NRF2 and the Phase II Response in Acute Stress Resistance Induced by Dietary Restriction
Received: 14-Jun-2012 / Accepted Date: 16-Jun-2012 / Published Date: 19-Jun-2012 DOI: 10.4172/2161-0681.S4-004
317448Introduction
The concept of immortality and the search for the fountain of youth is embedded in epic stories and fables going back thousands of years, and in all likelihood precedes recorded history. In the past century, we have seen dramatic increases in the expected mean lifespan of people throughout the world thanks to innovations in areas such as hygiene and medicine. In the United States in the past 30 years, death rates due to cancer and stroke - two leading causes of aging-related death - have declined 2.7% and 63%, respectively [1]. Despite these decreases, the absolute number of deaths from these conditions continues to increase [1].
Studies on the genetic and molecular basis of aging and longevity in lower organisms have given rise to the idea that a common process may drive both “normal” aging phenotypes experienced by most people (hair greying, reduced hormone levels, loss of muscle mass) as well as aging-related pathologies experienced only by some (cancer, stroke, heart disease and neurodegeneration). An important prediction of this concept is that interventions targeting the aging process itself will also delay or ameliorate a wide variety of aging-associated diseases.
The most potent intervention to retard the processes of aging is dietary restriction (DR) [2-4]. Generally defined as reduced food intake without malnutrition, DR describes a wide variety of nutritional interventions altering the composition and/or timing of food intake, including reduced daily calorie intake (also known as calorie restriction, or CR), reduced nutrient intake (e. g. protein, essential amino acids) or enforced periods of fasting between meals (e.g. every-other-day fasting, EOD). Modulating dietary intake as a means to defend again stagingrelated disease was established as a laboratory paradigm over 100 years ago [5]. It was first observed that restricting food intake of mice not only decreased the growth of implanted tumors, but also retarded angiogenesis and metastasis [5]. Life extension benefits of DR were first reported twenty years later [6]. In the ensuing decades, longevity benefits of DR have been reported in a wide variety of species, from single-celled yeast to non-human primates. The measured benefits of DR have also expanded to endpoints with arguably more immediate potential for clinical use, such as improved metabolic fitness and increased resistance to chemical [7] and radiation [8-10] stress or on both the cellular and organismal level [3,11-15].
Despite decades of research into the mechanistic (nutritional, physiological, molecular) basis of longevity extension by DR (as well as the aging process itself), a potentially unifying model of DR action has only recently emerged [16-18]. It draws on the concept of hormesis, or the beneficial adaptive response to the mild stress of nutrient/energy restriction. Here we focus on the potential hermetic role of reactive oxygen/nitrogen species (ROS) produced as a result of increased mitochondrial respiration or xenobiotic metabolism inactivation of Phase II antioxidant and detoxification systems [19,20] through stimulation of the NF-E2-related factor 2 (NRF2) transcription factor [21-23]. Although much of the experimental DR literature is focused on aging and longevity-related endpoints, in this review we will place special emphasis on DR-mediated benefits related to stress resistance with potential clinical relevance, such as resistance to surgical ischemia reperfusion injury, chemo- and radiation therapy, oxidative and electrophilic stressors.
Hormesis Hypothesis Of DR And DR Mimetics
Multiphasic Dose Response to Dietary Food Intake
It is commonly observed in biological systems that a given compound may have opposite biological effects at low and high doses [24,25]. This gives rise to a biphasic or multiphasic dose response when plotted over a range of concentrations, with a U or J shaped depending on the sign attached to the biological effect. For example, high doses of a chemotherapeutic agent such as cisplatin can be cytotoxic/cytostatic, while low doses can actually increase cell proliferation. Whether or not this low-dose effect is “beneficial” is entirely context-dependent –good in the case of a regenerating liver after tumor resection, bad in the case of there sected tumor itself. Hormesis is the term most often associated with “beneficial” actions in the low dose range of a compound that is toxic at higher doses. Abiphasic or multiphasic dose-response itself implies nothing about underlying mechanism (s). Nonetheless, hormetic responses are thought to involve adaptive changes altering signal transduction, transcription and translation and leading to such “beneficial” biological outcomes as increased resistance to subsequent stress.
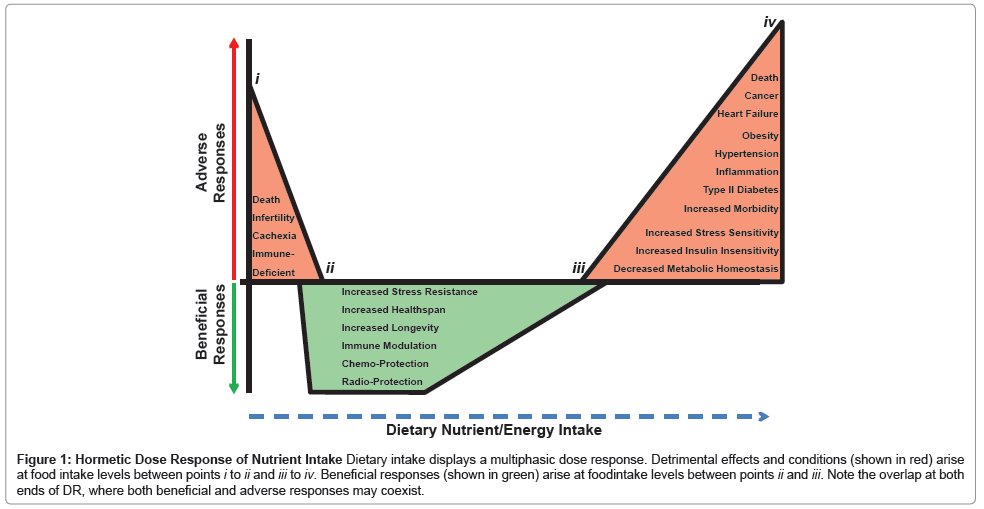
Dietary intake, similar to pharmaceuticals [26,27], radiation [28] and exercise [29], displays a multiphasic dose response [30] (Figure 1). Chronic underfeeding with malnutrition leads to reduced fecundity, cachexia, immunosuppression and even death [31-34] (Figure 1, points i to ii), while over nutrition leads to increased insulin resistance, inflammation and hypertension as well as increased morbidity and mortality due to diabetes, cardio-pulmonary diseases and cancer [35-38] (Figure 1, points iii to iv). Opposite biological effects are observed for these endpoints upon DR, defined here as the level of food intake between malnutrition (point ii) and that required for maintenance of body weight significantly below the set point achieved by adlibitum feeding (point iii).
Figure 1: Hormetic Dose Response of Nutrient Intake Dietary intake displays a multiphasic dose response. Detrimental effects and conditions (shown in red) arise at food intake levels between points i to ii and iii to iv. Beneficial responses (shown in green) arise at foodintake levels between points ii and iii. Note the overlap at both ends of DR, where both beneficial and adverse responses may coexist.
Critics of the hormesis hypothesis of DR have questioned its validity on at least two levels. First at issue is whether the benefits of DR reflect an experimental artifact of laboratory life -unlimited access to nutrient/ energy dense food. In Figure 1, this would imply that point iii, which we consider as optimal nutrition for rapid growth to adulthood and maintenance of adult body weight, actually represents a state of over nutrition, and that DR simply returns the animals to a less artificial level of food intake [39]. While we agree that the placement of point iii is somewhat arbitrary, the fact that the benefits of reduced food intake can be observed in prospective studies in relatively lean people [40] and in a dose-dependent fashion to near the point of starvation in rodents [6,41], put to rest fears that DR is an experimental artifact related to over-feeding of control subjects. A second criticism of the hormesis hypothesis is that neither the hormetic compound nor the underlying mechanism is well defined [42-44]. Below we address this concern by considering the ability of DR and DR mimetics to generate bona fide hormetic agents - reactive oxygen and electrophilic species - and the potential for the adaptive response to these stressors to underlie at least some of the benefits of DR.
DR, ROS and Mitohormesis
A common link among hormetic stimuli is their ability to generate reactive oxygen/nitrogen species (ROS) and other reactive metabolites either directly or indirectly [25,29,45]. At the hormetic dose, a transient increase in ROS may stimulate upregulation of antioxidant, detoxification and survival mechanisms on the cellular and organismal levels, thus providing a robust defense against larger and potentially more dangerous oxidative or toxicological stressors [46].
If ROS is the hormetic agent essential for the beneficial hormetic response, what is its source? Although not known for certain, emerging evidence points to an increase in mitochondrial respiration as a potential source of hormetic ROS. In the case of DR, evidence in yeast [47-49], C. elegans [46], flies [50] and rodents [51] points to increased rates of oxidative phosphorylation often accompanied by increased mitochondrial biogenesis. Altered energy metabolism, including a shift in energy substrate utilization from carbohydrate to fat [29,45-47,52-61], may also play a role in DR and DR mimetics like exercise.
Hormesis driven by oxidative stress derived specifically from mitochondrial respiration has been termed “mitohormesis” [55,58]. First proposed as a medical hypothesis, supporting experimental evidence has since been provided in a number of model systems. In worms, the DR mimetic 2-deoxyglucose inhibits glycolysis, increasing fatty acid oxidation and mitochondrial ROS while at the same time extending lifespan [46]. In humans, blocking oxidative stress by the addition of antioxidants vitamin C, vitamin E and/orn-acetyl-cysteine not only attenuates the beneficial results of acute bouts of exercise on insulin sensitivity [29], but also increases long-term oxidative damage and impaired healing of overworked muscles [62]. In dogs, where treadmill running preconditions against cardiac ischemic injury, inhibition of fatty acid oxidation with the acyl-CoA dehydrogenase inhibitor 5-hydroxydecanoate [63,64] prevents the benefits of exercise preconditioning without itself causing any damage [53]. Acyl-CoA dehydrogenase activity and fatty acid oxidation [65] are increased upon short-term starvation by Sirt3-mediated deacetylation in the mitochondria [66]. Sirt3 expression and activity are both increased upon exercise, fasting and caloric restriction but decreased on high fat diets [67,68]. This stimulation of Sirt3 activity not only promotes oxidation of fatty acids, but is also necessary for the phosphorylation/ activation of AMPK and CREB [68] as well as the mitochondrial superoxide dismutase SOD2 [69].
Can different energy substrates generate different amounts of ROS even in the absence of increased mitochondrial oxidative respiration? Long-chain fatty acids are broken down first in peroxisomes and then oxidized to completion at the inner mitochondrial membrane and in the matrix. β-oxidation of lipids is a complex pathway comprised by over 16 proteins and is controlled at several steps dependent on substrate and metabolite availability [70]. The production of reactive metabolites - superoxides and peroxides- is possible during β-oxidation of lipids in peroxisomes and in the mitochondria if it is not efficiently or directly coupled to ATP production. ROS production at complex II, QH2, and complex III from long-chain fatty acid oxidation derived FADH2 is greater than what is formed from NADH-linked substrates channeled first through complex I [71,72], although this is a still unresolved and controversial issue [73]. Dietary intake of moderately oxidized fats also results in increased antioxidant and detoxification systems in the livers of pigs [74].
It is still not well understood as to the relative and/or differential effects that increased respiration, increased fatty acid oxidation, differential substrate utilization, uncoupling, or increased mitochondrial biogenesis have on effective hormetic ROS production due to DR. Nonetheless, mitochondrial metabolism and activity is central and essential for the benefits of dietary restriction in eukaryotes.
DR Mimetics, ROS and Xenohormesis
“DR mimetic” is a term used loosely to define a compound or stimulus that imparts functional benefits of DR, but in the absence of reduced food intake [75]. Such functional benefits range from longevity extension in model organisms, as with rapamycin and metformin [48,76], to improved metabolic fitness in the face of over nutrition, as with resveratrol [77]. Aerobic exercise can also be considered a DR mimetic based on its ability to improve metabolic fitness and stress resistance, although itdoes not necessarily extend lifespan [78].
As with DR, most DR mimetics have multiple targets and pleotropic modes of action, and so their underlying mechanisms remain unproven or unknown. In principle, DR mimetics may work by creating a state of actual or perceived nutrient/energy deficiency, for example inhibition of glycolysis by 2-deoxyglucose, activation of AMP-Activated Protein Kinase (AMPK) by metformin [79], or inhibition of the nutrient/ energy sensor mTOR by rapamycin. In this way, DR mimetics may function like DR by transiently increasing ROS to hermetic levels as a result of increased fatty acid oxidation and mitochondrial respiration.
However, mitochondria are not the only potential source of ROS induced by DR mimetics. Many pharmacological DR mimetics, such as metformin, resveratrol and curcumin, are xenobiotics that activate Phase I detoxifying enzymes such as the cytochrome P-450 superfamily, also resulting in potential increases in oxidative stress [80,81]. These Phase I enzymes initiate detoxification and export of xenobiotics from the cell by oxidizing these molecules and making them more suitable substrates for Phase II enzymes. In doing so, they also create highly reactive metabolites with the potential to overwhelm the Phase II response and create oxidative stress. Many DR mimetics are natural substances used by their hosts in stress resistance. For example, rapamycin is a macrolide antibiotic made by soil bacteria; plant polyphenols such as resveratrol can defend against microorganisms, insects, herbivores, and even sunlight. The idea that such xenobiotics trigger stress responses in other organisms when ingested has been coined “xenohormesis” [82,83]. Thus, hormesis-inducing ROS produced as a result of mitochondrial respiration (mitohormesis) or Phase I oxidation reactions (xenohormesis) may be a common mechanistic link among different DR regimens and mimetics.
Table 1 summarizes DR regimens and mimetics reported to induce stress resistance together with the underlying pathways/genes implicated in that study. Three major conclusions are evident: 1) DR and DR mimetics are powerful tools to prevent damage from a variety of acute stressors including ischemia/ischemic reperfusion injury [84-87], toxic levels of chemicals and drugs [88-93], trauma [94], infection [95], inflammation [96] and radiological damage [9,2] 2) different DR regimens (type, duration, dose) and mimetics can provide protection against the same stressors across a variety of different species; and 3) maintenance of cellular antioxidant, detoxification and redox cycling programs is a common theme in protection against acute stress. These same pathways are also intricately involved in the Phase II response to toxicological and electrophilic stress, as described below.
| Type of DR & DR Mimetic | Duration/Dose of DR/ DR Mimetic | Organism and/or Cell Type | Pathways/Genes/Activity Altered (+/-) | Resistance Against Acute Stressor | Ref. |
|---|---|---|---|---|---|
| Protein/Amino Acid (Trp) Deficient | 6-14 days | Mice | (+) GCN2, (-) eIF2α | Renal and hepatic ischemia/reperfusion Injury | 84 |
| Glucose Deficient | 0.5g/L prior to insult | Rat and Human (Glial cells) | N/A | H2O2 and menadione (oxidative stress) Cyclophosphamide (chemotherapeutic) | 11 |
| Methionine Deficient | ~8.5-months | Mice | (-) in serum IGF-1, Insulin, and Glucose | Injection of toxic doses of acetaminophen | 88 |
| 25% Restriction | 140 days | Rats | N/A | Methylazoxymethanol (MAM) induced tumorigenesis | 7 |
| 30% Restriction | 2-4 weeks | Mice | (-) GH, (-) IGF-1, (+) HO-1, (+) GR, (+) Angiopoietin, (+)GSTs | Renal and hepatic ischemia/reperfusion Injury | 14 |
| 20-40% Restriction | >5 weeks to 42 weeks | Mice | (+) NRF2, (+) NQO1, (+) HO-1, (+) GCLC, (+) GST, (+) GPx | Induced Mutagenesis/Tumorigenesis by DMBA/TPA | 129 |
| 30-40% Restriction | N/A | Humans | N/A | Critically ill patients in a medical-surgical ICU | 252 |
| 40% Restriction | adult lifetime | Mice | (-) IGF-1, (-) Insulin | Excitotoxin kainic acid induced damage to the dorsal hippocampus | 89 |
| 40% restriction | 6 months | Rats | (+) deacetylation, (+) Sirtuins | Cardiac ischemia/ischemic reperfusion injury | 57 |
| 60 % Restriction (approximate) | 800 kcal/day for 2 weeks | Humans | No sig. diff. in weight/BMI between the two groups | Laparoscopic gastric bypass surgery | 251 |
| Every 2-Day Intermittent Fasting | N/A | C. elegans | (+) RHEB-1, (+) TOR | Heat and oxidative stress | 12 |
| EOD Intermittent Fasting | adult lifetime | Mice | (+) IGF-1, (-) Insulin | Excitotoxin kainic acid induced damage to the dorsal hippocampus | 89 |
| EOD Intermittent Fasting | 2-3 weeks | Mice | N/A | Lethal (8.7 Gy) and sub-lethal (5.26 Gy) total body ionizing radiation | 8 |
| EOD Intermittent Fasting | 3 weeks | Mice | (+) metabolic rate, (+) lipogenesis | Sub-lethal whole body gamma irradiation | 9 & 10 |
| Fasting | 48-180 hours before and 5-56 hours post- treatment | Stage I-IV cancer patients (44-78 yrs) | N/A | Chemotherapy for breast, esophageal, prostate and lung cancer | 253 |
| Fasting | 4 days | Rats | N/A | Hypoxic/Ischemic injury to the brain | 85 |
| Fasting | 1-4 days | Liver donor Rats | (+) GSH, (+) HO-1, (+) HSP-60, (+) HSP-70 | Liver ischemia and transplantation | 187 |
| Fasting | 3 days | Mice | (+) HO-1, (+) SOD, (+) GPx, (+) GSR, (-) IL-6 | Hepatic ischemia/reperfusion injury | 186 |
| Fasting | 3 days | Mice | (-) IGF-1, (+) IGFBPs | Doxorubicin (oxidative stress) | 90 |
| Fasting | 2-3 days | Mice | N/A | Etoposide | 11 |
| Fasting | 1-3 days | Mice | (-) GH, (-) IGF-1, (+) HO-1, (+) GR, (+) Angiopoietin, (+)GSTs | Renal and hepatic ischemia/reperfusion Injury | 14 |
| Fasting | 2 days | D. melanogaster | Independent of Hif-1 | Anoxia and reoxygenation injury | 13 |
| Water only media | 1 -2 days | Yeast | (-) RAS2 | H2O2,menadione, methylmethane sulfonate and cyclophosphamide | 11 |
| Fasting 24 hours post-brain injury | 24 hours | Rats | Insulin independent, Ketone dependent | Traumatic brain injury | 94 |
| Exercise (running) | 10 weeks of treadmill or voluntary wheel running | Rats | N/A | Doxorubicin induced cardiotoxicity | 91 |
| Exercise (running) | 5 x 5 min run/rest 10 min or 24 hr prior to insult | Dogs | Opening of mitochondrial KATP channels | Myocardial ischemia/ichemic reperfusion injury | 53 |
| AICAR | 100 mM for 48 hours | D. melanogaster | (+) AMPK | Anoxia and reoxygenation injury | 13 |
| Curcumin | 200 mg/kg per day for two weeks | Mice | (-) IFN-γ, (-) IL-17 | Interleukin (IL)-10 deficient mouse model of pro-inflammation | 96 |
| Curcumin | 200mg/kg a day for 4 days | Rats | (+) NRF2, (+) HO-1 | Dimethylnitrosamine (DMN)-induced hepatic injury | 189 |
| Impaired glucose metabolism by DOG | 5 mM for 6 days | C. elegans | (+)aak-2/AMPK, (+) CAT | Paraquat and sodium azide (inducers of toxic ROS levels) | 46 |
| Methylene Blue | 4x50 mg/day prior to chemotherapy | Humans with solid tumors | N/A | Ifosfamide (chemotherapeutic)-induced encephalopathy | 92 |
| Oltipraz | 2 days supplemented in food | D. melanogaster | (+) NRF2/CncC, (+) GST | Paraquat (inducers of toxic ROS levels) | 130 |
| Oltipraz | 10-100 µM prior to insult | Human (H9 cells) | (+) GSH, (+) NQO1 | Human Immunodeficiency Virus (HIV) infection | 95 |
| 3H-1,2-dithiole-3-thione (D3T)/Oltipraz | 25-100 µM prior to insult | Mouse (Cardiac cells) | (+) NRF2, (+) NQO1, (+) CAT, (+) GSH, (+)GR, (+)GST | XO/Xanthine and SIN-1 cytotoxicity | 98 |
| 3H-1,2-dithiole-3-thione (D3T)/Oltipraz | 50 µM prior to insult | Rat (Adrenal glad cells) | (+) NRF2 | Ethanol induced cytotoxicity and death | 93 |
| Plumbagin | 1 µM prior to insult | Human (Neuroblastoma cells) | (+) NRF2, (+) NQO1, (+) GCLM, (+) Trx, (+) HO-1 | tert-butyl-hydroperoxide (BHP) mediated oxidative stress and death | 149 |
| Plumbagin | 3 mg/kg 24-6 hours prior to stroke | Mice | (+) NRF2, (+) HO-1 | Cerebral focal ischemic stroke model | 149 |
| Procyanidin B2 | 1-20 µM for 20 hrs. | Human (Colonic cells) | (+) NRF2, (+) GSTP1, (+) MAPK, (+) ERKs | Pro-oxidant t-BOOH | 131 |
| Rapamycin | 0.25 mg/kg | Mice | Opening of mitochondrial KATP channels | Ischemia/reperfusion injury in isolated heart | 86 |
| Resveratrol | 5-40µM for 12 hours | Rat (Myocytes) | (+) deacetylation, (+) Sirtuins | Hypoxia/Reoxygenation | 57 |
| Resveratrol | 1x10-4 to 2x10-3 mg/kg 30 minutes before ischemia | Rats | (+) Estrogen Receptors, (+) NMDA Receptors | Ischemia/reperfusion injury in middle cerebral artery occlusion model | 87 |
| Resveratrol | 10 µM prior to insult | Human (Alveolar cells) | (+) NRF2, (+) GCL, (+) GSH | Cigarette Smoke | 122 |
| Sulforaphane | 3 µM prior to insult | Mouse (Embryonic Fibroblasts) | (+) NRF2, (+) GSTs, (+) GSH, (+) NQO1, (+) GR | Isothiocyanates, Diamide, Acrolein, Epoxides, and Peroxides | 132 |
Table 1: DR and DR Mimetic-Mediated Pathway Modulations and Induced Resistance to Acute Stress Various forms of DR, including specific nutrient/amino acid restrictions, reductions in caloric intake, intermittent fasting, fasting, and the use of physical (exercise) and chemical (xenobiotic and pharmaceutical) DR mimetics are shown to induce resistance to acute stressors in a variety of organisms, including humans. The table shows the type of DR and DR mimetic, the duration and/or dose used, the organism applied to, pathways/genes modulated by the treatment (+/-), and thestressor to which resistance was provided. Abbreviations: GSH= glutathione, GR= glutathione reductase, GPx= glutathione peroxidase, GST= glutathione transferase, GCL= glutamate cysteine ligase, NQO1= NAD(P)H dehydrogenase (quinone 1), Trx= thioredoxin, TrxR= thioredoxin reductase, CAT= catalase, HO-1= heme oxygenase 1, SOD= super oxide dismutase, DOG= 2-Deoxy-D-glucose.
Phase II Response
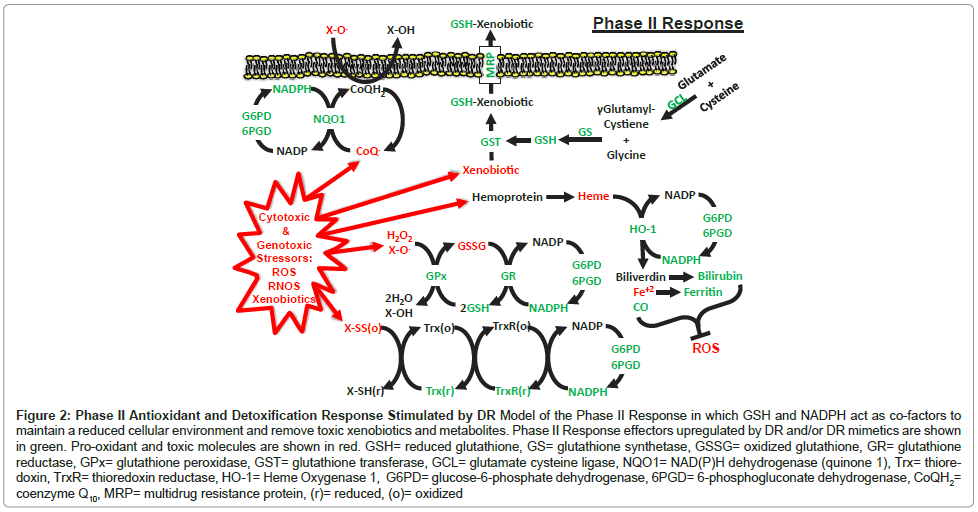
The Phase II response is an evolutionarily conserved adaptation to a wide variety of stressors, including ROS, xenobiotics and electrophiles, comprising the induction of over 200 genes and the repression of several dozen others [21,97-99]. The induced genes are largely responsible for the synthesis, use and/or recycling of cellular anti-oxidant and detoxification reducing agents, including glutathione (GSH), thioredoxin (Trx) and nicotinamide adenine dinucleotide phosphate (NADPH) [98] (Figure 2). The induction of the Phase II response is predominately facilitated by the transcription factor NRF2 binding to the antioxidant response element (ARE) in these gene promoters and stimulating their expression [21,98,100]. The major effector components of the Phase II Response induced by DR and DR mimetics are shown in Figure 2.
Figure 2: Phase II Antioxidant and Detoxification Response Stimulated by DR Model of the Phase II Response in which GSH and NADPH act as co-factors to maintain a reduced cellular environment and remove toxic xenobiotics and metabolites. Phase II Response effectors upregulated by DR and/or DR mimetics are shown in green. Pro-oxidant and toxic molecules are shown in red. GSH= reduced glutathione, GS= glutathione synthetase, GSSG= oxidized glutathione, GR= glutathione reductase, GPx= glutathione peroxidase, GST= glutathione transferase, GCL= glutamate cysteine ligase, NQO1= NAD(P)H dehydrogenase (quinone 1), Trx= thioredoxin, TrxR= thioredoxin reductase, HO-1= Heme Oxygenase 1, G6PD= glucose-6-phosphate dehydrogenase, 6PGD= 6-phosphogluconate dehydrogenase, CoQH2= coenzyme Q10, MRP= multidrug resistance protein, (r)= reduced, (o)= oxidized
Glutathione Metabolism
Glutathione, a tripeptide composed of γ-L-glutamyl-L-cysteinyl- glycine, exists ubiquitously in tissues and cells in its reduced or oxidized form (GSH or GSSG, respectively). The de novo synthesis of GSH occurs in the cytosol in a two-step process. The first and rate limiting step, catalyzed by the heterodimer glutamate cysteine ligase (GCL) - composed of catalytic (GCLC) and modifier (GCLM) subunits–conjugates the amino acids l-glutamate and l-cysteine to form γ-glutamyl-l-cysteine [101]. Activity of GCL is negatively regulated by low l-cysteine concentrations and/or high GSH levels [101]. In the second step, catalyzed by glutathione synthase (GS), γ-glutamyl-lcysteine is combined with l-glycine to form reduced glutathione (GSH). Expression of GS and both GCL subunits is under control of the Phase II response [102] due to the presence of antioxidant response elements (AREs) in each gene promoter [103,104]. Activation of GCL and GS gene expression increases intracellular GSH concentrations [101,105].
Once produced, GSH can be utilized for cellular functions throughout the cytoplasm, mitochondria, endoplasmic reticulum and even extracellular space. In the context of stress resistance, these functions include detoxification of reactive electrophiles, xenobiotics and free radicals including ROS; and the solubilization of reactive metabolites to facilitate their export from the cell [101]. Being just a tripeptide with no inherent catalytic activity, GSH action requires a collection of accessory enzymes, including glutathione S-transferases (GSTs), glutathione peroxidases (GPxs) and glutathione reductase (GR).
GSTs include over twenty mammalian genes in eight different classes, referred to as alpha, kappa, mu, omega, pi, theta, zeta, and microsomal to signify the location and substrate of that particular GST [106,107]. Enzymatically, GSTs catalyze the conjugation of GSH to the electrophilic group on a variety of reactive endogenous and exogenous substrates toxic to the cell. These substrates can include xenobiotics, reactive metabolites as well as oxidized lipids and proteins [108]. By doing so, GSTs detoxify the potentially harmful substrate and allow for its breakdown and/or transport out of the cell by way of multidrugresistance proteins (MRPs) [101,109,110]. GPxs are a family of intracellular and extracellular enzymes that use GSH to remove harmful peroxides, including ROS in the form of hydrogen peroxide, resulting in oxidized glutathione (GSSG) and water [101]. GPxs are the major antioxidants in the mitochondria, working in conjunction with SOD2 to detoxify superoxide into peroxide and then water. GR is responsible for recycling GSSG to GSH in order to prevent GSSG accumulation and to maintain the homeostatic GSH: GSSG ratio [111]. A decrease in this ratio above the normal 9.5:0.5 [112] can increase susceptibility to oxidative stress, protein dysfunction via glutathionylation, and cell death [113,114]. GR uses the reducing power of NADPH to reduce GSSG, producing two molecules of GSH and one molecule of NADP+ [108]. Due to the importance of GSSG recycling, both GR and NADPH production via the pentose phosphate pathway enzymes glucose-6- phosphate dehydrogenase and 6-phosphogluconate dehydrogenase are under control of the Phase II response [115-117].
A number of studies have demonstrated age-related changes in glutathione homeostasis and their modulation by DR. For example, age-related decline in GSH and reduced GSH:GSSG ratios attributable to reduced GCL expression [118] have been reported in ad libitum fed rodents [19] and humans [119], most notably those with diabetes [120], and may underlie vulnerability to cancer, Parkinson’s disease, Alzheimer’s disease [121] and heart disease [119]. In rodents, DR can blunt age-related GSH decreases [19], while in human epithelial lung cells the DR mimetic resveratrol can increase GSH levels and protect against cigarette smoke toxicity [122]. Deficiencies in GSTs, including an age-related decline in GST activity [123], are documented risk factors in several human pathologies. These include second hand smokeinduced childhood asthma [124], epoxide-induced cellular damage [125], pulmonary asbestosis [126], and cancer of the larynx, lung and bladder in smokers [127,128]. GSTs can be increased in abundance and activity by a variety of DR regimens, including fasting [14,102] and 30-40% DR [14,123,129], as well as DR mimetics Oltipraz [98,130], procyanidin B2 [131], and sulforaphane [132]. Age-related declines in Gpx activity, most notably in the intestinal mucosa, hepatocytes and kidney [20], can also be counteracted by DR and DR mimetics in rats [19,23]. Recent data from the CALERIE Trial of Human Caloric Restriction indicate that plasma GPx activity increases and plasma protein carbonyl levels decrease over a six month course of 10-30% DR in moderately overweight adults [133], indicative of a reduction in oxidative damage [134]. Exercise can also enhance GPx expression. In rats, 20 weeks of voluntary wheel running in rats increases cardiac and skeletal muscle GPx activities over 400% and 300%, respectively [135]. Stimulation of GPx can also be accomplished using 20-40% DR [129] or the DR mimetic sulphoraphane [136]. This stimulation of GPx via these preconditioning interventions correlates with and is thought to contribute to their protection against DMBA/TPA-induced skin carcinogenesis or azoxymethane/dextran sulfate sodium-induced colon carcinogenesis. Congenic deficiency in GR expression or activity in humans is rare [137], possibly signifying an essential role for GR function. However, GR expression can be increased by DR, possibly contributing to surgical stress resistance in mouse kidney and liver [14]. The DR mimetics Oltipraz [98] and sulforaphane [132] are also capable of inducing GR expression and providing protection against xenobiotic carcinogens and toxic compounds.
Thioredoxin and Thioredoxin Reductase
Thioredoxin (Trx) is a 12-kD protein enzyme with a conserved disulfide/dithiol sequence of -Trp-Cys-Gly-Pro-Cys- [138,139] that can directly reduce oxidized proteins, nucleic acids, lipids as well as small signaling molecules by itself becoming oxidized [139,140]. Thioredoxin reductase (TrxR) recycles oxidized Trx back to the reduced form [138] using NADPH as a reducing molecule, similar to GR. Expression levels and activity of Trx and TrxR are induced during the Phase II response [141,142]. Enhanced expression of Trx in transgenic mice has been linked to increased longevity, telomerase activity and resistance to stressors such as UV radiation [143,144]. Genetic overexpression of Trx and TrxR protects against mitomycin C and diepoxybutane- associated DNA damage and chromosomal instability in human fibroblasts obtained from donors with the accelerated aging/cancer predisposition syndrome Fanconi anemia [145,146]. An age-associated decline in Trx may be linked to the pathogenesis of Alzheimer’s disease (AD), as over expressing Trx in AD cells inhibits Ab amyloid toxicity [147]. Adult lifetime DR of 40% blunts Trx and TrxR decline in rats [19]. Similarly in C. elegans, Trxis upregulated upon DR and essential for lifespan extension upon DR as well as in a genetic model of DR [148]. The DR mimetic plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) protects human neuronal cells against tert-butyl-hydroperoxide (tBHP) mediated oxidative stress and cell death by the activation of several Phase II enzymes, including Trx [149]. Plumbagin treatment for up to 6 hours prior to focal cerebral ischemia also protects against neurologic damage and reduces infarct size in mice [149].
NAD (P) H-quinone oxidoreductase 1
An additional member of the Phase II Response that depends on the reducing power of NADPH to provide cellular protection against oxidative stressors is the NAD (P) H:quinone oxidoreductase (NQO1) [150]. NQO1 localizes to the cytoplasmic surface of the plasma membrane [151] and acts as a two-electron reductase on a number of substrates, mainly quinones. By transferring reducing potentials from NADPH onto quinones, the resulting hydroquinones [150,152], such as coenzyme Q (CoQ), keep the plasma membrane in a reduced state by maintaining exogenous antioxidants such as vitamin C (ascorbate) [153], inhibiting lipid peroxidation and activation of sphingomyelinase [154,155] and preventing subsequent ceramide release and ceramidedependent caspase activation [155].
NQO1 deficiency is a risk factor for therapy-related acute myeloid leukemia, basal cell carcinomas, pediatric leukemia and benzeneinduced liver toxicity [150]. Reduced NQO1 expression with age is associated with reduced CoQ and other plasma redox molecules and increased oxidative stress [156,157].
Evidence for a potential role of NQO1 in cellular protection induced by hormetic doses of carcinogens dates back to the 1960s [150]. Small amounts of azo dyes and aromatic olefins can stimulate NQO1 activity and protect mouse tissues against lethal subsequent doses of the carcinogen 7,12-dimethylbenzen (a) anthracene (DMBA) [158,159]. Long-term DR can also stimulate NQO1 expression, resulting in enhanced plasma membrane antioxidant capacity and decreased levels of 8-isoprostane and protein carbonyls in liver [156] and brain [4] of aged rats, and increased resistance to DMBA/TPA induced carcinogenesis in mice [129]. Short-term treatment with DR mimetics Oltipraz [98], plumbagin [149], and sulphoraphane [132] also stimulate NQO1 expression and increase protection against oxidative stress and chemical toxins.
Heme Oxygenase-1
Iron, including heme-bound iron can catalyze the formation of toxic hydroxyl radicals (OH.) from hydrogen peroxide (H2O2) through the Fenton reaction [160]. Under normal physiological conditions, this poses at most a minor threat to cells and tissues due to the matched rate of synthesis of heme and hemoproteins [160] including myoglobin [161], hemoglobin [162], neuroglobin [163] and heme scavenging protein hemopexin [164] in red blood cells, as well as intracellular proteins such as those of the cytochrome P-450 superfamily, peroxidases and catalases [160,165]. Increases in oxidative stress due to tissue injury, radiation and chemical toxins [166] can lead to disassociation of heme from hemoproteins [160], leading to hydroxyl radical generation through Fenton chemistry. Infectious or congenital diseases such as malaria [167], sickle cell anemia, thalassemia, glucose- 6-phosphate dehydrogenase (G6PD) deficiency and lupus can result in red blood cell lysis and further saturate the body’s heme scavenging defense systems [168].
Cellular defenses against free heme overload are centered on the inducible enzyme heme oxygenase-1 (HO-1) [169]. HO-1 can be induced directly by heme or indirectly by oxidative stress, which can increase heme levels by damaging heme-containing intracellular proteins. Oxidative stress can also activate the Phase II transcription factor NRF2 [170,171] via modulation of its redox-sensitive binding partner, KEAP1 (see below).
Increased HO-1 expression protects cells from both extracellular and intracellular free heme by catalyzing the transfer of electrons from NADPH to heme, keeping iron in the reduced state and allowing for molecular oxygen to induce the degradation of heme into free iron, biliverdin and CO [165]. Because free iron remains a threat, it is picked up and stored by ferritin [160]. Biliverdin is further processed by the enzyme biliverdin reductase using NADPH as the reducing agent to form bilirubin, an antioxidant that can itself lend protection against ischemia reperfusion injury and damage caused by sepsis [160]. CO mediates many of HO-1’s cytoprotective effects, for example by suppressing apoptosis [172,173], rejection of transplanted organs [174] and cardiac ischemic reperfusion injury [175,160].
How CO protects cells from oxidative damage is not entirely known, but may involve mitohormesis. Upon release from heme, CO binds to iron in other hemoproteins, including mitochondrial cytochrome c oxidase, resulting in a small, quick burst of mitochondrial ROS [60]. This potentially mitohormetic dose [55,176] can then lend protection by activating the p38 beta MAPK cell survival signal cascade [177] and transcription factors including PPARγ [178], HIF1-α [179] and NRF2 [180]. Activation of these cell survival and protection pathways, notably through NRF2, can further increase the expression of HO-1 as well as the other Phase II antioxidant and detoxification genes, enabling cells and tissues to fight off further free heme and oxidative stress-induced damage. Interestingly, this system of small amounts of free heme initiating a feed-forward cytoprotective response centered on the Phase II Response has been reported for the protection against atherosclerotic lesions and endothelial stress in a mouse model of sickle cell disease [181]. This brings into question whether the increases in hemolysis and free heme due to foot strike during running [182] can be seen not as detrimental, but positive and attributing to the benefits of moderate running. Additionally, future research on barefoot/ minimalist shoe- versus traditional shoe- running may shed light on which method best provides running hemolysis induced benefits.
Deficiencies in HO-1 expression have been reported in aging rat brains [183] and in several human clinical pathologies, including macular degeneration, a leading cause of age-related vision loss in of people aged 60 and older caused by increased oxidative damage to the retina [184], and chronic obstructive pulmonary disease (COPD) [185], a disease characterized by difficulty breathing due to long-lasting bronchitis and/or emphysema and also thought to be caused by an oxidant/antioxidant imbalance [185].
Stimulation of HO-1 through DR and DR mimetics is feasible and may contribute to preconditioning against acute stress. Long-term DR of 20-40% in mice increases expression of HO-1 in liver and ultimately protects against DMBA/TPA-induced tumorigenesis [129]. Shortterm 30% DR in mice for two-four weeks or fasting for up to three days increases HO-1 expression in the liver and kidneys and decreases organ damage due to ischemia reperfusion injury [14,186]. The beneficial effects of fasting can also be transferred from organ donors to recipients. In rats, one to four days of water-only fasting by the donor increases HO-1 expression and maintenance of GSH concentrations in the donated livers, correlating with increased short-term and longterm survival of the recipients [187,188].
Preconditioning with 200mg/kg a day for 4 days of the DR mimetic curcumin stimulates HO-1 expression in rats and conveys protection against acute stressors such as dimethylnitrosamine (DMN) -induced hepatic injury [189]. Another DR mimetic, plumbagin, stimulates HO-1 expression in human neuroblastoma cells and protects them against tBHP-mediated oxidative stress and death [149]. Preconditioning with plumbagin for 6 to 24 hours before the onset of a cerebral focal ischemic stroke also increases HO-1 expression and reduces total infarct size as well as general neurologic damage [149].
NRF2: The “On Switch” For The Phase II Response
The major evolutionarily conserved transcription factor required for induction of the suite of detoxification and antioxidant proteins constituting the Phase II response is NRF2. Known as SKN-1 [76,190] in worms and CncC [130] in flies, NRF2 is a member of the Cap’n’Collar transcription factor family with a conserved basic regionleucine zipper domain that binds to the antioxidant response element (ARE) [ (T/C) TGCTGA (C/G) TCA (T/C) ] [191] of gene promoters and has the ability to induce or repress expression of these genes. There are over 230 genes induced by NRF2, either directly or indirectly, and approximately 30-40 genes that are inhibited in an NRF2 dependent manner [142,192].
Activation of NRF2 is central to the induction of potent cellular antioxidant and detoxification systems. Many diseases, including the aging-related diseases of cancer, Alzheimer’s, Parkinson’s and diabetes, are correlated with decreased activity of NRF2 and show improvements of symptoms upon NRF2 activation through gene therapy or DR in experimental models [193].
NRF2/SKN1 was first connected to DR in worms, where it was shown to be required for DR-mediated lifespan increase [190]. Interestingly, in rodents, Nrf2 mediates the cancer protection induced by DR, but is not required for DR’s prolongevity characteristics [129]. Maintenance and stability of NRF2 levels and its activation are induced by oxidative and electrophilic stress [194-196]. In the scope of this review, we hypothesize that activation of NRF2 by DR regimens and mimetics depends on their ability to stimulate ROS production, either through increased mitochondrial fatty acid oxidation or activation of Phase I oxidases. The gene expression program engaged byNRF2 activation can then suppress the ROS prompted by mitohormesis or xenobiotic metabolism, and at the same time mount a total cellular defense against a multitude of stressors.
Genes Regulated by NRF2
The majority of genes induced by NRF2 are those of the Phase II antioxidant and detoxification response, including glutathione synthesis genes (GCLC, GCLM), glutathione reductase (GR), glutathione transferases (GSTs), glutathione peroxidases (GPxs), heme oxygenase-1 (HO-1), NADPH quinone reductase 1 (NQO1), multidrug resistance protein transporters, thioredoxin (Trx) and thioredoxin reductase (TrxR). Additional genes that play a role in survival and stress resistance stimulated by NRF2 include chaperone, heat shock and Cyp/cytochrome P450 genes, UDP-glucuronosyl transferases, carbonyl reductase, lysozyme M, and peroxiredoxin genes [142,192]. Other NRF2-stimulated gene groups include those involved in the ubiquitin-proteasome system, cell growth, metabolism, apoptosis, protein translation, and cytoskeletal organization. Interestingly, the NRF2 gene itself contains AREs and is thus highly inducible by the protein it encodes [192].
NRF2 also plays a role in negative regulation of genes and/or pathways involved in longevity and stress resistance, for example, insulin and growth hormone (GH) / insulin-like growth factor (IGF) signaling. Reduced signaling through these pathways, mediated by reduced levels of peptide hormones or their receptors upon DR, correlates with both extended longevity and increased stress resistance [3,197,198]. Genes encoding both growth hormone receptor (GHR), which extends lifespan when genetically ablated in mice [198], and insulin-like growth factor 2 (IGF-2), which is similar to IGF-1, promotes growth and acts as a hyperproliferative switch in the growth of tumors [199], are negatively regulated by NRF2 [142,192]. The gene encoding Insulin-Degrading Enzyme (IDE), which is responsible for the degradation of insulin and protection against hyperinsulinemia as well as degrading endogenous amyloid β-protein (Aβ) [200], is upregulated by NRF2 [142]. Thus, NRF2-based downregulation of insulin and GH/IGF signaling may contribute to longevity, stress resistance, and protection from age-related pathologies including and type 2 diabetes and Alzheimer’s disease.
NRF2 Regulation
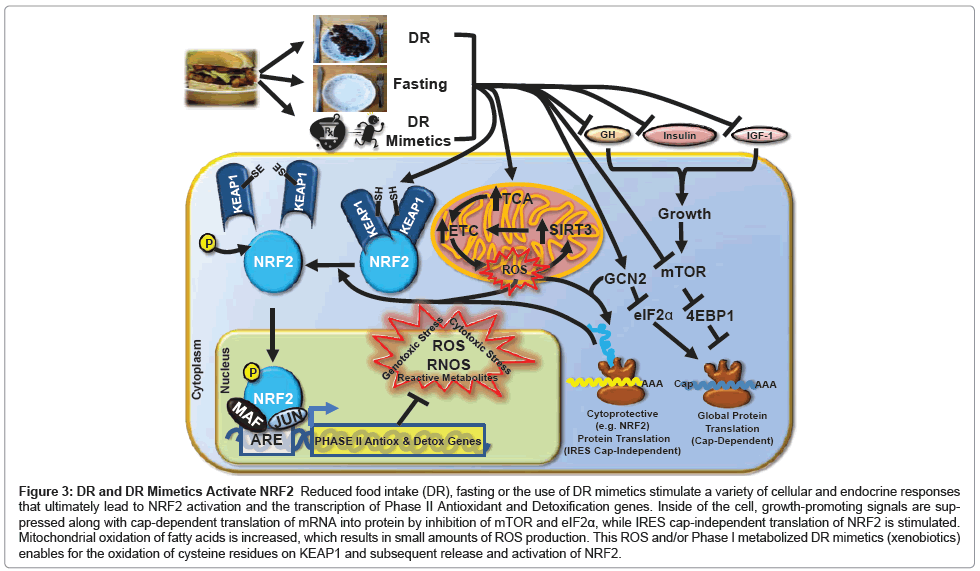
NRF2 is regulated at multiple levels, including transcription, translation, posttranslational modification and localization. The majority of studies have focused on negative regulation of NRF2 by KEAP1, which binds to and sequesters NRF2 in the cytoplasm, leading to its ubiquitination and proteasome-mediated degradation [201,202]. Obstruction of the NRF2-KEAP1 interaction allows for the release, nuclear translocation and activation of NRF2 [203]. Additional NRF2 regulation is KEAP1-independent [204-207], including transcriptional and translational mechanisms summarized in Figure 3 and described below.
Figure 3: DR and DR Mimetics Activate NRF2 Reduced food intake (DR), fasting or the use of DR mimetics stimulate a variety of cellular and endocrine responses that ultimately lead to NRF2 activation and the transcription of Phase II Antioxidant and Detoxification genes. Inside of the cell, growth-promoting signals are suppressed along with cap-dependent translation of mRNA into protein by inhibition of mTOR and eIF2a, while IRES cap-independent translation of NRF2 is stimulated. Mitochondrial oxidation of fatty acids is increased, which results in small amounts of ROS production. This ROS and/or Phase I metabolized DR mimetics (xenobiotics) enables for the oxidation of cysteine residues on KEAP1 and subsequent release and activation of NRF2.
Transcriptional Regulation of NRF2
Similar to other genes in the Phase II response, the NRF2 promoter contains two ARE-like sequences located at nucleotides -579 (GCCACAGTCA) and -317 (TGACTCCGC) relative to its transcriptional start site [206,207]. Phase II response inducers such as D3T increase levels of NRF2 in the nucleus by up to 6-fold within twenty minutes and steady-state levels of NRF2 mRNA by 2-fold within six hours. Despite this positive feedback loop, NRF2 protein and mRNA levels decline within 24-hours of the initial NRF2 activation [207]. Several factors are capable of breaking this positive feedback loop. One is NRF3, another NF-E2 family member that can compete with NRF2 ARE binding and repress the transcription of ARE-containing genes [208]. Another is MiR-28, a small, non-coding microRNA that can bind to the 3’ UTR of NRF2 mRNA and decrease its stability [204].
An additional mechanism for the induction of NRF2 transcription independent of NRF2 protein activity involves the aryl hydrocarbon receptor (AHR) and its interactions with xenobiotic response elements (XPE) in the NRF2 promoter region [206]. AHR is a transcription factor normally found in its inactive form in the cytoplasm. Upon binding to its many synthetic and naturally occurring ligands, including thiabendazole, guanabenz, lipoxin A4, bilirubin, prostaglandin G2, β-Naphthoflavone, tangeritin and diosmin, as well as DR mimetics like curcumin [209], AHR is imported into the nucleus, binds to the XPE in gene promoters and induces a variety of Phase I response drug metabolizing genes such as cytochrome p450s CYP1A1 and CYP1B1 [81,210]. Although necessary for proper drug metabolism, many Phase I response enzymes bioactivate their substrates into carcinogenic intermediates that can react with DNA and form adducts [81]. Because of the dangers posed by these bioactivated compounds, a swift induction of Phase II Response is required. This is mediated by AHR binding to three XPE domains in the NRF2 promoter located at -712 (GCGTG), +755 (CACGC) and +870 (CACGC) [206]. Thus, NRF2 transcription is induced by NRF2/ARE and AHR/XPE, furthering the robustness of the Phase II response against reactive species and xenobiotics.
The decline in antioxidant system activities and gene expression with age [19,211] may be related to a decline in NRF2 activity and decreased expression of Phase II response genes in several tissues, including vascular endothelial and smooth muscle cells of the carotid arteries [212] and liver [213]. Epigenetic changes to the NRF2 promoter may underlie these age-related changes, as silencing of the NRF2 promoter by methylation of CpG islands has been reported in mouse models of prostate cancer [214,215]. Microarray analysis of human prostate cancer samples indicate a reduction in NRF2 mRNA [216]. Reversal of NRF2 deficiency in the prostate, possibly by prevention or removal of DNA methylation at the NRF2 promoter, may help to prevent the onset of prostate cancer. The DR mimetic curcumin, which was previously shown to inhibit the development of prostate cancer in mouse models [217], also acts as a hypomethylation agent and/or DNA methyltransferase inhibitor and restores the expression of NRF2 in prostate cells [215]. Increased levels of NRF2 mRNA, on the other hand, correlate with decreased ischemia reperfusion injury and increased organ functioning following liver transplantation [218]. Taken together, maintenance and/or stimulation of NRF2 transcriptional expression appears to bevital for cancer suppression, successful organ transplantation and overall defense against ROSmediated damage.
Translational Regulation of NRF2
NRF2 protein translation can also be induced upon oxidative stress, for example in rat cardiomyocytes exposed to H2O2 [219]. The increased translation of NRF2 under cellular stress is attributed to an IRES element in the 5’ translated region of human NRF2 mRNA [220], which allows for translational initiation when cap-dependent translation is repressed. Exogenous addition of the DR mimetic/ phyto-oxidant sulphoraphane, but not that of the anti-oxidants GSH orn-acetylcysteine, greatly induces IRES-mediated NRF2 expression on polysomes. Simultaneously, stress, sulphoraphane, as well as the DR mimetic curcumin [221,222], induce the phosphorylation of eIF2α, the attenuation of global protein synthesis [220] and initiate translational derepression of genes with upstream ORFs or IRES in a eIF5B-dependent mode [223]. Thus, the production of ROS from DRstimulated mitochondrial activity and/or xenobiotic stress as well as pharmacological perturbation of classical cap-dependent translational machinery can stimulate the expression of NRF2.
Posttranslational Modification and Localization of NRF2
NRF2 was first identified as a positive regulator of the Phase II Response by Itoh et al. [21]. In this study, treatment of cells with oxidative stress and electrophilic agents increased NRF2 nuclear protein levels as well as its DNA binding activity to AREs without increasing its mRNA levels [21,201], suggesting a cytoplasmic factor responsible for NRF2 instability and inhibition. The half-life of NRF2 protein under normal physiological conditions is very short - only 10 to 40 minutes - further suggesting a powerful repressive mechanism that can also be turned off rapidly during oxidative and electrophilic stress [224,225]. This turns out to be a cytoplasmic inhibitor of NRF2 known as KEAP1, a homolog of the Drosophila actin cytoskeleton binding protein Kelch [201]. Mechanistically, each KEAP1 homodimer can bind a single NRF2 molecule and anchor it to the actin cytoskeleton in the cytoplasm [226,227]. KEAP1 not only blocks nuclear localization of NRF2, but also targets it for degradation by the E3/Cul3 mediated ubiquitin-proteasome pathway, hence the relatively short half-life of NRF2 [227,228]. NRF2’s escape from the grasp of KEAP1 and eventual activation primarily involves oxidation of and/or electrophile binding to the many cysteine thiol groups present in KEAP1 [142,194,226,227]. By oxidation or attachment of electrophiles to these KEAP1 cysteine thiols, cross-linking and/or disruption of the KEAP1 homodimer occurs and results in the inability of KEAP1 to target NRF2 for degradation [227,229].
In addition to liberation from KEAP1, NRF2 undergoes additional posttranslational modifications before it enters the nucleus. For example, phosphorylation at serine 40 by PKC enhances NRF2 mediated transcription of the Phase II Response [230]. Other possible kinase candidates include the MAP kinases p38, PI3K, PKC, JNK and ERK [231,232]. However, whether this or other known serine/ threonine phosphorylation events occur before or after release from KEAP1, and what exactly the roles these phosphates play in NRF2 stability and transcriptional activity remain largely unknown [231]. An additional factor that regulates NRF2 activity is the Parkinson’s associated protein DJ-1/PARK7. Mutations and/or deletions in DR-1/ PARK7 are associated with homozygous recessive forms of Parkinson’s in humans as well as decreased ability to initiate an antioxidant stress response [233]. While the function, either structural or enzymatic, of DJ-1/PARK7 is not entirely known, it has been shown to increase the stability and/or inhibit the degradation of NRF2 by KEAP1/ E3/CUL3 mediated ubiquitin-proteasome pathway and aid its translocation into the nucleus [234,235].
Once in the nucleus, NRF2 associates with several other transcription factors and undergoes additional posttranslational modifications to execute its stimulatory and repressive activities. Recent evidence suggests acetylation of lysine residues 588 and 591 by p300/CBP enhances NRF2’s interaction with the ARE elements in gene promoters and stimulates their expression while under stress [236,237]. Deacetylation by Sirt1 inhibits NRF2 activity and results in decreased nuclear and increased cytoplasmic pools of NRF2 [237]. On the contrary, histone deactylase 2 (HDAC2), but not HDAC1, aids in the transcriptional activity of NRF2, and blocking HDAC2’s enzymatic activity leads to decreased NRF2 activity [238]. Thus, both acetylation and deacetylation play important roles in NRF2 activity. These modifications and their modifiers will need to be studied more in depth to differentiate at what stages they impact or are required for NRF2 stability, localization and activity.
Small Maf transcription factors (MafG, MafK or MafF) form heterodimers with NRF2 that allows specific binding to the AREs of target genes (21; 239). Interestingly, the MafG gene itself is a target ofNRF2/Small Maf-mediated transcription under electrophilic cellular stress [240], reinforcing the idea that a strong Phase II Response requires a positive feedback loop. An additional group of transcription factors, Jun proteins, have also been shown to associate with NRF2 in the upregulation of detoxifying enzymes containing AREs [241]. These Jun proteins include c-Jun, Jun-B and Jun-D. However, elevated levels of c-Jun actually decrease NRF2 mediated transcription. This is possibly by the formation of negative regulatory c-Jun+c-Fos complexes which interfere with the binding of the positive regulatory heterodimer Nrf2+c-Jun atthe ARE binding site [241].
Balancing NRF2 Activation: Don’t leave the lights on
Although maintaining the ability to activate NRF2 is important for detoxification and prevention of aging-associated diseases, constitutive activation has dire consequences. This is seen in the deleterious and lethal phenotype of Keap1 knockout mice that die postnatally due to hyperkeratosis of the esophagus and forestomach [242]. This lends support to short-term rather than long-term use of DR and/or DR mimetics, as it may lessen negative side effects of long-term NRF2 activation while still offering health-promoting benefits. Additionally, oncogene-induced NRF2 transcription [243,244] or loss of function mutations in Keap1 [245] lead to overexpression and/or overactivation of NRF2 and directly increases tumor growth, chemoresistance and radioresistance. Due to DR eliciting a differential response between cancerous and non-cancerous tissue in that cancerous tissue becomes sensitized to chemotherapy and non-cancerous becomes more resistant [11,246], it seems prudent to give our normal, healthy tissue a fighting chance via DR against various cancer treatments. Doing so would hypothetically activate NRF2 and Phase II systems and increase resistance only in non-cancerous tissues, allowing for more aggressive treatments against endogenously resistance cancer cells.
Conclusions
In this review, we have touched upon several mechanistic topics related to stress resistance induced by DR and DR mimetics. Although not completely understood, these mechanisms shed light on a less appreciated side of DR quite apart from canonical agingrelated GH, IGF1, AMPK and TOR pathways. These include: 1) The hormetic nature of small, transient amounts of ROS derived from mitochondria upon the switch to fatty acid oxidation (mitohormesis) and/or xenobiotic stress produced by the Phase I metabolism of DR mimetics (xenohormesis). Dietary restriction and its xenobiotic and exercise mimetics thus generate beneficial responses not passively but rather actively stimulate the hormetic response by generating mild amounts of reactive metabolites, oxygen species, and xenobiotic stress. 2) These small stressors produced by DR and DR mimetics activate the transcription factor NRF2 through transcriptional upregulation of NRF2 mRNA, the increased translation of NRF2 protein and, most importantly, its release from KEAP1 in the cytoplasm and subsequent translocation and activation in the nucleus. Upon activation, NRF2 promotes the expression of over 200 potential cytoprotective genes and represses several pro-growth and pro-inflammation genes by binding to the ARE sequence in their gene promoters and may mediate many of the benefits of DR. 3) NRF2-mediated induction of Phase II Response genes may be crucial to stress resistance afforded by DR and DR mimetics. These Phase II Response genes are predominantly involved in maintaining the proper cellular redox state as well as detoxification of reactive xenobiotics and metabolites through the utilization of cellular reducing molecules GSH, Trx and NADPH. Interestingly, longlived dwarf mice with defects in growth hormone releasing hormone also display constitutive increases in Phase II Response elements, particularly GSTs [247,248]. These mice are also more resistant to the stress of acetaminophen toxicity, although whether or not the Phase II response underlies their longevity or stress resistance phenotypes is not known. Similarly, further experiments are required to fully elucidate the role of the NRF2-mediated Phase II Response in DR benefits, as many of the existing studies are largely correlative in nature.
Besides reviewing the mechanisms of DR-derived benefits, we provided several examples of age-related reductions in cellular redox and detoxification defenses and how DR is capable of preventing and even reversing these deteriorations. Translation of DR benefits from preclinical models to the clinic is still rare, likely due to such reasons as unlikelihood of patient compliance and/or safety and efficacy of the dietary intervention prior to the stress of surgery and therapies. One such negative connotation of DR is its ability to decrease survival of aged mice in response to primary influenza infection [249], suggesting that DR use in the elderly or in relation to viral infection should be more closely examined. Nonetheless there are a few recent examples of studies examining the potential benefits of DR and DR mimetics in humans. These limited studies show that various forms of short-term DR can be employed as a means to increase favorable clinical outcomes in conjuncture with surgery and chemotherapy [250-253]. In a different context, large populations voluntarily and routinely undergo various DR and fasting regimens, mostly for religious reasons. Favorable outcomes in these populations include lowered BMI, cholesterol, blood pressure as well as biomarkers of tissue damage and oxidative stress [254]. Impressively, fasting just one day per month in among Mormons is associated with decreased cardiac mortality, coronary artery disease and diabetes, even when all other risk and health factors are taken into consideration [255]. Could the oppositehold true for the phenomena termed “Merry Christmas Coronary” and “Happy New Year Heart Attack”? During the period between Thanks giving and New Year’s Day in the U. S. A, a time frame associated with over indulgence in food and drink, there is a striking increase in deaths due to ischemic heart disease that is independent of the weather [256]. Could fasting and overeating have such opposite effects through a common pathway, namely activation and suppression ofNRF2 and the Phase II Response, respectively, thus modulating vulnerability to ischemic heart diseaserelated mortality?
We foresee the use of short-term DR and DR mimetics in the clinic to enhance patient outcomes as a viable, readily available and inexpensive intervention with great potential. Optimization of dietary interventions in preclinical and clinical trials, a better mechanistic understanding of its stimulation of target genetic pathways as well as changes made in the current dogma of preoperative/pretreatment dietary intake may someday soon usher in a new era of preventative and palliative medicine.
Acknowledgements
The authors wish to thank Pedro Mejia, Lauren Robertson, Jordan Gallinetti, EylulHarputlugil, Lear Brace and Dorathy Vargas for critical feedback on the text. This work was funded by the following sources: NIH (National Institute on Aging, AG036712; National Institute of Diabetes and Digestive and Kidney Diseases, DK090629), Ellison Medical Foundation, American Federation for Aging Research, and the Glenn Foundation for Medical Research.
References
- Jemal A, Ward E, Hao Y, Thun M (2005) Trends in the leading causes of death in the United States, 1970-2002. JAMA 294: 1255-1259.
- Weindruch R, Sohal RS (1997) Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med 337: 986-994.
- Fontana L, Partridge L, Longo VD (2010) Extending healthy life span--from yeast to humans. Science 328: 321-326.
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R (2006) Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci U S A 103: 19908-19912.
- Rous P (1914) THE INFLUENCE OF DIET ON TRANSPLANTED AND SPONTANEOUS MOUSE TUMORS. J Exp Med 20: 433-451.
- CM M, MF C, LA M: The effect of retarded growth upon length of lifespan and upon ultimate body size. Journal of Nutrition 1935:63-79
- Pollard M, Luckert PH, Pan GY (1984) Inhibition of intestinal tumorigenesis in methylazoxymethanol-treated rats by dietary restriction. Cancer Treat Rep 68: 405-408.
- KozubÃk A, PospÃsil M (1982) Adaptation to intermittent fasting as a factor modifying the radiation resistance of mice. Experientia 38: 958-959.
- KozubÃk A, PospÃsil M (1985) Intermittent feeding as a factor enhancing hemopoietic stem cell proliferation and spleen colony formation in irradiated mice. Acta Radiol Oncol 24: 357-361.
- KozubÃk A, PospÃsil M, Hosek B (1985) Stimulatory effect of intermittent feeding on hemopoietic recovery in sublethally gamma-irradiated mice. Acta Radiol Oncol 24: 199-204.
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, et al. (2010) Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 70: 1564-1572.
- Honjoh S, Yamamoto T, Uno M, Nishida E (2009) Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457: 726-730.
- Vigne P, Tauc M, Frelin C (2009) Strong dietary restrictions protect Drosophila against anoxia/reoxygenation injuries. PLoS One 4: e5422.
- Mitchell JR, Verweij M, Brand K, van de Ven M, Goemaere N,et al (2010) Short-term dietary restriction and fasting precondition against ischemia reperfusion injury in mice. Aging Cell 9: 40-53.
- Singh R, Lakhanpal D, Kumar S, Sharma S, Kataria H, et al. (2011) Late-onset intermittent fasting dietary restriction as a potential intervention to retard age-associated brain function impairments in male rats. Age (Dordr) .
- Sinclair DA (2005) Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev 126: 987-1002.
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, et al. (1999) Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54: B492-501.
- Masoro EJ (1998) Hormesis and the antiaging action of dietary restriction. Exp Gerontol 33: 61-66.
- Cho CG, Kim HJ, Chung SW, Jung KJ, Shim KH, et al. (2003) Modulation of glutathione and thioredoxin systems by calorie restriction during the aging process. Exp Gerontol 38: 539-548.
- Rao G, Xia E, Richardson A (1990) Effect of age on the expression of antioxidant enzymes in male Fischer F344 rats. Mech Ageing Dev 53: 49-60.
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, et al. (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313-322.
- McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ,et al. (2001) The Cap'n'Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res 61: 3299-3307
- Rao G, Xia E, Nadakavukaren MJ, Richardson A (1990) Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J Nutr 120: 602-609.
- Calabrese EJ, Mattson MP (2011) Hormesis provides a generalized quantitative estimate of biological plasticity. J Cell Commun Signal 5: 25-38.
- Dudekula N, Arora V, Callaerts-Vegh Z, Bond RA (2006) The temporal hormesis of drug therapies. Dose Response 3: 414-424.
- Son TG, Camandola S, Mattson MP (2008) Hormetic dietary phytochemicals. Neuromolecular Med 10: 236-246.
- Thompson RE (2010) Epidemiological Evidence for Possible Radiation Hormesis from Radon Exposure: A Case-Control Study Conducted in Worcester, MA. Dose Response 9: 59-75.
- Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, et al. (2009) Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106: 8665-8670.
- Mattson MP: Dietary factors, hormesis and health. Ageing Res Rev 2008 7: 43-48
- Ng MF (2010) Cachexia - an intrinsic factor in wound healing. Int Wound J 7: 107-113.
- Muscaritoli M, Anker SD, Argilés J, Aversa Z, Bauer JM,et al (2010) Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) "cachexia-anorexia in chronic wasting diseases" and "nutrition in geriatrics". Clin Nutr 29: 154-159
- Stewart DE (1992) Reproductive functions in eating disorders. Ann Med 24: 287-291.
- Cunningham-Rundles S, McNeeley DF, Moon A (2005) Mechanisms of nutrient modulation of the immune response. J Allergy Clin Immunol 115: 1119-1128.
- Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, et al. (2011) Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A 108: 15324-15329.
- Mollica MP, Lionetti L, Putti R, Cavaliere G, Gaita M, et al. (2011) From chronic overfeeding to hepatic injury: role of endoplasmic reticulum stress and inflammation. Nutr Metab Cardiovasc Dis 21: 222-230.
- Tam CS, Viardot A, Clément K, Tordjman J, Tonks K, et al. (2010) Short-term overfeeding may induce peripheral insulin resistance without altering subcutaneous adipose tissue macrophages in humans. Diabetes 59: 2164-2170.
- Molon-Noblot S, Keenan KP, Coleman JB, Hoe CM, Laroque P (2001) The effects of ad libitum overfeeding and moderate and marked dietary restriction on age-related spontaneous pancreatic islet pathology in Sprague-Dawley rats. Toxicol Pathol 29: 353-362.
- Shanley DP, Kirkwood TB (2006) Caloric restriction does not enhance longevity in all species and is unlikely to do so in humans. Biogerontology 7: 165-168
- Lagerpusch M, Bosy-Westphal A, Kehden B, Peters A, Müller MJ (2011) Effects of brief perturbations in energy balance on indices of glucose homeostasis in healthy lean men. Int J Obes (Lond) .
- Weindruch R, Walford RL (1982) Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science 215: 1415-1418.
- Wilson R (2000) Does caloric restriction cause hormesis? Comments on paper by Turturro, Hass and Hart. Hum Exp Toxicol 19: 353-359.
- Sprott RL (2000) Is caloric restriction hormetic or is ad libitum feeding toxic? Hum Exp Toxicol 19: 351-352.
- Boxenbaum H: (2000) Commentary: does caloric restriction induce hormesis?. Hum Exp Toxicol 19: 330-331
- Ristow M, Schmeisser S (2011) Extending life span by increasing oxidative stress. Free Radic Biol Med 51: 327-336.
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, et al. (2007) Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab 6: 280-293.
- Sharma PK, Agrawal V, Roy N (2011) Mitochondria-mediated hormetic response in life span extension of calorie-restricted Saccharomyces cerevisiae. Age (Dordr) 33: 143-154.
- Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS (2007) Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metab 5: 265-277.
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, et al. (2002) Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418: 344-348.
- Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, et al. (2009) 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell 139: 149-160.
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, et al. (2005) Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science 310: 314-317.
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H,et al. (2009) Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297: H13-20.
- Domenech R, Macho P, Schwarze H, Sánchez G (2002) Exercise induces early and late myocardial preconditioning in dogs. Cardiovasc Res 55: 561-566.
- López-Lluch G, Hunt N, Jones B, Zhu M, Jamieson H, et al. (2006) Calorie restriction induces mitochondrial biogenesis and bioenergetic efficiency. Proc Natl Acad Sci U S A 103: 1768-1773.
- Ristow M, Zarse K (2010) How increased oxidative stress promotes longevity and metabolic health: The concept of mitochondrial hormesis (mitohormesis). Exp Gerontol 45: 410-418.
- Schulz TJ, Westermann D, Isken F, Voigt A, Laube B, et al. (2010) Activation of mitochondrial energy metabolism protects against cardiac failure. Aging (Albany NY) 2: 843-853.
- Shinmura K, Tamaki K, Sano M, Nakashima-Kamimura N, Wolf AM,et al. (2011) Caloric restriction primes mitochondria for ischemic stress by deacetylating specific mitochondrial proteins of the electron transport chain. Circ Res 109: 396-406
- Tapia PC: 2006) Sublethal mitochondrial stress with an attendant stoichiometric augmentation of reactive oxygen species may precipitate many of the beneficial alterations in cellular physiology produced by caloric restriction, intermittent fasting, exercise and dietary phytonutrients: "Mitohormesis" for health and vitality. Med Hypotheses 66: 832-843.
- Zarse K, Bossecker A, Müller-Kuhrt L, Siems K, Hernandez MA,et al (2011) The phytochemical glaucarubinone promotes mitochondrial metabolism, reduces body fat, and extends lifespan of Caenorhabditis elegans. Horm Metab 43: 241-243.
- Zuckerbraun BS, Chin BY, Bilban M, d'Avila JC, Rao J, et al. (2007) Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J 21: 1099-1106.
- Balogun E, Hoque M, Gong P, Killeen E, Green CJ, et al. (2003) Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371: 887-895.
- Childs A, Jacobs C, Kaminski T, Halliwell B, Leeuwenburgh C (2001) Supplementation with vitamin C and N-acetyl-cysteine increases oxidative stress in humans after an acute muscle injury induced by eccentric exercise. Free Radic Biol Med 31: 745-753.
- Hanley PJ, Mickel M, Löffler M, Brandt U, Daut J (2002) K(ATP) channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol 542: 735-741.
- Hanley PJ, Dröse S, Brandt U, Lareau RA, Banerjee AL, et al. (2005) 5-Hydroxydecanoate is metabolised in mitochondria and creates a rate-limiting bottleneck for beta-oxidation of fatty acids. J Physiol 562: 307-318.
- Ross NS, Hoppel CL (1987) Acyl-CoA dehydrogenase activity in the riboflavin-deficient rat. Effects of starvation. Biochem J 244: 387-391.
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, et al. (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464: 121-125.
- Jing E, Emanuelli B, Hirschey MD, Boucher J, Lee KY, et al. (2011) Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A 108: 14608-14613.
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, et al. (2009) Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 1: 771-783.
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D (2010) Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab 12: 662-667.
- Bartlett K, Eaton S (2004) Mitochondrial beta-oxidation. Eur J Biochem 271: 462-469.
- Leeuwenburgh C, Heinecke JW (2001) Oxidative stress and antioxidants in exercise. Curr Med Chem 8: 829-838.
- Seifert EL, Estey C, Xuan JY, Harper ME (2010) Electron transport chain-dependent and -independent mechanisms of mitochondrial H2O2 emission during long-chain fatty acid oxidation. J Biol Chem 285: 5748-5758.
- Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417: 1-13.
- Varady J, Ringseis R, Eder K (2012) Dietary moderately oxidized oil induces expression of fibroblast growth factor 21 in the liver of pigs. Lipids Health Dis 11: 34.
- Lane MA, Roth GS, Ingram DK (2007) Caloric restriction mimetics: a novel approach for biogerontology. Methods Mol Biol 371: 143-149.
- Onken B, Driscoll M (2010) Metformin induces a dietary restriction-like state and the oxidative stress response to extend C. elegans Healthspan via AMPK, LKB1, and SKN-1. PLoS One 5: e8758.
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, et al. (2008) Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8: 157-168.
- Holloszy JO (1997) Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol 82: 399-403.
- Hawley SA, Gadalla AE, Olsen GS, Hardie DG (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51: 2420-2425.
- Guengerich FP (2001) Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem Res Toxicol 14: 611-650.
- Harrigan JA, McGarrigle BP, Sutter TR, Olson JR: (2006)Tissue specific induction of cytochrome P450 (CYP) 1A1 and 1B1 in rat liver and lung following in vitro (tissue slice) and in vivo exposure to benzo(a)pyrene. Toxicol In Vitro 20: 426-438
- Lamming DW, Wood JG, Sinclair DA (2004) Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol 53: 1003-1009.
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, et al. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191-196.
- Peng W, Robertson L, Gallinetti J, Mejia P, Vose S, et al. (2012) Surgical stress resistance induced by single amino acid deprivation requires Gcn2 in mice. Sci Transl Med 4: 118ra11.
- Go KG, Prenen GH, Korf J (1988) Protective effect of fasting upon cerebral hypoxic-ischemic injury. Metab Brain Dis 3: 257-263.
- Khan S, Salloum F, Das A, Xi L, Vetrovec GW, et al. (2006) Rapamycin confers preconditioning-like protection against ischemia-reperfusion injury in isolated mouse heart and cardiomyocytes. J Mol Cell Cardiol 41: 256-264.
- Saleh MC, Connell BJ, Saleh TM (2010) Resveratrol preconditioning induces cellular stress proteins and is mediated via NMDA and estrogen receptors. Neuroscience 166: 445-454.
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, (2005) Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4: 119-125.
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, et al. (2003) Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A 100: 6216-6220.
- Lee C, Safdie FM, Raffaghello L, Wei M, Madia F, et al. (2010) Reduced levels of IGF-I mediate differential protection of normal and cancer cells in response to fasting and improve chemotherapeutic index. Cancer Res 70: 1564-1572.
- Hydock DS, Lien CY, Jensen BT, Schneider CM, Hayward R (2011) Exercise preconditioning provides long-term protection against early chronic doxorubicin cardiotoxicity. Integr Cancer Ther 10: 47-57.
- Pelgrims J, De Vos F, Van den Brande J, Schrijvers D, Prové A, et al. (2000) Methylene blue in the treatment and prevention of ifosfamide-induced encephalopathy: report of 12 cases and a review of the literature. Br J Cancer 82: 291-294.
- Dong J, Yan D, Chen SY (2011) Stabilization of Nrf2 protein by D3T provides protection against ethanol-induced apoptosis in PC12 cells. PLoS One 6: e16845.
- Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG (2008) Fasting is neuroprotective following traumatic brain injury. J Neurosci Res 86: 1812-1822.
- Prochaska HJ, Yeh Y, Baron P, Polsky B (1993) Oltipraz, an inhibitor of human immunodeficiency virus type 1 replication. Proc Natl Acad Sci U S A 90: 3953-3957.
- Ung VY, Foshaug RR, MacFarlane SM, Churchill TA, Doyle JS, et al. (2010) Oral administration of curcumin emulsified in carboxymethyl cellulose has a potent anti-inflammatory effect in the IL-10 gene-deficient mouse model of IBD. Dig Dis Sci 55: 1272-1277.
- Talalay P, Dinkova-Kostova AT, Holtzclaw WD (2003) Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv Enzyme Regul 43: 121-134.
- Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579: 3029-3036.
- An JH, Blackwell TK (2003) SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev 17: 1882-1893.
- Kwak MK, Wakabayashi N, Kensler TW (2004) Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res 555: 133-148.
- Lu SC (2009) Regulation of glutathione synthesis. Mol Aspects Med 30: 42-59.
- Vázquez-Medina JP, Zenteno-SavÃn T, Forman HJ, Crocker DE, Ortiz RM (2011) Prolonged fasting increases glutathione biosynthesis in postweaned northern elephant seals. J Exp Biol 214: 1294-1299.
- Yang H, Magilnick N, Lee C, Kalmaz D, Ou X, et al. (2005) Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol Cell Biol 25: 5933-5946.
- Lee TD, Yang H, Whang J, Lu SC (2005) Cloning and characterization of the human glutathione synthetase 5'-flanking region. Biochem J 390: 521-528.
- Huang ZA, Yang H, Chen C, Zeng Z, Lu SC (2000) Inducers of gamma-glutamylcysteine synthetase and their effects on glutathione synthetase expression. Biochim Biophys Acta 1493: 48-55.
- Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360: 1-16.
- Boyer TD (1989) The glutathione S-transferases: an update. Hepatology 9: 486-496.
- Meister A (1988) Glutathione metabolism and its selective modification. J Biol Chem 263: 17205-17208.
- Morrow CS, Smitherman PK, Townsend AJ (2000) Role of multidrug-resistance protein 2 in glutathione S-transferase P1-1-mediated resistance to 4-nitroquinoline 1-oxide toxicities in HepG2 cells. Mol Carcinog 29: 170-178.
- Paumi CM, Ledford BG, Smitherman PK, Townsend AJ, Morrow CS (2001) Role of multidrug resistance protein 1 (MRP1) and glutathione S-transferase A1-1 in alkylating agent resistance. Kinetics of glutathione conjugate formation and efflux govern differential cellular sensitivity to chlorambucil versus melphalan toxicity. J Biol Chem 276: 7952-7956.
- Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113: 484-490.
- Owen JB, Butterfield DA (2010) Measurement of oxidized/reduced glutathione ratio. Methods Mol Biol 648: 269-277.
- Harvey CJ, Thimmulappa RK, Singh A, Blake DJ, Ling G, et al. (2009) Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic Biol Med 46: 443-453.
- Filomeni G, Rotilio G, Ciriolo MR (2003) Glutathione disulfide induces apoptosis in U937 cells by a redox-mediated p38 MAP kinase pathway. FASEB J 17: 64-66.
- Wu KC, Cui JY, Klaassen CD (2011) Beneficial role of Nrf2 in regulating NADPH generation and consumption. Toxicol Sci 123: 590-600.
- Sachan DS, Das SK (1982) Alterations of NADPH-generating and drug-metabolizing enzymes by feed restriction in male rats. J Nutr 112: 2301-2306.
- Li J, Lee JM, Johnson JA (2002) Microarray analysis reveals an antioxidant responsive element-driven gene set involved in conferring protection from an oxidative stress-induced apoptosis in IMR-32 cells. J Biol Chem 277: 388-394.
- Chen CN, Brown-Borg HM, Rakoczy SG, Ferrington DA, Thompson LV (2010) Aging impairs the expression of the catalytic subunit of glutamate cysteine ligase in soleus muscle under stress. J Gerontol A Biol Sci Med Sci 65: 129-137.
- Kennedy BP, Rao F, Botiglieri T, Sharma S, Lillie EO, et al. (2005) Contributions of the sympathetic nervous system, glutathione, body mass and gender to blood pressure increase with normal aging: influence of heredity. J Hum Hypertens 19: 951-969.
- Samiec PS, Drews-Botsch C, Flagg EW, Kurtz JC, Sternberg P Jr, et al. (1998) Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med 24: 699-704.
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, et al. (2009) Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 390: 191-214.
- Kode A, Rajendrasozhan S, Caito S, Yang SR, Megson IL, et al. (2008) Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L478-488.
- Leakey JA, Cunny HC, Bazare J Jr, Webb PJ, Lipscomb JC, et al. (1989) Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the Fischer 344 rat. II: Effects on conjugating enzymes. Mech Ageing Dev 48: 157-166.
- Kabesch M, Hoefler C, Carr D, Leupold W, Weiland SK, et al. (2004) Glutathione S transferase deficiency and passive smoking increase childhood asthma. Thorax 59: 569-573.
- Wiencke JK, Kelsey KT, Lamela RA, Toscano WA Jr (1990) Human glutathione S-transferase deficiency as a marker of susceptibility to epoxide-induced cytogenetic damage. Cancer Res 50: 1585-1590.
- Smith CM, Kelsey KT, Wiencke JK, Leyden K, Levin S, et al. (1994) Inherited glutathione-S-transferase deficiency is a risk factor for pulmonary asbestosis. Cancer Epidemiol Biomarkers Prev 3: 471-477.
- McWilliams JE, Sanderson BJ, Harris EL, Richert-Boe KE, Henner WD (1995) Glutathione S-transferase M1 (GSTM1) deficiency and lung cancer risk. Cancer Epidemiol Biomarkers Prev 4: 589-594.
- Lafuente A, Pujol F, Carretero P, Villa JP, Cuchi A (1993) Human glutathione S-transferase mu (GST mu) deficiency as a marker for the susceptibility to bladder and larynx cancer among smokers. Cancer Lett 68: 49-54.
- Pearson KJ, Lewis KN, Price NL, Chang JW, Perez E, et al. (2008) Nrf2 mediates cancer protection but not prolongevity induced by caloric restriction. Proc Natl Acad Sci U S A 105: 2325-2330.
- Sykiotis GP, Bohmann D (2008) Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev Cell 14: 76-85.
- RodrÃguez-Ramiro I, Ramos S, Bravo L, Goya L, MartÃn MA (2011) Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur J Nutr .
- Higgins LG, Kelleher MO, Eggleston IM, Itoh K, Yamamoto M, et al. (2009) Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol Appl Pharmacol 237: 267-280.
- Meydani M, Das S, Band M, Epstein S, Roberts S (2011) The effect of caloric restriction and glycemic load on measures of oxidative stress and antioxidants in humans: results from the CALERIE Trial of Human Caloric Restriction. J Nutr Health Aging 15: 456-460.
- Chevion M, Berenshtein E, Stadtman ER (2000) Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radic Res 33 Suppl: S99-108.
- Siu PM, Pei XM, Teng BT, Benzie IF, Ying M, et al. (2011) Habitual exercise increases resistance of lymphocytes to oxidant-induced DNA damage by upregulating expression of antioxidant and DNA repairing enzymes. Exp Physiol 96: 889-906.
- Krehl S, Loewinger M, Florian S, Kipp A, Banning A, (2011) Glutathione peroxidase-2 and selenium decreased inflammation and tumors in a mouse model of inflammation-associated carcinogenesis whereas sulforaphane effects differed with selenium supply. Carcinogenesis 33: 620-628.
- Kamerbeek NM, van Zwieten R, de Boer M, Morren G, Vuil H, et al. (2007) Molecular basis of glutathione reductase deficiency in human blood cells. Blood 109: 3560-3566.
- Holmgren A, Björnstedt M (1995) Thioredoxin and thioredoxin reductase. Methods Enzymol 252: 199-208.
- Holmgren A (1995) Thioredoxin structure and mechanism: conformational changes on oxidation of the active-site sulfhydryls to a disulfide. Structure 3: 239-243.
- Björnstedt M, Hamberg M, Kumar S, Xue J, Holmgren A (1995) Human thioredoxin reductase directly reduces lipid hydroperoxides by NADPH and selenocystine strongly stimulates the reaction via catalytically generated selenols. J Biol Chem 270: 11761-11764.
- Kim YC, Yamaguchi Y, Kondo N, Masutani H, Yodoi J (2003) Thioredoxin-dependent redox regulation of the antioxidant responsive element (ARE) in electrophile response. Oncogene 22: 1860-1865.
- Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, et al. (2003) Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem 278: 8135-8145.
- Brown-Borg HM (2006) Longevity in mice: is stress resistance a common factor? Age (Dordr) 28: 145-162.
- Mitsui A, Hamuro J, Nakamura H, Kondo N, Hirabayashi Y, et al. (2002) Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid Redox Signal 4: 693-696.
- Kontou M, Adelfalk C, Ramirez MH, Ruppitsch W, Hirsch-Kauffmann M, et al. (2002) Overexpressed thioredoxin compensates Fanconi anemia related chromosomal instability. Oncogene 21: 2406-2412.
- Ruppitsch W, Meisslitzer C, Hirsch-Kauffmann M, Schweiger M (1998) Overexpression of thioredoxin in Fanconi anemia fibroblasts prevents the cytotoxic and DNA damaging effect of mitomycin C and diepoxybutane. FEBS Lett 422: 99-102.
- Akterin S, Cowburn RF, Miranda-Vizuete A, Jiménez A, Bogdanovic N, et al. (2006) Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ 13: 1454-1465.
- Fierro-González JC, González-Barrios M, Miranda-Vizuete A, Swoboda P (2011) The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem Biophys Res Commun 406: 478-482.
- Son TG, Camandola S, Arumugam TV, Cutler RG, Telljohann RS, et al. (2010) Plumbagin, a novel Nrf2/ARE activator, protects against cerebral ischemia. J Neurochem 112: 1316-1326.
- Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, et al. (2000) NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 129: 77-97.
- Navarro F, Navas P, Burgess JR, Bello RI, De Cabo R, et al. (1998) Vitamin E and selenium deficiency induces expression of the ubiquinone-dependent antioxidant system at the plasma membrane. FASEB J 12: 1665-1673.
- Arroyo A, Kagan VE, Tyurin VA, Burgess JR, de Cabo R, et al. (2000) NADH and NADPH-dependent reduction of coenzyme Q at the plasma membrane. Antioxid Redox Signal 2: 251-262.
- Arroyo A, Navarro F, Gómez-DÃaz C, Crane FL, AlcaÃn FJ, et al. (2000) Interactions between ascorbyl free radical and coenzyme Q at the plasma membrane. J Bioenerg Biomembr 32: 199-210.
- Hyun DH, Kim J, Moon C, Lim CJ, de Cabo R, et al (2012) The plasma membrane redox enzyme NQO1 sustains cellular energetics and protects human neuroblastoma cells against metabolic and proteotoxic stress. Age (Dordr) 34: 359-370
- Villalba JM, Navas P (2000) Plasma membrane redox system in the control of stress-induced apoptosis. Antioxid Redox Signal 2: 213-230.
- De Cabo R, Cabello R, Rios M, López-Lluch G, Ingram DK, et al. (2004) Calorie restriction attenuates age-related alterations in the plasma membrane antioxidant system in rat liver. Exp Gerontol 39: 297-304.
- Suh JH, Shenvi SV, Dixon BM, Liu H, Jaiswal AK, et al. (2004) Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A 101: 3381-3386.
- WILLIAMS-ASHMAN HG, HUGGINS C (1961) Oxydation of reduced pyridine nucleotides in mammary gland and adipose tissue following treatment with polynuclear hydrocarbons. Med Exp Int J Exp Med 4: 223-226.
- Talalay P, Dinkova-Kostova AT (2004) Role of nicotinamide quinone oxidoreductase 1 (NQO1) in protection against toxicity of electrophiles and reactive oxygen intermediates. Methods Enzymol 382: 355-364.
- Gozzelino R, Jeney V, Soares MP (2010) Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol 50: 323-354.
- Graber SG, Woodworth RC (1986) Myoglobin expression in L6 muscle cells. Role of differentiation and heme. J Biol Chem 261: 9150-9154.
- BRUNS GP, LONDON IM (1965) THE EFFECT OF HEMIN ON THE SYNTHESIS OF GLOBIN. Biochem Biophys Res Commun 18: 236-242.
- Zhu Y, Sun Y, Jin K, Greenberg DA (2002) Hemin induces neuroglobin expression in neural cells. Blood 100: 2494-2498.
- Tolosano E, Altruda F (2002) Hemopexin: structure, function, and regulation. DNA Cell Biol 21: 297-306.
- Maines MD (1988) Heme oxygenase: function, multiplicity, regulatory mechanisms, and clinical applications. FASEB J 2: 2557-2568.
- Garratty G (2009) Drug-induced immune hemolytic anemia. Hematology Am Soc Hematol Educ Program .
- Pamplona A, Hanscheid T, Epiphanio S, Mota MM, Vigário AM (2009) Cerebral malaria and the hemolysis/methemoglobin/heme hypothesis: shedding new light on an old disease. Int J Biochem Cell Biol 41: 711-716.
- Dhaliwal G, Cornett PA, Tierney LM Jr (2004) Hemolytic anemia. Am Fam Physician 69: 2599-2606.
- Morse D, Choi AM (2005) Heme oxygenase-1: from bench to bedside. Am J Respir Crit Care Med 172: 660-670.
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, et al. (1999) Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071-26078.
- Alam J, Killeen E, Gong P, Naquin R, Hu B, et al. (2003) Heme activates the heme oxygenase-1 gene in renal epithelial cells by stabilizing Nrf2. Am J Physiol Renal Physiol 284: F743-752.
- Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, et al. (2000) Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med 192: 1015-1026.
- Günther L, Berberat PO, Haga M, Brouard S, Smith RN, et al. (2002) Carbon monoxide protects pancreatic beta-cells from apoptosis and improves islet function/survival after transplantation. Diabetes 51: 994-999.
- Sato K, Balla J, Otterbein L, Smith RN, Brouard S, et al. (2001) Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol 166: 4185-4194.
- Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graça-Souza AV, et al. (2004) Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemia reperfusion injury. FASEB J 18: 771-772.
- Schmeisser S, Zarse K, Ristow M (2011) Lonidamine extends lifespan of adult Caenorhabditis elegans by increasing the formation of mitochondrial reactive oxygen species. Horm Metab Res 43: 687-692.
- Silva G, Cunha A, Grégoire IP, Seldon MP, Soares MP (2006) The antiapoptotic effect of heme oxygenase-1 in endothelial cells involves the degradation of p38 alpha MAPK isoform. J Immunol 177: 1894-1903.
- Bilban M, Bach FH, Otterbein SL, Ifedigbo E, d'Avila JC, et al. (2006) Carbon monoxide orchestrates a protective response through PPARgamma. Immunity 24: 601-610.
- Chin BY, Jiang G, Wegiel B, Wang HJ, Macdonald T, et al. (2007) Hypoxia-inducible factor 1alpha stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci U S A 104: 5109-5114.
- Kim KM, Pae HO, Zheng M, Park R, Kim YM, et al. (2007) Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res 101: 919-927.
- Ghosh S, Tan F, Yu T, Li Y, Adisa O, et al. (2011) Global gene expression profiling of endothelium exposed to heme reveals an organ-specific induction of cytoprotective enzymes in sickle cell disease. PLoS One 6: e18399.
- Telford RD, Sly GJ, Hahn AG, Cunningham RB, Bryant C, et al. (2003) Footstrike is the major cause of hemolysis during running. J Appl Physiol 94: 38-42.
- Ewing JF, Maines MD (2006) Regulation and expression of heme oxygenase enzymes in aged-rat brain: age related depression in HO-1 and HO-2 expression and altered stress-response. J Neural Transm 113: 439-454.
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, et al. (2008) Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med 14: 194-198.
- Maestrelli P, Páska C, Saetta M, Turato G, Nowicki Y, et al. (2003) Decreased haem oxygenase-1 and increased inducible nitric oxide synthase in the lung of severe COPD patients. Eur Respir J 21: 971-976.
- Verweij M, van Ginhoven TM, Mitchell JR, Sluiter W, van den Engel S, et al. (2011) Preoperative fasting protects mice against hepatic ischemia/reperfusion injury: mechanisms and effects on liver regeneration. Liver Transpl 17: 695-704.
- van Ginhoven TM, Mitchell JR, Verweij M, Hoeijmakers JH, Ijzermans JN,et al. (2009) The use of preoperative nutritional interventions to protect against hepatic ischemia-reperfusion injury. Liver Transpl 15: 1183-1191.
- Uchida Y, Tamaki T, Tanaka M, Konoeda Y, Kaizu T, et al. (2000) De novo protein synthesis induced by donor nutritional depletion ameliorates cold ischemia and reperfusion injury in rat liver. Transplant Proc 32: 1657-1659.
- Farombi EO, Shrotriya S, Na HK, Kim SH, Surh YJ (2008) Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem Toxicol 46: 1279-1287.
- Bishop NA, Guarente L (2007) Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447: 545-549.
- Andrews NC, Kotkow KJ, Ney PA, Erdjument-Bromage H, Tempst P, et al. (1993) The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci U S A 90: 11488-11492.
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA (2003) Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278: 12029-12038.
- MartÃn-Montalvo A, Villalba JM, Navas P, de Cabo R (2011) NRF2, cancer and calorie restriction. Oncogene 30: 505-520.
- Wang XJ, Hayes JD, Wolf CR (2006) Generation of a stable antioxidant response element-driven reporter gene cell line and its use to show redox-dependent activation of nrf2 by cancer chemotherapeutic agents. Cancer Res 66: 10983-10994.
- Bell KF, Al-Mubarak B, Fowler JH, Baxter PS, Gupta K, et al. (2011) Mild oxidative stress activates Nrf2 in astrocytes, which contributes to neuroprotective ischemic preconditioning. Proc Natl Acad Sci U S A 108: E1-2.
- Imhoff BR, Hansen JM (2009) Extracellular redox status regulates Nrf2 activation through mitochondrial reactive oxygen species. Biochem J 424: 491-500.
- Chiba T, Yamaza H, Shimokawa I (2007) Role of insulin and growth hormone/insulin-like growth factor-I signaling in lifespan extension: rodent longevity models for studying aging and calorie restriction. Curr Genomics 8: 423-428.
- Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A,et al (2003) Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology 144: 3799-3810.
- Christofori G, Naik P, Hanahan D (1994) A second signal supplied by insulin-like growth factor II in oncogene-induced tumorigenesis. Nature 369: 414-418.
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, et al. (2003) Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 100: 4162-4167.
- Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, et al. (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76-86.
- Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, et al. (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A 99: 11908-11913.
- Kobayashi M, Yamamoto M (2005) Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal 7: 385-394.
- Yang M, Yao Y, Eades G, Zhang Y, Zhou Q (2011) MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res Treat 129: 983-991.
- Higgins LG, Hayes JD (2011) Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev 43: 92-137.
- Miao W, Hu L, Scrivens PJ, Batist G (2005) Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem 280: 20340-20348.
- Kwak MK, Itoh K, Yamamoto M, Kensler TW (2002) Enhanced expression of the transcription factor Nrf2 by cancer chemopreventive agents: role of antioxidant response element-like sequences in the nrf2 promoter. Mol Cell Biol 22: 2883-2892.
- Sankaranarayanan K, Jaiswal AK (2004) Nrf3 negatively regulates antioxidant-response element-mediated expression and antioxidant induction of NAD(P)H:quinone oxidoreductase1 gene. J Biol Chem 279: 50810-50817.
- Wu RP, Hayashi T, Cottam HB, Jin G, Yao S, et al. (2010) Nrf2 responses and the therapeutic selectivity of electrophilic compounds in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A 107: 7479-7484.
- Denison MS, Nagy SR (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 43: 309-334.
- Sanz N, DÃez-Fernández C, Andrés D, Cascales M (2002) Hepatotoxicity and aging: endogenous antioxidant systems in hepatocytes from 2-, 6-, 12-, 18- and 30-month-old rats following a necrogenic dose of thioacetamide. Biochim Biophys Acta 1587: 12-20.
- Ungvari Z, Bailey-Downs L, Sosnowska D, Gautam T, Koncz P, et al. (2011) Vascular oxidative stress in aging: a homeostatic failure due to dysregulation of NRF2-mediated antioxidant response. Am J Physiol Heart Circ Physiol 301: H363-372.
- Shih PH, Yen GC (2007) Differential expressions of antioxidant status in aging rats: the role of transcriptional factor Nrf2 and MAPK signaling pathway. Biogerontology 8: 71-80.
- Yu S, Khor TO, Cheung KL, Li W, Wu TY, et al. (2010) Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice. PLoS One 5: e8579.
- Khor TO, Huang Y, Wu TY, Shu L, Lee J, et al. (2011) Pharmacodynamics of curcumin as DNA hypomethylation agent in restoring the expression of Nrf2 via promoter CpGs demethylation. Biochem Pharmacol 82: 1073-1078.
- Frohlich DA, McCabe MT, Arnold RS, Day ML (2008) The role of Nrf2 in increased reactive oxygen species and DNA damage in prostate tumorigenesis. Oncogene 27: 4353-4362.
- Barve A, Khor TO, Hao X, Keum YS, Yang CS, et al. (2008) Murine prostate cancer inhibition by dietary phytochemicals--curcumin and phenyethylisothiocyanate. Pharm Res 25: 2181-2189.
- Zaman MB, Leonard MO, Ryan EJ, Nolan NP, Hoti E, et al. (2007) Lower expression of Nrf2 mRNA in older donor livers: a possible contributor to increased ischemia-reperfusion injury? Transplantation 84: 1272-1278.
- Purdom-Dickinson SE, Sheveleva EV, Sun H, Chen QM (2007) Translational control of nrf2 protein in activation of antioxidant response by oxidants. Mol Pharmacol 72: 1074-1081.
- Li W, Thakor N, Xu EY, Huang Y, Chen C, et al. (2010) An internal ribosomal entry site mediates redox-sensitive translation of Nrf2. Nucleic Acids Res 38: 778-788.
- Beevers CS, Li F, Liu L, Huang S (2006) Curcumin inhibits the mammalian target of rapamycin-mediated signaling pathways in cancer cells. Int J Cancer 119: 757-764.
- Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, et al. (2009) Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res 69: 1000-1008.
- Thakor N, Holcik M (2012) IRES-mediated translation of cellular messenger RNA operates in eIF2α- independent manner during stress. Nucleic Acids Res 40: 541-552.
- McMahon M, Thomas N, Itoh K, Yamamoto M, Hayes JD (2004) Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J Biol Chem 279: 31556-31567.
- Kobayashi A, Kang MI, Okawa H, Ohtsuji M, Zenke Y, et al. (2004) Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol 24: 7130-7139.
- Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, et al. (2004) Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci U S A 101: 2040-2045.
- Taguchi K, Motohashi H, Yamamoto M (2011) Molecular mechanisms of the Keap1–Nrf2 pathway in stress response and cancer evolution. Genes Cells 16: 123-140.
- McMahon M, Itoh K, Yamamoto M, Hayes JD (2003) Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278: 21592-21600.
- Yamamoto T, Suzuki T, Kobayashi A, Wakabayashi J, Maher J, et al. (2008) Physiological significance of reactive cysteine residues of Keap1 in determining Nrf2 activity. Mol Cell Biol 28: 2758-2770.
- Huang HC, Nguyen T, Pickett CB (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem 277: 42769-42774.
- Sun Z, Huang Z, Zhang DD (2009) Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS One 4: e6588.
- Surh YJ (2003) Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer 3: 768-780.
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. (2003) Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299: 256-259.
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP (2006) DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A 103: 15091-15096.
- Malhotra D, Thimmulappa R, Navas-Acien A, Sandford A, Elliott M, et al. (2008) Decline in NRF2-regulated antioxidants in chronic obstructive pulmonary disease lungs due to loss of its positive regulator, DJ-1. Am J Respir Crit Care Med 178: 592-604.
- Sun Z, Chin YE, Zhang DD (2009) Acetylation of Nrf2 by p300/CBP augments promoter-specific DNA binding of Nrf2 during the antioxidant response. Mol Cell Biol 29: 2658-2672.
- Kawai Y, Garduño L, Theodore M, Yang J, Arinze IJ (2011) Acetylation-deacetylation of the transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2) regulates its transcriptional activity and nucleocytoplasmic localization. J Biol Chem 286: 7629-7640.
- Mercado N, Thimmulappa R, Thomas CM, Fenwick PS, Chana KK, et al. (2011) Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem Biophys Res Commun 406: 292-298.
- Motohashi H, Katsuoka F, Engel JD, Yamamoto M (2004) Small Maf proteins serve as transcriptional cofactors for keratinocyte differentiation in the Keap1-Nrf2 regulatory pathway. Proc Natl Acad Sci U S A 101: 6379-6384.
- Katsuoka F, Motohashi H, Engel JD, Yamamoto M (2005) Nrf2 transcriptionally activates the mafG gene through an antioxidant response element. J Biol Chem 280: 4483-4490.
- Venugopal R, Jaiswal AK (1998) Nrf2 and Nrf1 in association with Jun proteins regulate antioxidant response element-mediated expression and coordinated induction of genes encoding detoxifying enzymes. Oncogene 17: 3145-3156.
- Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, et al. (2003) Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35: 238-245.
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, et al. (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475: 106-109.
- Lister A, Nedjadi T, Kitteringham NR, Campbell F, Costello E, et al. (2011) Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol Cancer 10: 37.
- Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, (2010) Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth. Mol Cancer Ther 9: 336-346.
- Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, et al. (2012) Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med 4: 124ra27.
- Amador-Noguez D, Yagi K, Venable S, Darlington G (2004) Gene expression profile of long-lived Ames dwarf mice and Little mice. Aging Cell 3: 423-441.
- Leiser SF, Miller RA (2010) Nrf2 signaling, a mechanism for cellular stress resistance in long-lived mice. Mol Cell Biol 30: 871-884.
- Gardner EM (2005) Caloric restriction decreases survival of aged mice in response to primary influenza infection. J Gerontol A Biol Sci Med Sci 60: 688-694.
- Raffaghello L, Safdie F, Bianchi G, Dorff T, Fontana L, et al. (2010) Fasting and differential chemotherapy protection in patients. Cell Cycle 9: 4474-4476.
- Van Nieuwenhove Y, Dambrauskas Z, Campillo-Soto A, van Dielen F, Wiezer R, et al. (2011) Preoperative very low-calorie diet and operative outcome after laparoscopic gastric bypass: a randomized multicenter study. Arch Surg 146: 1300-1305.
- Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, et al. (2011) Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 93: 569-577.
- Safdie FM, Dorff T, Quinn D, Fontana L, Wei M, et al. (2009) Fasting and cancer treatment in humans: A case series report. Aging (Albany NY) 1: 988-1007.
- Trepanowski JF, Bloomer RJ (2010) The impact of religious fasting on human health. Nutr J 9: 57.
- Horne BD, May HT, Anderson JL, Kfoury AG, Bailey BM,et al (2008) Study IHC: Usefulness of routine periodic fasting to lower risk of coronary artery disease in patients undergoing coronary angiography. Am J Cardiol 102: 814-819.
- Kloner RA (2004) The "Merry Christmas Coronary" and "Happy New Year Heart Attack" phenomenon. Circulation 110: 3744-3745.
Citation: Hine CM, Mitchell JR (2012) NRF2 and the Phase II Response in Acute Stress Resistance Induced by Dietary Restriction. J Clin Exp Pathol S4:004. DOI: 10.4172/2161-0681.S4-004
Copyright: © 2012 Hine CM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15749
- [From(publication date): 0-2012 - Nov 30, 2024]
- Breakdown by view type
- HTML page views: 11024
- PDF downloads: 4725