Review Article Open Access
Omega-3 Fatty Acids and Cardiovascular Disease
John H Lee1*, Amit Prasad1, Tara Kay Jarreau1, James H O’Keefe2, Richard Milani1 and Carl J Lavie11John Ochsner Heart and Vascular Institute, Ochsner Clinical School, The University of Queensland, School of Medicine, New Orleans, LA
2Mid America Heart Institute, University of Missouri-Kansas City
- *Corresponding Author:
- Dr. John H Lee, MD
John Ochsner Heart and Vascular Institute
Ochsner Clinical School, The University of Queensland School of Medicine
1514 Jefferson Highway, New Orleans, LA 70121, USA
Tel : 504-842- 4135
Fax : 504-842-4465
E-mail: johlee@ochsner.org
Received date: June 28, 2011; Accepted date: August 18, 2011; Published date: September 07, 2011
Citation: Lee JH, Prasad A, Jarreau TK, O’Keefe JH, Milani R, et al. (2011) Omega-3 Fatty Acids and Cardiovascular Disease. J Marine Sci Res Development S1:001. doi:10.4172/2155-9910.S1-001
Copyright: © 2011 Lee JH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Keywords
Omega-3 fatty acids; Eicosapentaenoic acid; Docosahexaenoic acid; α-Linolenic acid; Coronary disease prevention; Fish oils
Introduction
The use of omega-3 fatty acids for the secondary prevention of cardiovascular (CV) events has been endorsed by the American Heart Association (AHA). A number of key epidemiologic and randomized trials have been the basis for this recommendation. The AHA recommends 1 g/d of omega-3 fatty acids with a mixture of docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). The preferential source is oily fish, however fish oil supplements are considered an acceptable option as well [1]. In addition, the Food and Drug Administration (FDA) has approved an omega-3 fatty acid formulation for the treatment of high triglycerides at a dose of 4 g/d (3.4 g/d of EPA + DHA). This review will summarize current scientific data on omega-3 fatty acids as well as discuss the sources and sustainability of fish oil production.
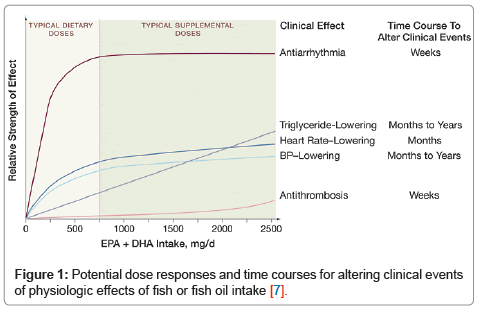
Benefits of omega-3 fatty acidsOver the past several decades, an abundance of epidemiological, experimental and randomized controlled studies have been published on the CV effects of omega-3 fatty acids [2]. The bulk of evidence has demonstrated clear CV protective effects [3]. DHA and EPA are the two specific omega-3 fatty acids that have been associated with CV benefit and triglyceride lowering. DHA and EPA are present in varying ratios in oily fish [4]. Commonly consumed fish such as salmon contain EPA to DHA in a ratio of about 1:2. DHA can partially be retro-converted to EPA [5]; however, EPA supplementation has only a modest effect at raising tissue or blood levels of DHA [6]. A meta-analysis [7] of prospective clinical trials and epidemiologic studies demonstrated that the majority of reduction in risk of CHD death is conferred with modest omega-3 fatty acid consumption: 250 to 500 mg/d of DHA + EPA (Figure 1). This diet corresponds to approximately 1-2 servings/ wk of oily fish
Alpha-linolenic acid (ALA) is another form of omega-3 that is found in flaxseed, nuts, and in trace amounts in green leafy vegetables. Humans convert less than 5% of ALA to EPA, and ALA gets converted into DHA even more sparingly [8], so ALA is inadequate as the sole dietary source of omega-3 fatty acids. ALA intake has been inversely associated with CV events in some epidemiological studies, but this has not been corroborated by subsequent studies [3].
Omega-3 fatty acids provides CV benefits through DHA and EPA enrichment of membrane phospholipids [9] which confers several potentially cardio-protective effects, including an increase arrhythmic thresholds [10], reduction in blood pressure [11,12], improved arterial and endothelial function [13], reduced platelet aggregation [14], and a favorable autonomic balance [11,15]. In a large population study of over 100,000 subjects, omega-3 fatty acid intake was shown to be inversely related to the development of type 2 diabetes in women [16]. A meta-analysis by Geleijnse et al. [17], of 22 double blind studies revealed that omega-3 fatty acid intake of about 4 g/d was associated with significant reductions of 1.7 and 1.5 mm Hg in systolic and diastolic pressures, respectively, an effect that was noted to be more pronounced in older patients and in those with higher baseline blood pressures. Lowering systolic blood pressure by as little as 2 mm Hg can yield mortality reductions of up to 4% from CHD [18]. The anti-platelet, anti-inflammatory, and triglyceride-lowering effects of omega-3 fatty acids are seen with higher doses (3-4 g/d) of DHA + EPA, whereas the reduction in sudden cardiac death (SCD) risk can be achieved at lower intakes (0.5 to 1.0 g/d) [19,21]. Omega-3 fatty acids have been shown to have anti-inflammatory effects suppressing the production of cytokines such as interleukin-6, interleukin-1β, and tissue necrosis factor-α [22]. In a study by Noori et al. [23]. of 145 patients on hemodialysis, a higher omega-6 fatty acid to omega-3 fatty acid ratio was associated with higher C-reactive protein (CRP) levels. Additionally when EPA is administered to obese individuals, an increase in the levels of adiponectin, which may reduce inflammation and improve insulin sensitivity, [24] has been observed. Very high doses of omega-3 fatty acids (i.e., 8 g/d) have anti-inflammatory effects and improves body composition in heart failure (HF) patients [25]. A dose of DHA + EPA (810 mg/d) given to stable CHD patients decreased resting heart rate, increased post-exercise heart rate recovery, and increased beat-to-beat heart rate variability (in the high frequency band) [11]. These changes are consistent with an augmented vagal tone, indicating the role of omega-3 fatty acids in improving autonomic sympatho-vagal balance [11,15]. Recent work by Moyer et al. [26] also corroborated the effects of omega-3 fatty acid supplementation on vagal tone by illustrating a significant association between omega-3 fatty acid levels and heart rate recovery, exercise capacity and exercise time.
Some of the cardio-protective effect of omega-3 fatty acids is related to the favorable effects on the lipid profile. Davidson et al. [27] reported reductions in triglycerides and non-high density lipoprotein (HDL) cholesterol by 29% and 9%, respectively, with the addition of 3.4 g/d of omega-3 fatty acids to baseline simvastatin therapy. There was also a small, but significant, increase in HDL. In a review of 25 trials by Harris et al. [20], the risk of CHD events was related to in vivo omega-3 fatty acid levels. The risk of CV events was inversely related to the amount of DHA in plasma and cellular phospholipids, which correlates with the amount of DHA in the myocardium [20,28].
Evidence in CV disease
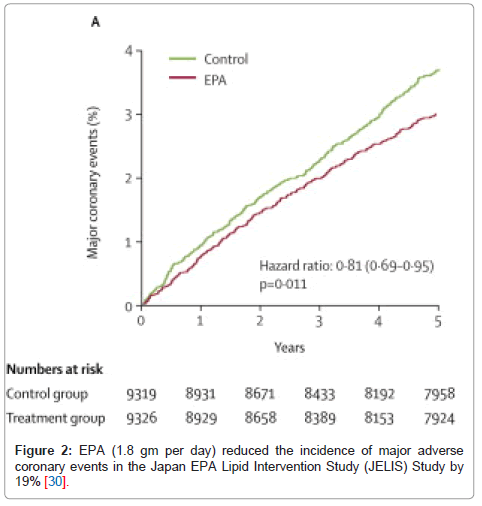
It has been shown that omega-3 fatty acid supplementation has a profound impact on patient survival and CV events [21]. Starting from a number of early randomized trials, omega-3 fatty acids, either in the form of oily fish or fish oil capsules, reduced all-cause mortality in post-myocardial infarction (MI) patients [29]. Subsequently, 2 major randomized controlled trials examined the effects of supplemental omega-3 fatty acids on CHD risk. The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione study randomized 11,323 post-MI patients to omega-3 acid ethyl esters (1 capsule/d providing 850 mg of DHA + EPA) or usual care. Treatment with omega-3 fatty acids significantly reduced the risk of death from any cause by 28%. By 4 months, the risk for SCD was decreased by 45% [19]. The next major randomized controlled trial to be published was the Japan EPA Lipid Intervention Study (JELIS) [30] which studied 18,645 hypercholesterolemic patients who were randomly assigned to either statin alone or statin + pure EPA (1.8 g/d). By the end of the 5-year follow up period, the patients who received EPA had 19% lower major adverse CV events (Figure 2).
Several older studies have shown conflicting results, of which the most commonly quoted are the randomized trials reported by Burr et al. [29] and Nilsen et al. [32]. In Burr’s study, patients with angina treated with fish oil capsules had higher rates of SCD than untreated controls. This study was criticized as being “well designed” but sub-optimally “conducted and reported”, and thus its results are questionable [33]. The Nilsen study is thought to be flawed because of the high background intake of fish oils in the Norwegian subjects, possibly masking the treatment effects.
Some of the more recent trials such as the OMEGA trial [34] and the Alpha Omega Trial [35] have also shown some conflicting results. The OMEGA trial [34] is a randomized controlled multi-center trial which enrolled 3851 patients and compared patients receiving 1 g/d of omega-3 fatty acids versus placebo for one year and was unable to show a significant difference in CV outcomes in post -MI patients. This discrepancy could have potentially been from the improvements in medical therapy and stent technology from the decade older trials, thus resulting in a lower number of events in the placebo-treated patients. In addition, enrolled subjects had high levels of fish consumption that increased significantly during the course of the study in both arms. The Alpha Omega trial [35] enrolled 4837 patients into their randomized controlled trial which investigated the supplementation of omega-3 in the form of 18.8 g of margarine per day and also showed no significant differences in CV events is patients with a history of MI. The patients in this trial were also on a modern regimen of CV medications and only received a small supplement of omega-3 (400 mg EPA + DHA or 2 g ALA, each alone, or placebo) which may have not been enough to exert an effect.
Omega-3 fatty acids have been strongly associated with antiarrhythmic effects. Calo et al. [36] showed a significant reduction of post-operative atrial fibrillation (AF) in coronary artery bypass surgical patients. However, in Kowey et al.’s trial [37] of 663 AF patients, omega-3 supplementation did not reduce the recurrence of AF in the 6 month study period. Others have described trends towards reduction in malignant ventricular arrhythmias with omega-3 supplementation
[10,38-40]. Although omega-3 fatty acids appear to be effective for reducing SCD in the setting of CHD with reduced left ventricular systolic function [41], 3 trials using omega-3 fatty acids in implantable cardioverter defibrillator patients have shown mixed results [38-40]. The most recent trial evaluating major arrhythmic events was a substudy of the GISSI Heart Failure (HF) trial that showed fewer events in the omega-3 arm, but this finding was not statistically significant [42].
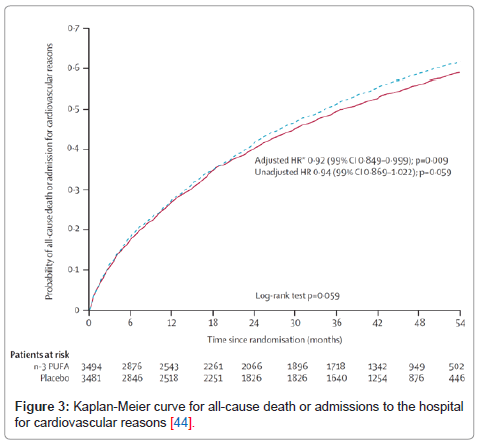
Levitan et al. [43] further linked omega-3 fatty acids and CV disease by showing that moderate intake of fatty fish and marine omega-3 fatty acids in 39,367 middle-aged or older men was associated with lower rates of HF. In GISSI HF [44], a large randomized placebo-controlled trial of 7046 HF patients, 850 mg/d of EPA + DHA significantly reduced
mortality and CV admissions by 8%. (Figure 3) The Cardiovascular Health Study showed an inverse association in the intake of baked or broiled fish and incidence of HF [45,46]. Further evidence to support the benefit of omega-3 fatty acids in HF patients was reported in the Atherosclerosis Risk in Community study ,which showed an inverse relationship between omega-3 polyunsaturated fatty acids(PUFA) and intake and incidence of HF in women [47]. A recent randomized controlled trial conducted by Nodari et al. [48] evaluated 133 patients with chronic HF secondary to non-ischemic cardiomyopathy and demonstrated significant improvements in many HF and major CV parameters at 12 month follow up in the subjects who received 2 g/ day omega-3 fatty acid supplementation versus placebo Left ventricular ejection fraction in the omega-3 arm was increased by 10.4% compared to a decrease of 5.0% in the placebo arm (p<0.001). Significant improvements in exercise capacity, New York Heart Association functional class, re-hospitalization rates, and inflammatory cytokines were also seen with omega-3 PUFA [48].
Low blood levels of DHA + EPA have been reported to be a markers of cardiovascular risk [9]. Red blood cell membrane DHA + EPA levels of > 8% appear to be associated with the lowest risk for CHD death [9,49] and are associated with reduced risk for acute coronary syndromes [50]. Individuals with DHA + EPA < 4% are considered to be at higher risk for CV events [50]. In addition, low blood omega-3 fatty acid levels are associated with increased risk of SCD [51]. Optimal membrane levels of omega-3 fatty acid usually are achieved with consumption of 1 to 1.5 g of DHA + EPA per day, which is an intake similar to that of the average Japanese adult [49].
Best source of omega-3 fatty acids
The AHA recommends a total of 1 g/d of DHA and EPA preferably from oily fish for patients with coronary heart disease (CHD) but considers fish oil supplements as an acceptable alternative [1]. Oily fish is the preferred source of the AHA, but not all fish have the same amount or ratio of DHA and EPA. (Table 1) The question of whether fish or fish oils confer the same beneficial CV effects has been addressed in several studies evaluating the various modes of consumption. A recent study compared the effects of supplementing with walnuts versus fatty fish and found that walnut supplementation significantly lowered cholesterol while fatty fish significantly lowered triglycerides [52]. Another study of cod protein supplementation showed significant reductions in CRP when compared to diets containing similar quantities of other types of protein [53]. This would suggest that there may be other elements in a diet of fish intake that may contribute to the CV benefits. The fact that several important nutrients such as vitamin D, phospholipids, and naturally occurring antioxidants are missing in fish oil supplements have some experts advocating dietary intake of fatty fish as the main source of omega-3 fatty acids. However, there is much data from landmark omega-3 trials such as the GISSIPrevenzione [19] and JELIS [30] trials that clearly indicate that fish oil supplements confer CV benefits. In addition, Harris and colleagues have illustrated that equivalent amounts of EPA and DHA from either a fish diet or a fish oil supplement enrich the omega-3 content of red blood cell membranes equally well [9,54].
| Type | DHA (gm per 100 g) | EPA (gm per 100 g) | DHA+EPA | Ratio DHA:EPA |
|---|---|---|---|---|
| Tuna, Bluefin | 1.141 | 0.363 | 1.504 | 3.1 : 1.0 |
| Tuna, Light, canned-in water | 0.223 | 0.047 | 0.27 | 4.8 : 1.0 |
| Tuna, Albacore, canned-in water | 0.629 | 0.233 | 0.862 | 2.7 : 1.0 |
| Salmon, Atlantic, farmed | 1.457 | 0.69 | 2.147 | 2.1 : 1.0 |
| Salmon, Atlantic, wild | 1.429 | 0.411 | 1.84 | 3.5 : 1.0 |
| Salmon, Chinook | 0.727 | 1.01 | 1.737 | 1.0 : 1.4 |
| Salmon, Sockeye | 0.7 | 0.53 | 1.23 | 1.3 : 1.0 |
| Mackerel, Atlantic | 0.699 | 0.504 | 1.203 | 1.4 : 1.0 |
| Herring, Atlantic | 1.105 | 0.909 | 2.014 | 1.2 : 1.0 |
| Trout, rainbow, farmed | 0.82 | 0.334 | 1.154 | 2.5 : 1.0 |
| Trout, rainbow, wild | 0.52 | 0.468 | 0.988 | 1.1 : 1.0 |
| Halibut | 0.374 | 0.091 | 0.465 | 4.1 : 1.0 |
| Cod | 0.154 | 0.004 | 0.158 | 38.5 : 1.0 |
| Haddock | 0.162 | 0.076 | 0.238 | 2.1 : 1.0 |
| Catfish, channel, farmed | 0.128 | 0.049 | 0.177 | 2.6 : 1.0 |
| Catfish, channel, wild | 0.137 | 0.1 | 0.237 | 1.4 : 1.0 |
| Swordfish | 0.681 | 0.087 | 0.768 | 7.8 : 1.0 |
| Grouper | 0.213 | 0.035 | 0.248 | 6.1 : 1.0 |
| Shrimp | 0.144 | 0.171 | 0.315 | 1.0 : 1.2 |
Table 1: Fish content of EPA + DHA [4,21]
A potential drawback to fish consumption over fish oil supplements is that certain fish can contain environmental toxins such as mercury, polychlorinated biphenyls (PCBs), chlordane, dioxins, and dichlorodiphenyltrichloroethane (DDT). Concentrations of these contaminants increase as fish move up the food chain, so top predators (such as swordfish, shark, king mackerel, largemouth bass, and walleye) may not be safe to eat on a frequent basis. (Table 2) On the other hand, many of the most popular fish varieties, such as salmon, shrimp, canned light tuna, pollock, and catfish, are relatively low in these chemical contaminants and are safe to eat up to several times per week [55]. Unfortunately, except for salmon, they are relatively poor sources of EPA and DHA.
| Species | Range (ppm) | Average (ppm) |
|---|---|---|
| Domestic Samples | ||
| Catfish | ND - 0.16 | ND |
| Cod | ND-0.17 | 0.13 |
| Crab | ND-0.27 | 0.13 |
| Flounder | ND | ND |
| Hake | ND | ND |
| Halibut | 0.12 - 0.63 | 0.24 |
| Pollock | ND | ND |
| Salmon (canned) | ND | ND |
| Salmon (fresh or frozen) | ND | ND |
| Shark | 0.30 - 3.52 | 0.84 |
| Swordfish | 0.36 - 1.68 | 0.88 |
| Tuna (canned) | ND - 0.34 | 0.20 |
| Tuna (fresh or frozen) | ND - 0.76 | 0.38 |
| Import Samples | ||
| Pollock | ND - 0.78 | 0.16 |
| Shark | ND - 0.70 | 0.36 |
| Swordfish | 0.80 - 1.61 | 0.86 |
| Tuna (canned) | ND - 0.39 | 0.14 |
| Tuna (fresh of frozen) | ND - 0.75 | 0.27 |
Table 2: Levels of Methyl Mercury in Commonly Consumed Fish [55]
Sustainability of wild fish stocks
Reports have documented a rapid worldwide decline in fish stocks over the past 50 years. Populations of some commercially popular fish species have collapsed to only 10% of their historic maximum with over 100 confirmed cases of marine-species extinctions. Worldwide fish stocks could be depleted within 40 years if harvests continue at the current rate [56,57]. This forecast has, however, been severely criticized [58] and may be overly pessimistic. With the current recommendations for omega-3 supplementation, increased pressure on fish stocks can be expected to continue. Furthermore, recommendations for higher levels of fish and/or fish oil consumption for variety of conditions such as hypertriglyceridemia, depression, inflammation, and cognitive impairment will exert further pressure on wild fish stocks . Whether the demand for fish and fish oil supplements can be met in a sustainable manner remains to be seen. Currently, close to 90% of Americans have suboptimal intake of omega-3 fatty acids [59]. Repletion of deficient omega 3 levels should be an important goal both for individual patient care and for public health but may seriously impact our fish sources. Major changes in the way we source omega 3 fatty acid supplements, such as aquaculture (fish farming) and bioengineering seem to be logical and increasingly practical solutions to this conundrum. Nontraditional sources of marine-based omega-3 fatty acids such as algae to produce DHA and yeast to produce EPA are being developed and may help to offload some of the burden of demand. Additionally, the plantderived omega-3 fatty acids, alpha-linolenic acid (ALA) and stearidonic acid (the delta-6 desaturase product of ALA which can be produced by biotechnology in soybeans) might provide some of the benefits of DHA and EPA but that remains to be firmly established [60].
Conclusions
Although more recent trials have not shown all the robust benefits that were seen in the earlier ones, the balance of evidence still favors the beneficial effects of omega-3 fatty acids in the primary and secondary prevention of CV diseases. We would recommend the use of omega-3 fatty acids in patients with CV disease and in HF, and suggest that the evidence is appealing for it’s use in the primary prevention of CHD.
References
- Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106: 2747-2757.
- Lavie CJ, Milani RV, Mehra MR, Ventura HO (2009) Omega-3 polyunsaturated fatty acids and cardiovascular diseases. J Am Coll Cardiol 54: 585-594.
- Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM (2006) n-3 Fatty acid from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr 84: 5-17.
- Nutrient Data: USDA Nutrient Laboratory (2006).
- Conquer JA, Holub BJ (1997) Dietary docosahexaenoic acid as a source of eicosapentaenoic acid in vegetarians and omnivores. Lipids 32: 341-345.
- Park Y, Harris WS (2003) Omega-3 fatty acid supplementation accelerates chylomicron triglyceride clearance. J Lipid Res 44: 455-463.
- Mozaffarian D, Rimm EB (2006) Fish intake, contaminants, and human health: Evaluating the risks and the benefits. JAMA 296: 1885-1899.
- Plourde M, Cunnane SC (2007) Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Appl Physiol Nutr Metab 32: 619-637.
- Harris WS (2007) Omega-3 fatty acids and cardiovascular disease: a case for omega-3 index as a new risk factor. Pharmacol Res 55: 217-223.
- Anand RG, Alkadri M, Lavie CJ, Milani RV (2007) The role of fish oil in arrhythmia prevention. J Cardiopulm Rehabil Prev 28: 92-98.
- O’Keefe JH, Abuissa H, Sastre A, Steinhaus DM, Harris WS (2006) Effects of omega-3 fatty acids on resting heart rate, heart rate recovery after exercise, and heart rate variability in men with healed myocardial infarctions and depressed ejection fractions. Am J Cardiol 97: 1127-1130.
- Ventura HO, Milani RV, Lavie CJ, Smart FW, Stapleton DD, et al. (1993) Cyclosporine-induced hypertension. Efficacy of omega-3 fatty acids in patients after cardiac transplantation. Circulation 88: II281-285.
- Thies F, Garry JM, Yaqoob P, Rerkasem K, Williams J, et al. (2003) Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomized controlled trial. Lancet 361: 477-485.Din JN, Harding SA, Valerio CJ, Sarma J, Lyall K, et al. (2008) Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis 197: 290-296.
- Din JN, Harding SA, Valerio CJ, Sarma J, Lyall K, et al. (2008) Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis 197: 290-296.
- Abuissa A, O’Keefe JH, Harris WS, Lavie CJ (2005) Autonomic function, omega-3 and cardiovascular risk Chest 127: 1088-1091.
- Villegas R, Xiang YB, Elasy T, Li HL, Yang G, et al. (2011) Fish, shellfish, and long-chain n-3 fatty acid consumption and risk of incident type 2 diabetes in middle-aged Chinese men and women. Am J Clin Nutr 94: 543-551.
- Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ (2002) Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens 20: 1493-1499.
- Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ (2007) Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure: INTERMAP study. Hypertension 50: 313-319.
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, et al. (2002) Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation 105: 1897-1903.
- Harris WS, Poston WC, Haddock CK (2007) Tissue n-3 and n-6 fatty acids and risk for coronary heart disease events. Atherosclerosis 193: 1-10.
- Lee JH, O’Keefe JH, Lavie CJ, Marchioli R, Harris WS, et al. (2008) Omega3 fatty acids for cardioprotection. Mayo Clin Proc 83: 324-332.
- Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, et al. (2007) Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr 85: 385-391.
- Noori N, Dukkipati R, Kovesdy CP, Sim JJ, Feroze U, et al. (2011) Dietary Omega-3 Fatty Acid, Ratio of Omega-6 to Omega-3 Intake, Inflammation, and Survival in Long-term Hemodialysis Patients. Am J Kidney Dis 58: 248-256.
- Itoh M, Suganami T, Satoh N, Tanimoto-Koyama K, Yuan X, et al. (2007) Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol 27: 1918-1925.
- Mehra MR, Lavie CJ, Ventura HO, Milani RV (2006) Fish Oils produce antiinflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant 25: 834-838.
- Moyers B, Farzaneh-Far R, Harris WS, Garg S, Na B, et al. (2011) Relation of whole blood n-3 fatty acid levels to exercise parameters in patients with stable coronary artery disease (from the heart and soul study). Am J Cardiol 107: 1149-1154.
- Davidson MH, Stein EA, Bays HE, Maki KC, Doyle RT, et al. (2007) Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther 29: 1354-1367.
- Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, et al. (2007) Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr 85: 1222-1228.
- Burr ML, Fehily AM, Gilbert JF, Rogers S, Holliday RM, et al. (1989) Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet 2: 757-761.
- Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, et al. (2007) Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomized open label, blinded endpoint analysis. Lancet 369: 1090-1098.
- Ness AR, Hughes J, Elwood PC, Whitley E, Smith GD, et al. (2002) The longterm effect of dietary advice in men with coronary disease: follow-up of the Diet and Reinfarction trial (DART). Eur J Clin Nutr 56: 512-518.
- Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, et al. (2001) Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr 74: 50-56.
- Von Schacky C, Harris WS (2007) Cardiovascular benefits of omega-3 fatty acids. Cardiovasc Res 73: 310-315.
- Rauch B, Schiele, Schneider S, Diller F, Victor N, et al. (2010) OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation 122: 2152-2159.
- Kromhout D, Giltay EJ, Geleijnse JM (2010) n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med 363: 2015-2026.
- Calo L, Bianconi L, Colivicchi F, Lamberti F, Loricchio ML, et al. (2005) N-3 fatty acids for the prevention of atrial fibrillation after coronary artery bypass surgery: A randomized, controlled trial. J Am Coll Cardiol 45: 1723-1728.
- Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM (2010) Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA 304: 2363- 2372.
- Raitt MH, Connor WE, Morris C, Kron J, Halperin B, et al. (2005) Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators. JAMA 293: 2884-2891.
- Brouwer IA, Zock PL, Camm AJ, Böcker D, Hauer RN, et al. (2006) Effects of fish oil on ventricular tachyarrhythmia and death in patients with implantable cardioverter defibrillators. JAMA 295: 2613-2619.
- Leaf A, Albert CM, Josephson M, Steinhaus D, Kluger J, et al. (2005) Prevention of fatal arrhythmias in high-risk subjects by fish oil n-3 fatty acid intake. Circulation 112: 2762-2768.
- Macchia A, Levantesi G, Franzosi MG, Geraci E, Maggioni AP, et al. (2005)Left ventricular systolic dysfunction, total mortality, and sudden death in patients with myocardial infarction treated with n-3 polyunsaturated fatty acids. Eur J Heart Fail. 7: 904-909.
- Finzi AA, Latini R, Barlera S, Rossi MG, Ruggeri A, et al. (2011) Effects of n-3 polyunsaturated fatty acids on malignant ventricular arrhythmias in patients with chronic heart failure and implantable cardioverter-defibrillators: A substudy of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca (GISSI-HF) trial. Am Heart J 161: 338-343.e1.
- Levitan EB, Wolk A, Mittleman MA (2009) Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: a population-based prospective study of middle-aged and elderly men. Eur Heart J 30: 1495-1500.
- Gissi-HF Investigators, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, et al. (2008) Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 372: 1223-1230.
- Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS (2005) Fish intake and risk of incident heart failure. J Am Coll Cardiol 5: 2015-2021.
- Lavie CJ, O’Keefe JH, Milani RV, Ventura HO, Mehra MR (2009) New data on the clinical impact of exercise training, fish oils, and statins in heart failure. Phys Sportsmed 37: 22-28.
- Yamagishi K, Nettleton JA, Folsom AR (2008) Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 156: 965-974.
- Nodari S, Triggiani M, Campia U, Manerba A, Milesi G, et al. (2011) Effects of n-3 polyunsaturated fatty acids on left ventricular function and functional capacity in patients with dilated cardiomyopathy. J Am Coll Cardiol 57: 870- 879.
- Harris WS, VonSchaky C (2004) The omega-3 index: a new risk factor for death from coronary artery disease? Prev Med 39: 212-220.
- Block RC, Harris WS, Reid KJ, Sands SA, Spertus JA (2008) EPA and DHA in blook cell membranes from acute coronary syndrome patients and controls. Atherosclerosis 197: 821-828.
- Albert CM, Campos H, Stampfer MJ, Ridker PM, Manson JE, et al. (2002) Blood levels of long-chain n-3 fatty acids and the risk of sudden death. N Engl J Med 346: 1113-1118.
- Rajaram S, Haddad EH, Mejia A, Sabaté J (2009) Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr 89: 1657S-1663S.
- Ouellet V, Weisnagel SJ, Marois J, Bergeron J, Julien P, et al. (2008) Dietary cod protein reduces plasma C-reactive protein in insulin-resistant men and women. J Nutr 138: 2386-2391.
- Harris WS, Pottala JV, Sands SA, Jones PG (2007) Comparison of the impact of fish and fish oil capsules on the n-3 fatty acid content of blood cells and plasma phospholipids. Am J Clin Nutr 86: 1621-1625.
- Foulke JE (1994) Mercury In Fish: Cause For Concern? : U.S. Food and Drug Administration.
- Jenkins DJ, Sievenpiper JL, Pauly D, Sumaila UR, Kendall CW, et al. (2009) Are dietary recommendations for the use of fish oils sustainable? CMAJ 180: 633-637.
- Lee JH, O’Keefe JH, Lavie CJ, Harris WS (2009) Omega-3 fatty acids: cardiovascular benefits, sources and sustainability. Nat Rev Cardiol 6: 753- 758.
- Murawski S, Methot R, Tromble G (2007) Biodiversity loss in the ocean: how bad is it? Science 316: 1281-1284.
- Sands SA, Reid KJ, Windsor SL, Harris WS (2005) The impact of age, body mass index, and fish intake on the EPA and DHA content of human erythrocytes. Lipids 40: 343-347.
- Harris WS (2008) Cardiovascular risk and alpha-linolenic acid: can Costa Rica clarify? Circulation 118: 323-324.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 16262
- [From(publication date):
June-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11517
- PDF downloads : 4745