Research Article Open Access
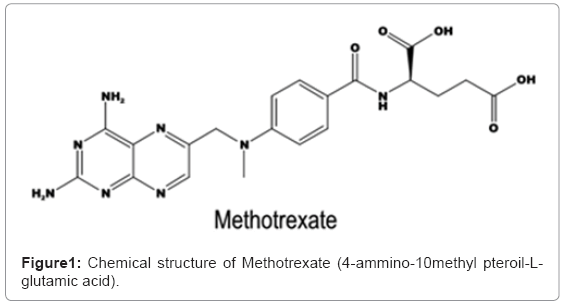
Obtaining of SiO2 Nanostructured Materials for Local Drug Delivery of Methotrexate.
López T1,2,5*, Alvarez M2, Arroyo S2,6, Sánchez A3, Rembao D5 and López R21Depto. de Atención a la Salud, Universidad Autónoma Metropolitana-Xochimilco, Calz. Del Hueso 1100, Col. Villa Quietud, 04960, Tlalpan, México, DF
2Laboratorio de Nanotecnología, Instituto Nacional de Neurología y Neurocirugía “MVS”. Insurgentes Sur 3877, Col. La Fama, C. P. 14269, Tlalpan, México
3Laboratorio de Neuropatología, Instituto Nacional de Neurología y Neurocirugía “MVS”. Insurgentes Sur 3877, Col. La Fama, C. P. 14269, Tlalpan, México
4Laboratorio de Patología Experimental, Instituto Nacional de Neurología y Neurocirugía “MVS”. Insurgentes Sur 3877, Col. La Fama, C. P. 14269, Tlalpan, México
5Department of Chemical and Biomolecular Engineering, Tulane University, New Orleans, LA 70118 USA
6Escuela Superior de Medicina. Instituto Politécnico Nacional. Plan de San Luis y Díaz Mirón s/n, Col. Casco de Santo Tomas, Delegación Miguel Hidalgo, C.P. 11340, México, DF
- Corresponding Author:
- Tessy López, PhD
Insurgentes Sur 3877, Col La Fama, CP 14269, Tlalpan, México, DF
Tel: +52 55 56063822 ext 5034
Fax: +52 55 5528 8036
E-mail: tessy3@prodigy.net.mx
Received date: June 03, 2010; Accepted date: November 13, 2011; Published date: November 13, 2011
Citation: López T, Alvarez M, Arroyo S, Sánchez A, Rembao D, et al. (2011) Obtaining of SiO2 Nanostructured Materials for Local Drug Delivery of Methotrexate. J Biotechnol Biomaterial S4:001. doi:10.4172/2155-952X.S4-001
Copyright: © 2011 López T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Local delivery of drugs has become an alternative to overcome several side effects of conventional drug administration methods. Because of their novel properties, nanomaterials for controlled drug release have shown enormous potential in the treatment of cancer. In the present work a chemotherapeutic agent, methotrexate (MTX) was encapsulated into a sol-gel SiO2 matrix, as a reservoir for local controlled release in the treatment of a solid brain tumor like Glioblastoma Multiforme (GBM) in an animal model. In order to study interactions between the drug and the SiO2, several physicochemical characterization techniques were carried out. FTIR spectroscopy showed evidence drug incorporation into the silica matrix as the presence of bands at 1504 cm-1, 1604 cm-1 and 1637 cm-1 reveals. This is supported by UV-Vis results, since silica spectrum acquired new features. The materials are mesoporous with a mean pore diameter about 78 Å. In vitro drug release was performed by UV-Vis spectroscopy following the main absorption band of MTX at 348 nm. Drug release was followed and monitored by 16 days. For in vivo evaluation GBM model was performed in Wistar rats once the tumor was developed a reservoir was placed by stereotactic surgery. After three months of tumor inoculation animals treated with MTX-SiO2 survived while in those untreated survival did not exceed 30 days.
Keywords
Nanomaterials; Methotrexate; Sol-gel silica; Cancer; Controlled drug release
Abbreviations
MTX: Methotrexate; GBM: Glioblastoma Multiforme; FTIR: Fourier Transformed Infrared; TGA: Thermogravimetric Analysis; H-E: Hematoxiline Eosine
Introduction
Due to the possibility to avoid undesirable side effects, local administration of therapeutics represents an excellent alternative to treat some refractory diseases like cancer [1]. Methotrexate (MTX) is an anti-cancer drug that interferes with folic acid pathway, by competitively inhibiting dihydrofolate reductase, preventing its activation to folinic acid [2]. This drug is administered for the treatment of neoplastic diseases such as gestational choriocarcinoma, invasive chorioadenoma, acute lymphocytic leukemia, meningeal leukemia, breast cancer certain types of cancer of skin, head, neck and lung and recently in brain tumors [3]. Its side effects involve: stomach pain, nausea, vomiting, anorexia, diarrhea and mouth ulcers.
Nanomaterials and particularly inorganic nanoparticleshave emerged as efficient carriers for a variety of drugs [4,5] and research in this field has increased during the last years. The search for efficient and non-toxic carriers to deliver drugs into cells has been a challenging and interesting area of research. In the past decades, many carriers have been investigated extensively. These carriers can be classified into four major groups: viral carriers, organic cationic compounds, recombinant proteins and inorganic nanoparticles [6]. Among the advantages of inorganic nanoparticles, suitability to be used at physiological conditions and low toxicity are the most relevant. To make possible their use in biomedical applications, surface functionalization is essential and – NH2, -COOH and –SH groups play an important role for anchoring of biomolecules [7]. Regardless to the synthesis of inorganic nanoparticles there are several reported techniques, but one of the most versatile is the sol-gel process, because of it is helpful for encapsulation of drugs and biological molecules [8,9]. Besides, functionalization is easy at the beginning of the synthesis. By controlling synthesis parameters, sol-gel nanomaterials with desirable properties can be obtained and almost any drug can be encapsulated. With the development of nanotechnology and the subsequent advances in nanomedicine, diseases like cancer, which commonly require systemic therapeutic approach, could be clinically managed with local or regional strategies directly at the tumor site [10]. In this research, we investigated a biocompatible silica- based nanomaterial with MTX encapsulated for controlled drug release applications in order to use it as a reservoir for local treatment of GBM.
Materials and Methods
MTX-SiO2 synthesis
10 g of Sol-gel SiO2 were prepared at pH 7, using tetraethoxisilane (TEOS, SIGMA 98%) and ethanol, as previously reported [11-13]. 500 mg of MTX (Medsatrexate®) were added during the synthesis, in order to encapsulate the drug. The obtained gel was dried at room temperature until solvent was completely removed. The solid was crushed into a fine powder. A SiO2 reference sample (without MTX) was prepared under the same conditions.
Characterization
FTIR: Infrared spectra were taken in a NICOLET FTIR spectrometer model MAGNA 560 with resolution of 4 cm-1, 100 scans and equipped with DTGS detector. The solid (5%wt) was milled with KBr and then 100 mg of this mixture were compressed until a thin and transparent wafer was obtained.
UV-Vis spectroscopy: Measurements were performed in a Varian Spectrometer, Cary-1 with integration sphere. The spectra of the powders were taken as obtained.
TGA: The resulting gels were analyzed in a Shimadzu model DT-30 in conditions of static atmosphere. The heating rate applied was 10°C min-1.
In vitro drug release: 50 mg wafers of MTX-SiO2 were made and placed in 50 ml of deionized water at room temperature. The same procedure was carried out using synthetic cerebrospinal fluid. The release test was carried out twice. Aliquots were taken at periodical intervals and analyzed by UV-Vis spectroscopy following the band at 348 nm. Calibration curve was made and concentration at time t was calculated using the Lambert- Beer law.
| CONCENTRATION 0.15 mg/ml |
LIVING CELLS | DEAD CELLS | % OF LIVING CELLS | % OF DEAD CELLS | TOTAL CELL |
|---|---|---|---|---|---|
| CONTROL | 135, 000 | 15, 000 | 90 | 10 | 1.5X105 |
| SiO2 | 23, 400 | 106, 600 | 18 | 82 | 1.3X105 |
| Methotrexate/SiO2 | 3, 200 | 156, 800 | 2 | 98 | 1.6x105 |
Table 2: Cytotoxic effect of Mtx/SiO2 compared to SiO2 and to a control group without treatment at 12 hours of exposure in a dilution of 0.15 mg/ml.
| group | Description | Findings |
|---|---|---|
| 1 | surgical procedure | Inflammation associated to injection |
| 2 | Tumor | 17% survival. Fast cell proliferation, inflammation and highly invasive cell behaviour |
| 3 | Tumor+MTX | 75% of the animals died 33 days later of infiltration. No significant therapeutic effect was observed |
| 4 | MTX/SiO2 | 100% survival. Mild inflammation and macrophages were observed around particles |
| 5 | Tumor+ MTX/SiO2 | 80% survival. Small lesion area, moderate chronic inflammation with abundant macrophages, lymphocytes and hemosiderophages. After 6 months no tumor activity was observed . |
Table 3: Summarized in vivo findings.
In vitro cytotoxic test
Cells of the T98G cell line were cultivated in 24-well plates until get the 90% of confluence and treated with different compounds (CisPlatin, SiO2 and MTX/SiO2) with different doses (0.15 mg, 0.25 mg, 0.5 mg and 0.75 mg/mL) during 12 hours to reach the cytotoxic effect. The medium was removed by suction and then washed twice with sterile PBS, methanol was added up to completely cover and left it to stand for 10 minutes, the cells were stained with crystal violet (ethanol at 10%) for 30 minutes and finally, the plates were washed extensively. Cellular count was made to estimate cell dead percentage.
Animal model: Animal experiments were made according to the Mexican Norm (NOM-062-ZOO-1999), in the animal care facility from the National Institute of Neurology and Neurosurgery. The studies were made with the following model: 1 x106 C6 (ATCC) cells were suspended in 2 μL of modified Dulbecco’s medium and were inoculated in the frontal cortex of male Wistar rats (225-250 g) through stereotactic surgery. The coordinates were AP=1.6 (A), L=3.0 y V=2.0.The animals were randomly allocated in 5 groups (Table 1).
| Group | n | Description |
|---|---|---|
| 1 | 6 | Control of the procedure. Sterotactic injection of saline solution without tumor. |
| 2 | 6 | Control of tumor growth without any treatment. |
| 3 | 10 | Tumor + MTX (260µg/2µl) |
| 4 | 5 | MTX/SiO2 without tumor |
| 5 | 10 | Tumor + MTX/SiO2 |
Table 1: Animal test.
5 ±0.5 mg of nanoparticles were slightly compressed into cylinders of approximately 2x1 mm for intratumoral application in groups 4 and 5. The corresponding cylinder was placed at the 14th day after cell inoculation in the same coordinates where cells were previously injected.
In order to determine therapy effectiveness, the animals were kept under close observation and tested with physical proves for clinical evaluation. At the end of the experiment, the animals were sacrificed and brains processed. H-E stain was performed for the histological analysis of the tumor growth and the therapy effect and GFAP Immunohistochemistry for tumor cells detection.
Results and Discussion
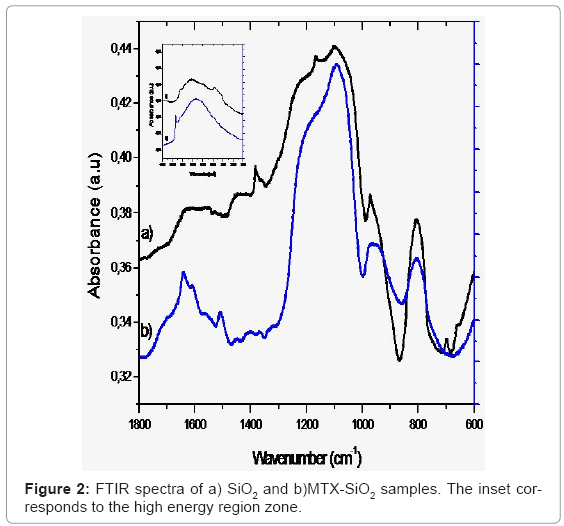
FTIR spectra of the SiO2 reference and the MTX-SiO2 materials are shown in figure 2. In the high energy region for the SiO2 we observed a broad band centered at 3437 cm-1 corresponding to O-H stretching due to the presence of water. The band at 3643 cm-1 is attributed to O-H bonded to Si atoms. In the MTX-SiO2 sample, these bands appear at 3332 cm-1 and 3652 cm-1 respectively. The intensity of this broad band is due to the addition of MTX, which contributes with vibrations of its COOH and N-H groups [14]. The main difference between both SiO2 and MTX-SiO2 spectra is in the mid-energy region since three bands located at 1504 cm-1, 1604 cm-1 and 1637 cm-1 assigned to C=N and N-H stretching vibrations can be observed, these bands has been previously reported for pure MTX [15].
Both samples exhibited same feature bands in the low-energy region, the observed bands at 1099 cm-1 and 808 cm-1 are attributable to the asymmetric and symmetric siloxane (O-Si-O) bond vibrations, respectively.
UV-vis spectroscopy
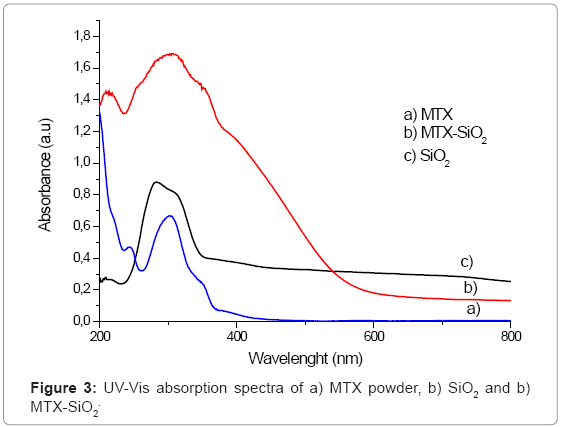
UV-Vis spectroscopic analysis provides more information about the incorporation of the drug into the silica matrix. This technique is the most commonly employed for MTX determinations. MTX absorbs strongly in the UV-Vis region due to the presence of heteroaromatic pterine chromophore. The main absorption bands of pure MTX are situated at the wavelengths of 255 nm, 302 nm and 352 nm. When MTX is incorporated into SiO2, the UV-Vis spectrum of SiO2 is modified (Figure 3) since a broad band around 302 nm is observed, but the presence of two small shoulders at 254 and 352 nm can be detected. These bands can be attributable to the presence of MTX and the width of the band is due to overlapping of MTX and SiO2 characteristic bands.
N2 adsorption analysis
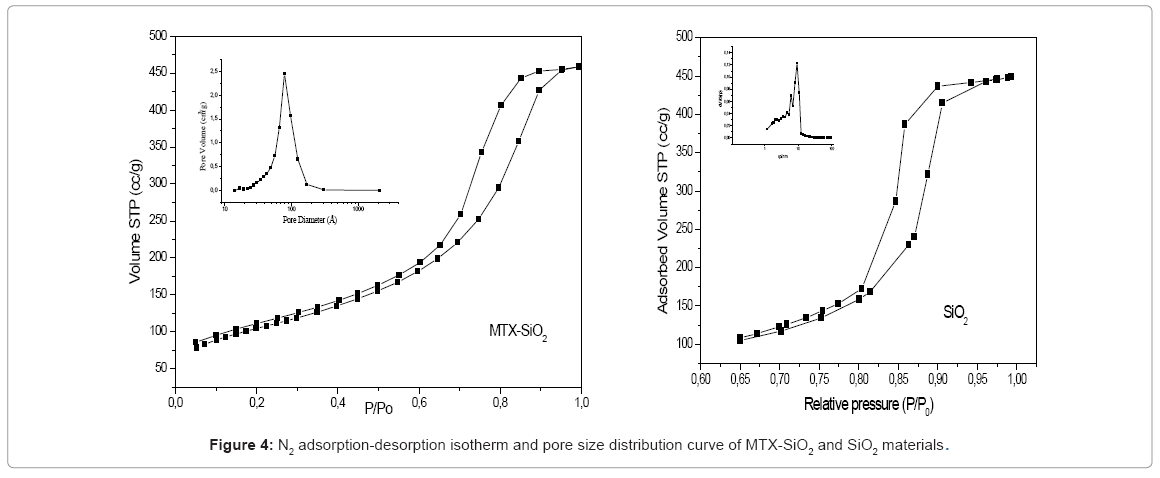
The adsorption-desorption isotherms of SiO2 and MTX-SiO2 are showed in figure 4 and corresponds to a type IV (IUPAQ classification). Its hysteresis loop is associated with capillary condensation taking place in mesopores, and the limiting uptake over a range of high p/p°. The initial part of the Type IV isotherm is attributed to monolayer-multilayer adsorption since it follows the same path as the corresponding part of a Type II isotherm obtained with the given adsorptive on the same surface area of the adsorbent in a non—porous form. The BET [16] surface area value was 371 m2/g, while pore size distribution curve showed a broad peak from 30 to 170 Å centered at 78Å. The pores of the obtained material are in the mesopore range. Hysteresis of the isotherm is type H2 , characteristic of solids consisting of particles crossed by nearly cylindrical channels or made by aggregates of spherical particles and pores with relative non-uniform size or shape [17]. The observed hysteresis even at lower relative pressure can be associated with presence of some micropores [18].
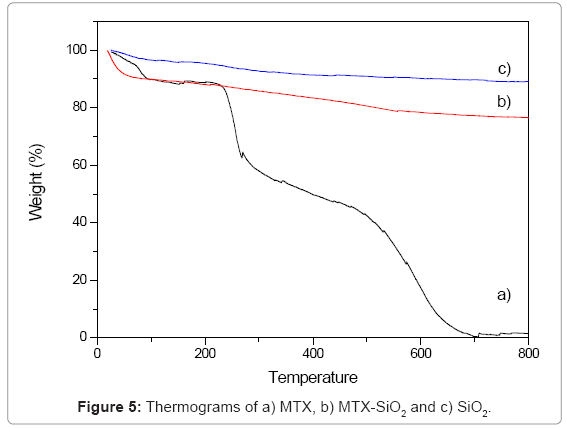
Thermal analysis: Thermograms of the samples are showed in figure 5. In MTX-SiO2 a mass loss observed between 24°C to 100°C is related with decomposition of the drug, solvent residues and removal of the surface adsorbed water of the material. This first loss was about 10%. Between 100°C and 800°C the sample experiments a gradual variation in weight with a final mass loss of 26%. This second step is related with the partial dehydroxylation of the SiO2 surface as well as the functionalizing agent. In pure MTX, a three-step process is observed: the first loss occurred at 85ºC and is associated with endothermic dehydration of the commercial sample associated with a mass loss of about 11% while decomposition of MTX occurs at 252ºC.
MTX release study
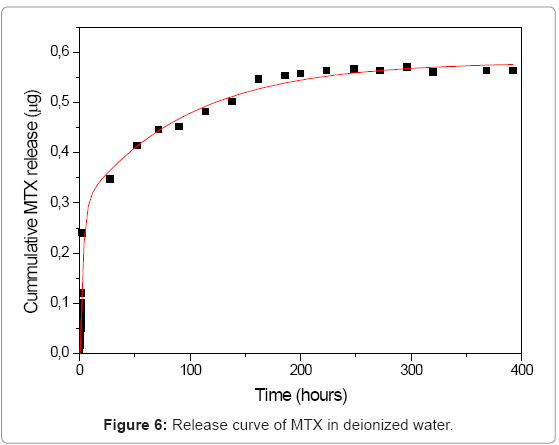
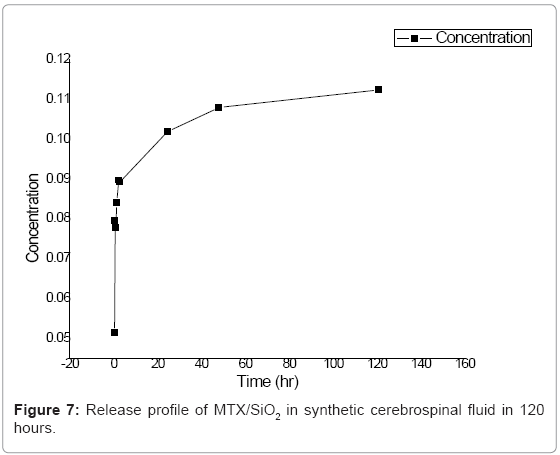
Several studies of drug release from silica materials have been made and have proved their suitability for controlled release applications [19,20]. In our silica sol-gel materials, we followed the release for a month in deionized water and for 5 days in synthetic cerebrospinal fluid. In deionized water, MTX was released in two steps as can be seen in the curve (Figure 6). The first step is related with the release of the drug located on or near the surface of the nanoparticles and with the poorly entrapped drug molecules. In the first two hours of the experiment, data showed an exponential growing. At t=72, release process becomes slower; this can be explained due to the diffusion of the medium into the SiO2 network, releasing molecules that are deeper occluded. In synthetic cerebrospinal fluid the profiles showed that methotrexate releases in controlled and sustained way (Figure 7).
The synthesis method allows the formation of a net of SiO2 encapsulating the drug by weak interactions to be released by diffusion through the pores.
These results suggest that the use of non-degradable materials represents a good alternative to biodegradable polymers in which is common to observe more than two steps, not allowing an adequate kinetic study. Narayani et al. [21] reported on the synthesis of biodegradable microspheres where MTX was covalently linked to gelatin. They carried out in vitro release test and the period was about 7-11 days, in their experiment release rate was dependent of surface area they found that the largest surface the slower release. Sol-gel materials exhibit large surface area values, moreover porosity represent and additional advantage, since pores with different sized can be obtained leading to different release rates of the drug.
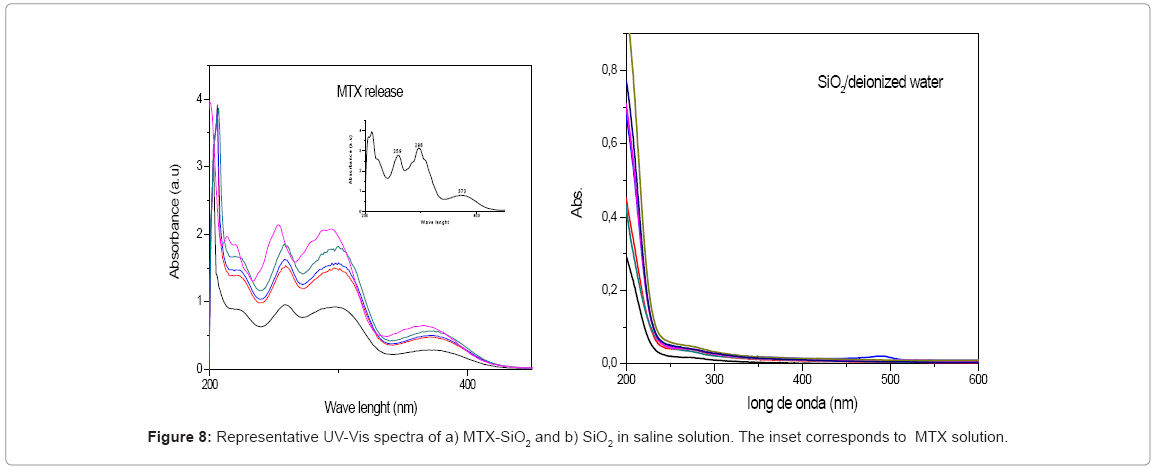
Since drug stability must be assed, full spectra in the release test were collected. In Figure 8 some spectra are showed. We observed that drug was release without modification and the characteristics bands were preserved after 12 days. In order to verify that no other compound can be desorbed from silica surface, same procedure was followed with pure SiO2. Our material was used without any treatment and the observed spectra indicate that after 5 days no modification that can be attributable to any other molecule present in silica surface was detected.
In vitro cytotoxic test
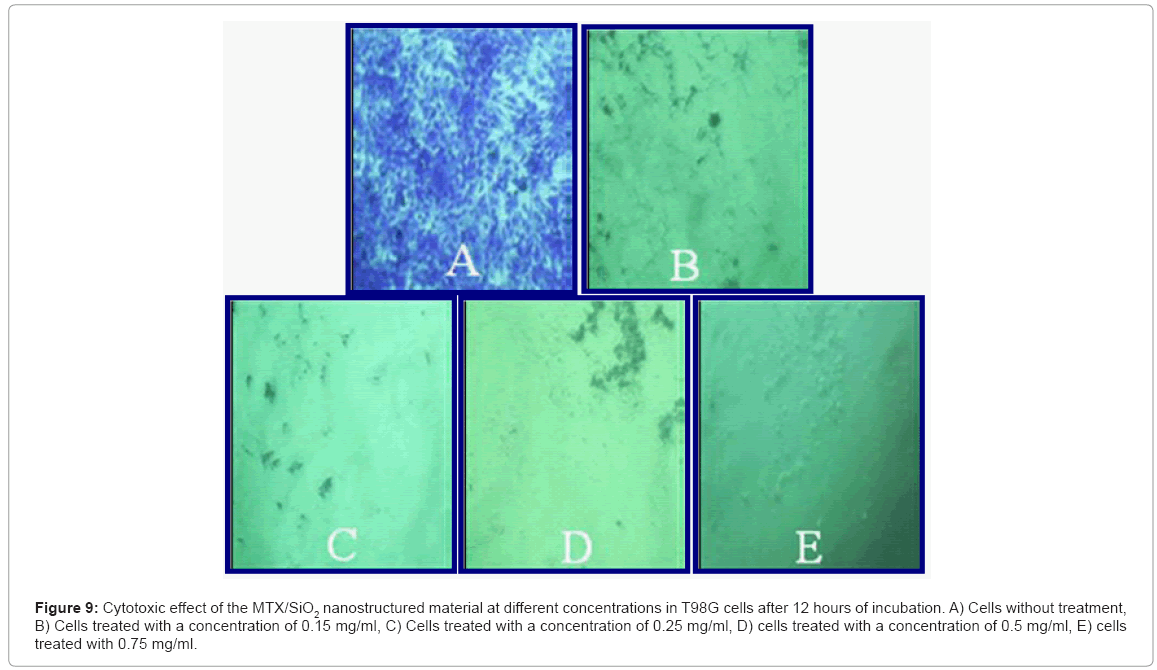
Cell culture: The MTX/SiO2 showed a clear effect on T98G cells, observing no cellular damage in the cells without treatment (Figure 9A) whereas in the rest is notable the increase on cell death percentage as nanoparticles concentration increases (concentration of 0.15 mg/ml with 98% of cell death, 0.25mg/ml with 98.8% of cell death, 0.50mg/ml and 0.75mg/ml get 100% of cell death).
Figure 9: Cytotoxic effect of the MTX/SiO2 nanostructured material at different concentrations in T98G cells after 12 hours of incubation. A) Cells without treatment, B) Cells treated with a concentration of 0.15 mg/ml, C) Cells treated with a concentration of 0.25 mg/ml, D) cells treated with a concentration of 0.5 mg/ml, E) cells treated with 0.75 mg/ml.
In other work, polymeric chito-PEG nanoparticles with MTX where synthesized [22], they found that higher feed ratios of drug leaded to lower drug loading efficiency, and in vitro cytotoxicity in melanoma cells showed that cell killing is directly related with MTX content, more over pure MTX cytotoxicity was higher than MTX-incorporated polymeric nanoparticles.
Animal model
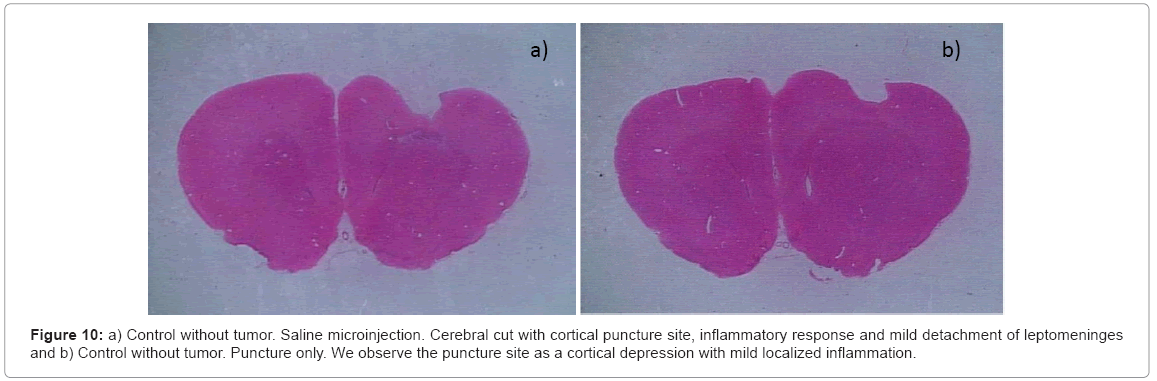
Group 1: Control – without tumor or drug
a) Saline solution: We did not find any change in the clinical behavior. In the histological study we found the cortical puncture in the cerebral section without inflammatory response and presenting a mild detachment of the leptomeninges (Figure 10a).
b) Puncture: We did not observe changes in the physical examination. Histological analysis: we found the puncture site like a cortical depression with the presence of a slight astrocytary reactive phenomenon, movement of the microglia (inflammation at the puncture site) and leptomeningeal swelling (Figure 10b).
Group 2: Control - tumor growth without drug administration
The rats showed changes in the physical exam from day 16 post-infiltration mainly characterized by predominant right muscular weakness of the hindlimbs, adding in the next days, a general affection of motor capacity.
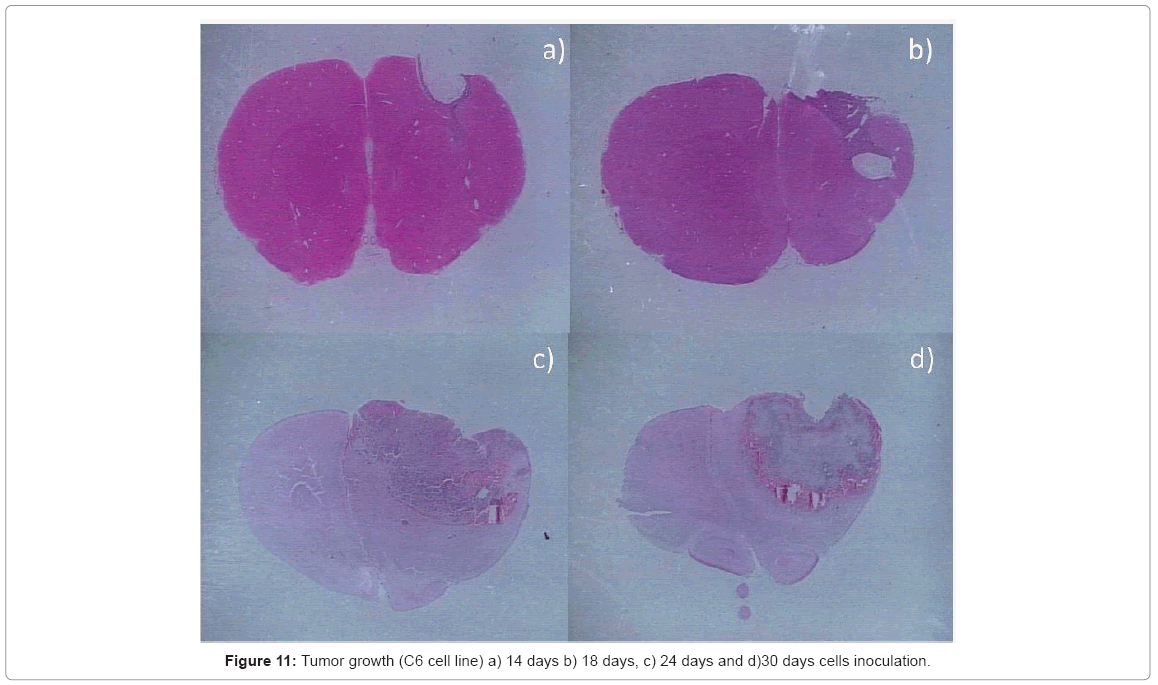
The rats died from day 19 to 30 post-infiltration, just one rat in free evolution survived without clinical alterations, sacrificed at 6 months post-infiltration to study the tumor viability by histology analysis finding ventriculomegaly and a little area of necrosis (no viable tumor) proving a failed tumor inoculation.
Histopathological analysis: the intracerebral inoculation of C6 line has a similar behavior to Glioblastoma Multiforme (GBM), presenting the characteristic palisading necrosis with a high mitotic rate, a great cellular pleomorphism, anaplasia, intense nuclear hyperchromasia, endothelial hyperplasia, with a distinct expansion phenomena and different presentation patterns. This tumor grows rapidly affecting almost the entire cerebral hemisphere causing the death of the animal model ( Figure 11).
Group 3: Methotrexate with tumor
Physical test: the majority of the group showed a similar behavior to the control group of tumor growing. Just tree rats had a different behavior; one of them showed a delay in the presentation of the clinical alterations; the rest, two rats, did not show changes in the physical examination. About survival, eight rats died from day 21 to 33 post-infiltration. In the histopathological analysis we observed the natural evolution of the tumor growth without changes due to the drug. We sacrificed the two survival rats 6 months later to verify the tumor viability, finding in the histological analysis and immunohistochemistry the presence of mild inflammation reaction and suggestive results of non-viable tumor.
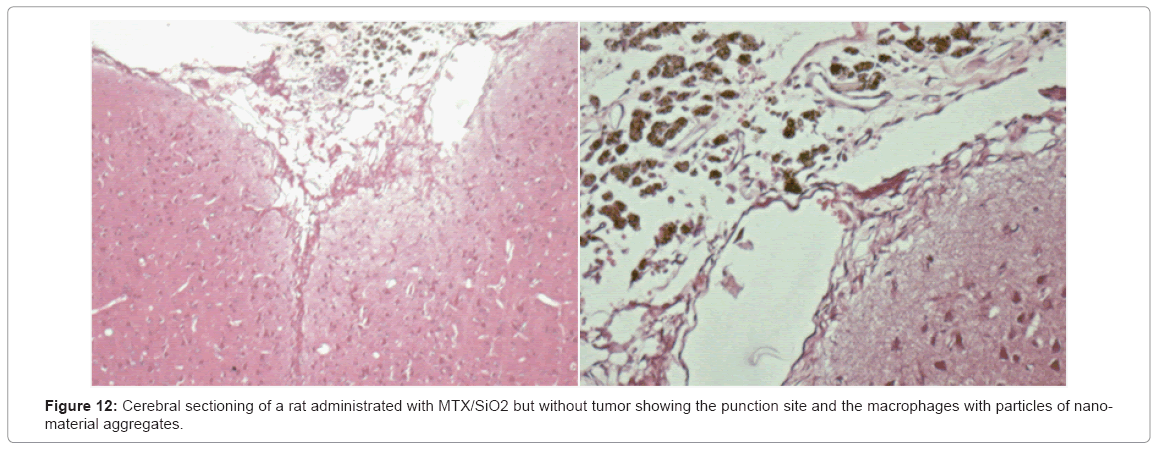
Group 4: Methotrexate/SiO2 without tumor
Physical exam: without clinical alterations. Survival: the five rats survived without any clinical changes. They were sacrificed 6 months post-infiltration for the histological analysis finding macrophages with particles of nanomaterial and a mild inflammatory response. These results suggest the biocompatibility of the compound (Figure 12).
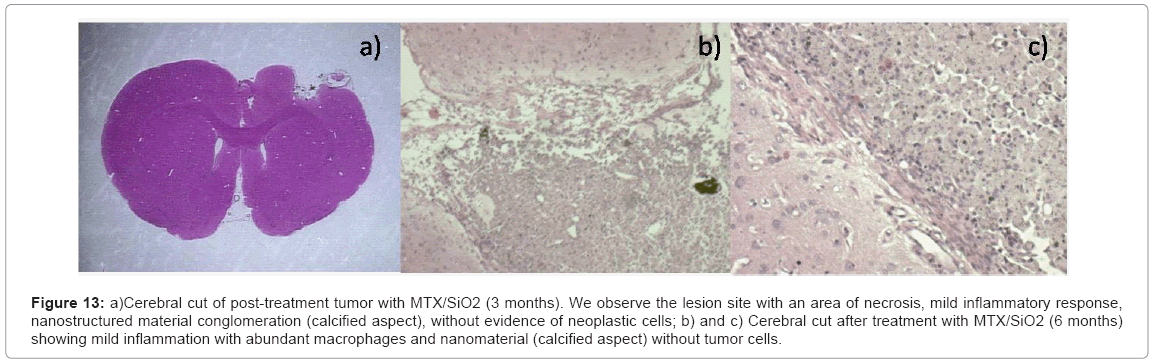
Figure 13: a)Cerebral cut of post-treatment tumor with MTX/SiO2 (3 months). We observe the lesion site with an area of necrosis, mild inflammatory response, nanostructured material conglomeration (calcified aspect), without evidence of neoplastic cells; b) and c) Cerebral cut after treatment with MTX/SiO2 (6 months) showing mild inflammation with abundant macrophages and nanomaterial (calcified aspect) without tumor cells.
Group 5: Methotrexate/SiO2 with tumor
It was applied MTX/SiO2 (5.2 to 6.5 mg) to ten rats with a tumor growth of 14 days, leaving in free evolution and making clinical evaluation every day. In the physical exam five rats showed a slight weakness of the right hindlimb at 19th day post-infiltration of C6, however, two of them recovered without later clinical alterations. One rat showed muscular weakness at 25th day post-infiltration but it recovered in a few days without later alterations. Another of those rats showed a fast tumor growth evolution and died at 29th day postinfiltration (natural tumor evolution). The fifth rat progressed slowly showing motor dysfunction evident at 35th day post-infiltration and died at 38th day. The five missing rats maintained without clinical alterations. The survival eight rats were sacrificed in two groups, 4 rats were sacrificed 4 months post-infiltration to verify the tumor viability, and the other 4 rats were sacrificed 6 months post-infiltration for the histopathological analysis.
Histological analysis: At the microscope in the slides of the survival rats sacrificed 4 months post-infiltration we found lesion area, moderate chronic inflammation with abundant macrophages, lymphocytes and hemosiderophages. We observed the presence of a material with calcified appearance.
We found meningothelial hyperplasia and necrosis areas in the lesion site. We did not find neoplastic cells. In the slides of the 4 rats sacrificed 6 months post-infiltration we found similar results with mild chronic inflammation with abundant macrophages and the presence of a calcified material (nanoparticles aggregates) without evidence of tumor cells.
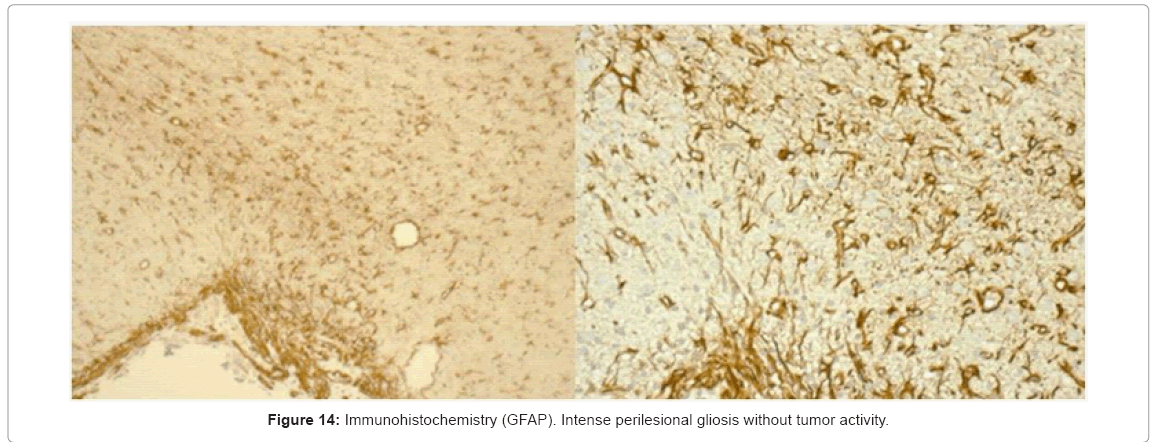
In the immunohistochemical analysis we found an intense perilesional gliosis (healing) without tumor activity (Figure 14). The use of PLGA microspheres containing MTX in an animal model of sarcoma 180 was reported by Singh and Udupa [23], they showed that PLGA-MTX loaded material exhibited higher antitumor activity than plain MTX, however once the drug loaded has been released cell proliferation continue and tumor grew.
About the histopathological analysis of the MTX/SiO2 therapy, we can say that it is a favorable treatment with a cell response to an area of necrosis and corresponding to foreign body (mild reaction) without evidence of neoplasia, where it should be noted the clinical behavior of the animals, found without systemic involvement. The drug alone did not affect natural evolution of the disease in the vast majority of animals and, with the immunohistochemestry, we demonstrated the meningothelial hyperplasia (a normal healing process) discarding tumor activity. The MTX/SiO2 therapy showed a significant result when compared with the MTX finding that 80% of the treated rats with MTX/ SiO2 survive up to 6 months post-infiltration of C6 cells instead with methotrexate survived only 2% of rats.
The effect of this drug against cancer is well know [24-26], but the main difference found in this research was the significant effect obtained by the controlled release of the drug (methotrexate) with a minimum dose within the inorganic matrix of SiO2 and demonstrating that the same dose of the single drug do not cause this effect, furthermore the advantage of being a local therapy avoiding the characteristic adverse effects of the chemotherapy. In our materials, MTX-SiO2 material showed clearly higher cytotoxic effect than MTX drug, both in vitro and in vivo. This can be explained by the enhancement due to the synergistic effect between our sol-gel modified silica (pH and additives) and the drug. Our materials showed to be active against malignant cells, however we need to remark that in healthy animals (without tumor) no cytotoxic effect was observed.
Conclusions
A functionalized inorganic nanostructured reservoir was obtained. In vitro experiments showed that MTX was encapsulated and released in a controlled way from the SiO2 structure, due to the appropriate control of physicochemical properties of the oxide, since the beginning of the synthesis. Pore size and surface area of the material as well as the presence of surface OH groups play an important role. Sol gel nanostructured materials have potential as drug delivery vehicles for local administration of chemotherapeutic agents, with the possibility to eradicate adverse side effects.
The MTX/SiO2 therapy has shown an important modification of the natural evolution of brain tumors GBM type in animal models, increasing the survival significantly of the affected animals with an important reduction in tumor size. In contrast, the drug itself to such small doses showed no significant changes in tumor behavior. These results strengthened the MTX/SiO2 therapy as a promising treatment for significant anti-carcinogenic effect avoiding the adverse effects caused by systemic therapies.
Acknowledgements
Authors want to thank to CONACYT 99243 and FONCICYT-CONACYT 96095 projects for financial support. To ICyTDF, UAM, INNN for the facilities and to I. Sánchez, E. Ortiz-Islas, J. Bustos and J. Manjarrez for technical assistance.
References
- Haley B, Frenkel E (2008) Nanoparticles for drug delivery in cancer treatment. Urol Oncol 26: 57-64.
- Borsa J, Whitmore GF (1969) Cell killing studies on the mode of action of methotrexate on L-cells in vitro. Cancer Res 29: 737-744.
- Dukic S, Heurtaux T, Kaltenbach M, Hoizey G, Lallemand A, et al. (2000) Influence of schedule of administration on methotrexate penetration in brain tumours. Eur J Cancer 36: 1578-1584.
- Son S, Bai X, Lee S (2007) Inorganic hollow nanoparticles and nanotubes in nanomedicine: Part 1. Drug/gene delivery applications. Drug Discov Today 12: 650.
- Faraji A, Wipf P (2009) Nanoparticles in cellular drug delivery. Bioorganic & Medicinal Chemistry 17: 2950-2962.
- Ping Xu Z, Hua Zeng Q, Qing Lu G, Bing Yu A (2006) Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci 61: 1027-1040.
- Duguet E (2009) Functionalisation of inorganic nanoparticles for biomedical applications in Nanoscience. Springer Link Chap 4.
- Vidinha P, Augusto V, Almeida M, Fonseca I, Fidalgo A, et al. (2006) Sol-gel encapsulation: an efficient and versatile immobilization technique for cutinase in nonaqueous media. J Biotechnol 121: 23-33.
- Bottcher H, Slowik P, Süb W (1998) Sol-Gel Carrier Systems for Controlled Drug Delivery. Sol-Gel Sci Technol 13: 277-281.
- Huntera W, Burta H, Machan L (1997) Local delivery of chemotherapy: a supplement to existing cancer treatments. A case for surgical pastes and coated stents Adv Drug Deliver 26:199.
- Lopez T, Manjarrez J, Rembao D, Vinogradova E, Moreno A, et al. (2006) An implantable sol-gel derived titania–silica carrier system for the controlled release of anticonvulsants. Mat Lett 602: 2903-2908.
- López T, Quintana P, Martínez J, Esquivel D (2007) Stabilization of dopamine in nanosilica sol-gel matrix to be used as a controlled drug delivery system Non-Cryst Solids 353: 987-989.
- Aguirre G, Arriola G, Gómez J, López T, Picquart M, et al. (2004) Thermal characterization during dehydration of nitrifying and denitrifying microbiological mud encapsulated in silica gel Acta 421: 211-215.
- Chadha R, Arora P, Kaur R, Saini A, Singla M, et al. (2009) Characterization of solvatomorphs of methotrexate using thermoanalytical and other techniques. Acta Pharm 59: 245-247.
- Dong-Hyuk S, Young-Il J, Don-Gon K, Min-Ja J, Mi-Kyeong J, et al. (2009) Methotrexate-incorporated polymeric nanoparticles of methoxy poly(ethylene glycol)-grafted chitosan. Colloids and Surfaces B Biointerfaces 69: 157-163.
- Brunnauer S, Emmet P, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60: 309-319.
- Leofanti G, Padovan M, Tozzola G, Venturelli B (1998) Surface area and pore texture of catalysts. Catalysis Today 41: 207-219.
- Sing K (1982) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 54: 2201-2218.
- Botterhuis N, Sun Q, Magusin P, van Santen R, Sommerdijk N (2006) Hollow Silica Spheres with an Ordered Pore Structure and Their Application in Controlled Release Studies. Chemistry a European Journal 12:1448-1456.
- Vallet-Regí M, Balas F, Arcos D (2007) Mesoporous materials for drug delivery. Angew Chem Int Ed 46: 7548-7558.
- Narayani R, Panduranga RK (1996) Biodegradable microspheres using two different gelatin drug conjugates for the controlled delivery of methotrexate. Int J Pharma 128: 261-268.
- Seo DH, Jeong YI, Kim DG, Jang MJ, Jang MK, et al. (2009) Methotrexate-incorporated polymeric nanoparticles of methoxy poly(ethylene glycol)-grafted chitosan, Col and Surf. B Biointerfaces 69: 157-163.
- Singh UV, Udupa N (1997) In vitro characterization of methotrexate loaded poly (lactic-co-glycolic) acid microspheresand antitumor efficacy in Sarcoma-180 mice bearing tumor, Pharma. Acta Helvetiae 72: 165-173.
- Altamirano E, Dreizzen E, Corrons F, Gonzalez P, Spinelli O (2004) Apoptosis: el metotrexate y sus efectos como agente inductor. Revista de la Facultad de Ciencias Médicas 1: 42-46.
- Tardi P, Boman N, Cullis P (1996) Liposomal Doxorubicin. J Drug Tar 4: 129-140.
- Han S, Joung J, Kim T, Jeong IG, Seo HK, et al. (2008). Methotrexate, vinblastine, doxorubicin and cisplatin combination regimen as salvage chemotherapy for patients with advanced or metastatic transitional cell carcinoma after failure of gemcitabine and cisplatin chemotherapy. British J Cancer 98: 86-90.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15706
- [From(publication date):
June-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11064
- PDF downloads : 4642