither disturbs the heart rhythm, nor causes arrhythmias associated with the effects of electric current.
Within 10 minutes after modeling MI animals’ behavioral reactions completely returned to preoperative level. No mortality was recorded. Assessment of animals’ behavior at 24 hours revealed no signs of abnormal behavioral reactions: animals were active; they reacted to sound and light stimulation without any delay. Signs of respiratory failure, nutrition or fluid intake disorders weren’t noted as well.
The study was performed on 30 outbred male rats weighing 180- 240 g that were divided into 2 groups, 15 animals in each group: MI was induced in the study group according to the authors’ method; the control group didn’t undergo surgical intervention.
Animals were taken out for the study on days 1, 5 or 7 after being anesthetized with 40 mg/kg of aethaminalum-natrium intraperitoneally.
In harvesting hearts for subsequent histological evaluation, gross observations in all cases showed presence of distinctly infarcted myocardial area; meanwhile, no changes in lungs or pleural effusion were noted; gross observations didn’t reveal any visible changes in abdominal organs (liver, spleen, intestinal loops) or other tissues.
Myocardium of all heart parts was morphologically studied after fixation. In addition to visual assessment there was light-optical examination of infracted and peri-infarcted myocardial areas, as well as left ventricular areas far from injured tissues. Paraffin blocks were made according to common histological method. Series of sections of 5-6 micron thickness were stained with H&E after van Gieson and Weigert [17,18].
Blood for biochemical analysis (3 ml) was collected by means of heart puncture with subsequent centrifugation and serum separation. Serum activity of creatine phosphokinase (CPK), aspartate aminotransferase (AST), isoenzymes 1 and 2, lactate dehydrogenase (LDH1-2) before and in the process of MI development was examined (at days 1, 5 and 7).
Laboratory blood tests were performed using the following devices and diagnostic systems:
• “Immunochemistry Systems” biochemical analyzer by Beckman Coulter, Inc. (Brea, USA)
• Diagnostic Systems by DSL, Inc. (Webster, USA)
• “Multiscan” Spectrophotometer by Labsystems Ltd. (Helsinki, Finland)
• “Glycomat DS5” Automatic Analyzer by Drew Scientific Ltd. (Dallas, USA)
Statistical analysis. The differences in mean value of the various groups were analyzed by Scheffe’s multiple comparisons in one-way analysis of variance (ANOVA). The comparison of parametric variables was performed by unpaired sample t test. All the data were expressed as mean ± SD. A p value < 0.05 was considered statistically significant.
Results
Results of L-17 compound effects on blood biochemical values in the process of experimental MI development are shown in Table 1.
| Values | Intact rats | Experimental MI Animals | ||
|---|---|---|---|---|
| Day 1 MI | Day 5 MI | Day 7 MI | ||
| (n = 15) | (n = 5) | (n = 5) | (n = 5) | |
| CPK (µmol/L-min) | 146,92±22,6 | 234,9±60,1* | 168,54±21,6* | 248,12±41,5* |
| AST (µmol/L-24hrs) | 0,193±0,014 | 0,415±0,033** | 0,290±0,05* | 0,288±0,023* |
| LDH1-2 (µmol/L-24hrs) | 165,15±34,6 | 515,82±60,1* | 262,28±22,1* | 346,46±52,9* |
Note: Significance of differences between intact animals and experimental MI animals: * - Ã?Â? <0,05; ** - Ã?Â? <0,01; significance of differences between animal that received and didn’t receive L-17 compound: Ã?Â? - Ã?Â? <0,05; Ã?Â?Ã?Â? - Ã?Â? <0,01.
Table 1: Blood serum biochemical values in the process of an experimental acute MI development.
According to biochemistry analysis in experimental MI animals, levels of all detectable enzymes reliably exceeded the same values in intact animals (see Table 1).
Thus, the CPK level in the group of intact animals was 146,92 ± 22,6 μmol/L -min. In the group of experimental MI animals CPK level at day 1 was 234,9 ± 60,1 μmol/L -min, at day 5 CPK level was 168,54 ± 21,6 μmol/L -min, at day 7 CPK level was noted to increase to 248,12 ± 41,5 μmol/L -min.
The AST level in the group of intact animals was 0,193 ± 0,014 μmol/L-24hrs. Whereas AST level in experimental MI group at day 1 was 0,415 ± 0,033 μmol/L-24hrs, at day 5 AST level was 0,290 ± 0,05 μmol/L-24hrs, at day 7 AST level was 0,288 ± 0,023 μmol/L-24hrs.
The LDH1-2 level in the group of intact animals was 165,15 ± 34,6 μmol/L-24hrs. Whereas LDH1-2 level in experimental MI group at day 1 was 515,82 ± 60,1 μmol/L-24hrs, at day 7 of experimental MI an increase in enzyme activity was noted (346,46 ± 52,9 μmol/L-24hrs).
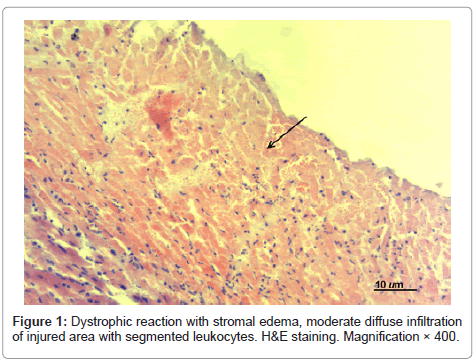
Histological examination of hearts of experimental MI animals showed the following: at day 1 postoperatively the infarcted area in the experimental MI animals that didn’t receive treatment was transmural and presented with cardiomyocytes with signs of karyolysis, plasmolysis and plasmorrhexis; there was moderate diffuse infiltration of the damaged area with segmented leukocytes without demarcation zone formation (see Figure 1). Edema, fullness of endomysial vessels with formation of sludge complexes were observed in adjacent structures.
Polymorphonucleocytes detected in the destruction area were indicative of reactive inflammation with exudative reaction. Erythrocyte “sludge complexes” and focal hemorrhages were detected in microvessels of the perifocal area. Significant changes were noted not only in the necrosis area, but also in adjacent tissues that demonstrated partial atrophy of myocardiocytes and pronounced dystrophic reaction with stromal edema, loss of cross and axial striation of myofibrils.
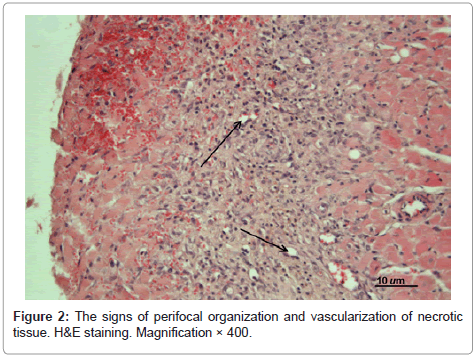
At day 5 postoperatively in the untreated experimental MI animals the infarcted area was characterized as predominantly transmural (see Figure 2). Necrotized cardiomyocytes were surrounded by demarcation ridge; just signs of granulation tissue formation were observed; fibroblasts and hemocapillaries began to appear. Adjacent structures demonstrated infiltration of endomysium.
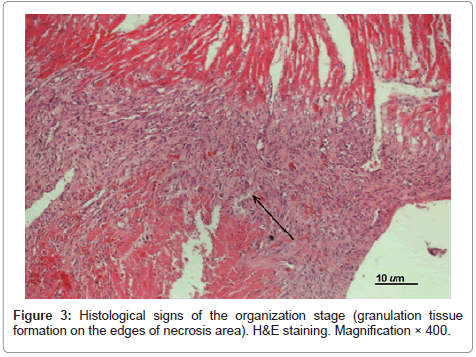
At day 7 postoperatively in all untreated experimental MI animals the infarcted area in the left ventricular wall was characterized as transmural (see Figure 3). Histological signs of the organization stage appeared (i.e. granulation tissue formation on the edges of necrosis area with large quantities of fibroblasts, macrophages and sinusoidal hemocapillaries replacing the injured segment), however, disintegration of muscular cells and infiltration of myocardium with lymphocytes and segmented leukocytes persisted. In some cases margination with signs of leukopedesis was detected in vessels.
Discussion
Based on the study results, dimensions of anatomical damaged areas in experimental MI animals were not significantly different, which indicates the standard level of affecting the coronary artery and similar amount of ischemic myocardium; meanwhile, histomorphological findings confirmed the standard formation of transmural myocardial infarction.
Staging of MI development correlated with the staging of MI development in rodents according to Dewald [12]. It is important to note that resultant changes in myocardium beyond the infarction area correlate with other researchers’ data [19,20] indicating the presence of significant changes in early period of MI not only in the necrosis area, but also in other parts of myocardium. So, at day 1 pronounced dystrophic reaction was noted with the stromal edema, loss of cross and axial striation with subsequent granular and lumpy disintegration in myocardium beyond the infarction area. Besides, tissues adjacent to the infarction area were subject to more significant changes, which manifested in partial atrophy of myocardiocytes and structural changes similar to those in the necrotized area up to dystrophic and necrobiotic changes both in individual muscle fibers and fascicles, which was detected by other researchers as well.
Histomorphological findings were confirmed by biochemistry data (see Table 1). In particular, an increased level of blood enzymes was detected at day 1 with decline in dynamics of enzymemia in the progress of the experiment. Besides, decreased enzymemia values at day 5 of experimental MI indicated the completion of necrotized myocardial tissue resorption period, which, according to literature, occurs at day 4 or 5 in small rodents [12,13]. At the same time, special attention deserves the fact that at day 7 in the experimental MI group there was a significant increase in CPK and LDH1-2 levels indicating the recurrence of MI, possibly provoked by active motions of the animals.
Conclusions
1. The presented method of experimental MI modeling is relatively simple for use and provides a high level of reproducibility of results.
2. MI modeling according to the authors’ method is not associated with risks of cardiac rhythm disturbance and provides a 100 % survival rate among laboratory animals.
3. The presented method can be used in laboratory studies on large groups of small laboratory animals.
Acknowledgements
This research project would not have been possible without the support of many people. The authors wish to express their gratitude to the supervisor, Prof., MD B.G. Ushkov, who was abundantly helpful and offered invaluable assistance, support and guidance. Deepest gratitude are also due to the members of the Laboratory of Morphology and Biochemistry (Institute of Immunology and Physiology RAS, Ekaterinburg) without whose knowledge and assistance this study would not have been successful
The authors would also like to convey thanks to the Ural Branch of the RAS for providing the financial means and laboratory facilities.
This study was supported by a Special Research Grant of Interdisciplinary project of the Institute of Immunology and Physiology and the Institute of Organic Synthesis, Ural Branch of RAS (2009-2011).
References
- Arzamastsev AP, Severina IS, Grigor'ev NB, Granik VG (2003) Exogenic donors of nitric oxide and inhibitors of NO-sintases (chemical aspect). Vestn Ross Akad Med Nauk 12: 88-95.
- Abakumov MM, Golikov PP, Nikolaeva NIu, Klychnikova EV, Stotskaia TV, et al. (2004) Nitric oxide in clinical emergency. Vopr Med Khim 48: 286-292.
- Saprunova VB (2003) Ultra-structure of mitochondrial mechanism of apoptotic rat cardiomyocytes induced by prolonged anoxia. Cytology 11: 1074-1082.
- Mamedov JD (1989) Myocardial infarction. The lymphatic system of the heart. Pathophysiology and pathogenetic basis for treatment. Medicine, Moscow.
- James JF, Hewett TE, Robbins J (1998) Cardiac physiology in transgenic mice. Circ Res 82: 407-415.
- Franz WM, Mueller OJ, Hartong R, Frey N, Katus HA (1997) Transgenic animal models: new avenues in cardiovascular physiology. J Mol Med 75: 115-129.
- Michael LH, Entman ML, Hartley CJ, Youker KA, Zhu J, et al. (1955) Myocardial ischemia and reperfusion: a murine model. Am J Physiol 269: 2147-2154.
- Nossuli TO, Lakshminarayanan V, Baumgarten G, Taffet GE, Ballantyne CM, et al. (2000) A chronic mouse model of myocardial ischemia-reperfusion: essential in cytokine studies. Am J Physiol Heart Circ Physiol 278: 1049-1055
- Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, et al. (1999) Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 5: 1135-1142
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, et al. (2000) Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest 106: 55-62.
- Frangogiannis NG (2006) The mechanistic basis of infarct healing. Antioxid Redox Signal 8: 1907-1939.
- Dewald O, Ren G, Duerr GD, Zoerlein M, Klemm C, et al. (2004) Of mice and dogs: species-speci?c differences in the inflammatory response following myocardial infarction. Am J Pathol 164: 665-677.
- Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, et al. (2006) Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res 324: 475-488.
- McQueen M, Holder D, EI-Maraghi NR (1983) Assessment of the accuracy of serial electrocardiogram in the diagnosis of acute myocardial infarction. Am Heart J 105: 258-261.
- Zarling EJ, Sexton H, Milnor P Jr (1983) Failure to diagnose acute myocardial infarction. JAMA 250: 1177-1181.
- WHO Working group on establishment of ischemic heart disease registers. Report of fifth working group (1972). Copenhagen, World Health Organization.
- Bancroft JD, Stevens A (1996) Theory and Practice of Histological Techniques. (4th edn). Churchill Livingstone, New York.
- Lillie RD (1977) H.J. Conn's Biological Stains. (4th edn). Williams and Wilkins, Baltimore.
- Hirsh J, Anand S, Halperin JL, Fuster V (2001) Guide to anticoagulant therapy: Heparin. A statement for healthcare professionals from the American Heart Association. Circulation 103: 2994-3018.
- Struchkov A I, Lushnikov EF, Gornak KA (1967) Histochemistry of myocardial infarction. Medicine, Moscow.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15054
- [From(publication date):
November-2011 - Nov 26, 2025] - Breakdown by view type
- HTML page views : 10252
- PDF downloads : 4802