Research Article Open Access
Oil Recovery From Fuel Oil Storage Tank Sludge Using Biosurfactants
| Tânia M. S. Lima*, Andréia F. Fonseca, Bruna A. Leão, Ann H. Mounteer, Marcos R. Tótola and Arnaldo C. Borges | |
| Environmental Biodiversity and Biotechnology Laboratory, Microbiology Department, Federal University of Viçosa, 36571-000 – Viçosa, Minas Gerais, Brazil | |
| Corresponding Author : | Dr. Tânia Maria da Silva Lima Environmental Biodiversity and Biotechnology Laboratory Microbiology Department, Federal University of Viçosa Viçosa, Minas Gerais, Brazil Tel: +55-31-3899-2903 Fax: +55-31- 3899-2953 E-mail: tlima@vicosa.ufv.br |
| Received June 13, 2011; Accepted September 16, 2011; Published September 18, 2011 | |
| Citation: Lima TMS, Fonseca AF, Leão BA, Mounteer AH, Tótola MR(2011) Oil Recovery From Fuel Oil Storage Tank Sludge Using Biosurfactants. J Bioremed Biodegrad 2:125. doi: 10.4172/2155-6199.1000125 | |
| Copyright: © 2011 Lima TMS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The petroleum industry generates large amounts of solid and semisolid wastes known as oily sludges. The composition of oily sludge varies due to the large diversity in the quality of crude oils, differences in the processes used for oil–water separation, leakages during industrial processes, and also mixing with the existing oily sludge. Usually, the oily sludge contains water, sand, oils, grease, organic compounds, chemical elements, and metals. Those sludges can be generated in several steps of the petroleum production and refining, such as in oil/water separation steps and in the bottom of tanks. The accumulation of oily residues in petroleum industry poses a serious environmental problem. The purpose of this work was evaluate an alternative process to removal of oily sludges through the use of biosurfactants to reduce the viscosity and promote formation of oil/water emulsions making sludge pumping easier and permitting crude oil recovery after breaking the emulsion. Five bacterial isolates were selected for their biosurfactant production potential after screening microorganisms recovered from oil-contaminated sites. Supernatants obtained from autoclave cell suspensions (hereby referred to as autoclaved-supernatant) were mixed with oily sludge collected from fuel oil storage tanks to a final concentration of 0.01%, in order to separate the oil from the inert material. The process proved to be highly efficient for oil recovery, and resulted in up to 95% reduction in sludge volume. The use of cell-free supernatant medium obtained from biosurfactant-producing bacterial strains to treat oily sludges may be an economically and environmentally viable technology, considering the small volume of microbial culture required for the treatment.
| Keywords |
| Emulsification; Surface tension; Petroleum; Oily sludge; CMC |
| Introduction |
| During oil production and processing, large amounts of oily sludges are generated. These sludges are potential environmental contaminants and, at the same time, take up useful storage tank room [1]. Oily sludge composition is variable and includes oil, coarse solids and water. Oily sludges settle on the bottom of storage tanks and their removal is a costly and time consuming process that depends on additional operations to ensure final sludge disposition in an environmentally sound manner [2]. |
| The high stability of oily sludges is attributed to the adsorption of oil on to solid particles, leading to the build up of a protective layer. This stability is enhanced by the presence of polar fractions in the oil, especially resins and asphaltenes that are also responsible for the high viscosity of such sludges. Furthermore, this protective layer creates a favourable environment for microbial corrosion and may shelter a high and diverse microbial community, thus serving as a contamination reservoir each time the storage tank is used. |
| Oily sludge accumulation reduces tank storage capacity and this, together with the possibility of corrosion, makes it necessary to periodically remove these deposits. Conventional petroleum industry waste treatment strategies present various shortcomings, including high cost and need for specialized equipment and skilled personnel. Waste landfarming is a lengthy process and may cause groundwater contamination [3]. In addition, it has been shown that recalcitrant organics in petroleum residues can accumulate in soil. For example, Bosert et al. characterized the fate of hydrocarbons during a laboratory study of oily sludge application to soil, simulating an active petrochemical plant landfarming operation. During intensive landfarming of petroleum waste, a gradual accumulation of petroleum hydrocarbons occurred in the soil over time, amounting to 13.8% (ww-1). |
| Removal of oily sludge from storage tanks can be carried out by using biosurfactants to reduce viscosity and promote water/oil or oil/ water emulsification [5]. This process facilitates sludge pumping and allows crude oil recovery after emulsion is broken. Biosurfactants are amphipathic molecules containing hydrophilic and hydrophobic moieties, found mainly on the cell surface or excreted to culture medium by a broad range of microorganisms [6]. These products increase the aqueous dispersion of poorly soluble compound by many orders of magnitude and change the affinity between microbial cells and hydrocarbons by increasing cell surface hydrophobicity [7]. |
| Few evaluations of biosurfactant performance in storage tank cleaning processes have been reported in the literature. In this study, we report the efficacy of biosurfactant produced by different microbial species in removing oily sludge from fuel oil storage tank. |
| Materials and Methods |
| Oily sludge characterization |
| Oily sludge was collected from a fuel oil storage tank at the Capuava Refinary (RECAP/Petrobras) in Mauá, São Paulo state. Raw sludge and its organic fraction were partially characterized. The organic phase was extracted by mixing the oily sludge with methylene chloride at room temperature for 24h. After extraction, the solvent was removed in a rotary evaporator. Aqueous phase nitrogen, iron and phosphorous contents were determined by chemiluminescence (Antek 700) [8]. Organic phase nitrogen content was determined by the Kjeldahl method [9]. Raw sludge and organic phase water content were determined by the Karl Fisher method [10]. Raw sludge density [11], viscosity [12] and pH were also determined. |
| Biosurfactant producing microorganisms |
| The five bacterial isolates used in this study were isolated from the RECAP oily sludge (Dietzia maris sp. LBBMA 191 and Arthrobacter oxydans LBBMA 201) and from soil and water samples collected in areas contaminated by petroleum or petroleum by-products (Pseudomonas aeruginosa LBBMA 88A, Bacillus sp. LBBMA 111A and Bacillus subtilis LBBMA 155). Isolates used in this study are maintained in the culture collection of the laboratory of Biotechnology and Biodiversity for the Environment (LBBMA) – Federal University of Viçosa (LBBMA-UFV, Viçosa, Minas Gerais, Brazil). |
| Growth and biosurfactant production |
| Isolates were activated by overnight growth in R2A medium [13] and incubated at 30°C and 200 rpm for seven days in mineral medium [5] modified by Lima [14]. This medium was supplemented with 2% (wv-1) glucose. Oily sludge (2% vv-1) was added after glucose exhaustion, as determined by GOD PAP reagent (Merck System, Darmstadt, Germany). Biosurfactant production was evaluated daily in cell suspensions and in the culture supernatant, obtained by centrifugation at 12.000 g for 15 min. To check for biosurfactant presence, the surface tension between a cell suspension/supernatant layer and air was measured with a Fisher tensiometer (Fisher Surface Tensiomat, Model 21, Pittsburgh, USA), according to the du Nouy ring method [15]. |
| Critical micelle Dilution and emulsion stability |
| Critical micelle dilution (CMD) was determined by measuring water-air surface tension of dilutions of the autoclaved-supernatants by the du Nouy ring method [15]. Biosurfactant dilutions were prepared in distilled water. The CMD was defined as the dilution above which surface tension begins to increase [15]. A positive control, using a 1% (pv-1) Sodium Dodecyl Sulfate (SDS) solution, was included in each assay. |
| Emulsification and emulsion stability were evaluated by the method proposed by Das et al. [16]. 2mL of the autoclaved-supernatant were mixed with 2mL kerosene (Esso Brasileira de Petróleo, Contagem, Brazil) in a test tube (100mm x 15mm). The test tube was sealed with parafilm, vortexed for 2 min, and then left standing for two minutes, after which the emulsion height was measured. Emulsion stability was evaluated by measuring its height every two hours for up to 42 hours. Emulsion volume (EV) was calculated from the test tube height and diameter. An emulsion was considered stable if its volume, 24 hours after formation, corresponded to 50% or more of its original volume, as proposed by Willumsem and Karlson [17]. |
| Treatment of oily sludges with biosurfacants |
| Oily sludge was sterilized in an autoclave at 121°C for 20 min. Tap water (56mL), sterilized oily sludge (44 mL) and autoclaved-supernatant (10μL) were added to duplicate sterile flasks. Flask contents were mixed in an orbital shaker at 200 rpm for five days [5]. By the end of the 5th day, the oil present in the oily sludge had been emulsified. Emulsions were broken by adding 1mL of a 20% magnesium nitrate solution. The aqueous phase was discarded and the amount of oil recovered from the sludge was measured. The percent of oil recovered from the sludge was calculated on a volume basis. |
| Results and Discussion |
| Oily sludge characterization |
| RECAP oily sludge contained considerable amounts of nitrogen, iron and phosphorous (table 1). Most of the nitrogen was present as organic nitrogen, while iron and phosphorous were present mainly as inorganic ions. Inorganic nutrients availability, together with an elevated raw oily sludge water content and abundance of organic substrate, may promote microbial activity and multiplication in fuel oil storage tanks. |
| Sludge viscosity decreased substantially with increasing temperature (table 1). Freely flowing liquids have viscosities below 200 cP [18]. The high oily sludge viscosity (899.3 cP at room temperature) is an evidence of how difficult it is to remove this residue from the bottom of oil storage tanks. |
| Surface tension and Critical Micelle Dilution (DMC) |
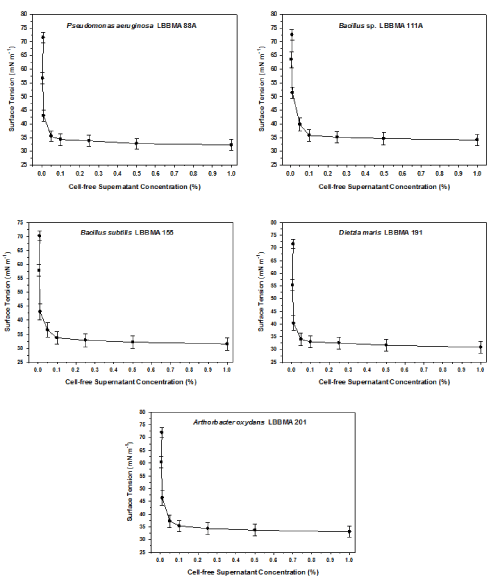
| The autoclaved-supernatant of the five isolates reduced surface tensions to below 40 mNm-1 (Figure 1), which is the value proposed by Cooper et al. (1979) [15] as an indicative of the presence of tensoactive compounds. |
| Utilization of oily sludge was effective for biosurfactant production. This effectiveness can be attributed to the composition of the organic phase of oily sludges, which contains basically heavy oil fractions whose presence appears to stimulate the excretion of biosurfactant by microorganisms [19]. Oily sludge may be considered as a potential substrate for large scale biosurfactant production. |
| The CMD’s of the autoclaved-supernatants of the five isolates was 0.01% (Figure 1). At higher dilutions, biosurfactant molecules could not form stable micelles and the surface tension increased abruptly. This CMD values indicate that biosurfactant concentrations in autoclaved-supernatants were 10,000-fold higher than the critical micelle concentration (CMC). These results are similar to those reported by Banat et al. [5]. |
| Emulsion formation and stability |
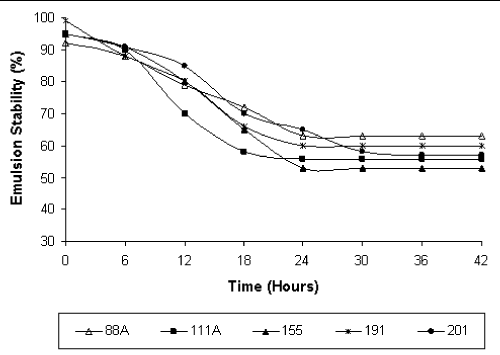
| The biosurfactants produced by the five bacterial strains formed stable emulsions with kerosene (Figure 2) for up to 42 hours [16], working with biosurfactants produced by Micrococcus sp., reported stable emulsion for only 20 hours. The ability to form stable emulsions is a specific characteristic of amphiphilic molecules [15,20]. The results obtained in this study are relevant, since the capacity to form stable emulsions is important for various biosurfactant applications, including solubilization of hydrophobic compounds in bioremediation processes [21,22], in microbiologically enhanced oil recovery [23], and in oil recovery from oily sludges [5], among others. |
| Haba et al. [24] reported one Arthrobacter oxydans with an emulsifying capacity of 1.9 % and Nazina et al. [25] reported an emulsifying capacity of 35 % for Dietzia. Our results show a higher emulsion capacity of the strains Dietzia sp. LBBMA 191 and Arthrobacter oxydans LBBMA 201 (60 % up to 42 hours of static storage). To our knowledge, this is the first report of high emulsion capacity for Dietzia and Arthrobacter oxydans, suggesting the potential application of these strains in bioremediation technologies. |
| Treatment of oily sludge with biosurfactants |
| It is important to distinguish between biosurfactant efficiency and efficacy. Efficiency measures the biosurfactant concentration necessary to significantly reduce water surface tension, while efficacy measures the lowest surface tension obtained with a particular biosurfactant [26]. Microbial growth media containing biosurfactants were used to treat oily sludge in order to separate oil from inert matter. Some of the growth media contained biosurfactants at concentrations capable of maintaining surface tension below 40mNm-1, even after a 10,000-fold dilution (Figure 1). Furthermore, biosurfactants resisted sterilization by autoclaving without loosing activity. Oily sludge hydrocarbons were emulsified by the biosurfactants during mixing, which was readily observed from mixing onset (Figure 3). Biosurfactants produced by the bacterial strains used in this study were efficient in removing oil retained in the oily sludge, recovering up to 95% of the total oily sludge volume as oil (Table 2). This recovery efficacy is higher than the 91% recovery reported by Banat et al. [5]. The oily sludge used in this study originated from catalytically cracked fuel oil and so is expected to be enriched in long-chain hydrocarbon. A temperature of about 50°C was used by Banat et al. [5], while in the present study the temperature was maintained at 30°C. Given that temperature has a significant effect on sludge viscosity (table 1), the results obtained in this study were highly satisfactory and the biosurfactants studied may be a promising alternative process for oil storage tank cleaning. This is especially evident when it is considered that only 2% of the oil present in the oily sludge was recovered in the absence of biosurfactant (Table 2) (Figure 3). |
| Conclusion |
| The biosurfactants produced by five bacterial isolates from the LBBMA culture collection proved to be highly efficient for oily sludge treatment, and their use led to a 95% recovery of the oil contained in a fuel oil storage tank oily sludge. Waste solids remaining after biosurfactant treatment contained an insignificant amount of residual oil. Such treatment on an industrial scale would reduce waste disposal cost and lower the risk of environmental contamination by oil present in disposed oily sludge residual solids. |
| The results showed that the isolates and its produced biosurfactants with effective surface and emulsifying properties represent a promising potential for application in bioremediation of soil environments polluted with hydrocarbons. |
| Acknowledgements |
| The authors would like to acknowledge the financial support granted by the |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 20117
- [From(publication date):
September-2011 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 14994
- PDF downloads : 5123
