Review Article Open Access
Newly Designed Magnetic and Non-Magnetic Nanoparticles for Potential Diagnostics and Therapy of Alzheimer's Disease
Hadas Skaat and Shlomo Margel*Department of Chemistry, Institute of Nanotechnology and Advanced Materials, Ramat-Gan 52900, Israel
- Corresponding Author:
- Shlomo Margel
Department of Chemistry
Institute of Nanotechnology and Advanced Materials
Ramat-Gan 52900, Israel
Tel: 972-3-5318861
Fax: 972-3-6355208
E-mail: shlomo.margel@mail.biu.ac.il
Received date: April 03, 2013; Accepted date: April 29, 2013; Published date: May 03, 2013
Citation: Skaat H, Margel S (2013) Newly Designed Magnetic and Non-Magnetic Nanoparticles for Potential Diagnostics and Therapy of Alzheimer’s Disease. J Biotechnol Biomater 3:156. doi:10.4172/2155-952X.1000156
Copyright: © 2013 Skaat H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The pathogenesis of many neurodegenerative diseases, including Alzheimer’s disease (AD) is characterized by protein aggregation into amyloid fibrils. In AD, the fibrils are of the amyloid-β (Aβ) peptide. The development of new approaches based on nanotechnology for early detection and potential treatment of AD is of high current interest. This review describes a pioneering approach involving the design, synthesis and utilization of new engineered magnetic and non-magnetic nanoparticles for inhibition and acceleration of conformational changes of the fibrilforming proteins, e.g. insulin and amyloid-β 40 (Aβ40) proteins. A novel method for detection of the location and removal of precursor protofibrils and fibrils by their selective marking by functional magnetic iron oxide nanoparticles was also demonstrated. These non-fluorescent and fluorescent iron oxide (maghemite, γ-Fe2O3) nanoparticles of narrow size distribution were synthesized by controlled nucleation and growth mechanism. Surface coatings of these nanoparticles with a functional fluorinated polymer and peptides, e.g. Leu-Pro-Phe-Phe-Asp (LPFFD) and Aβ40, through various activation methods, were performed. New uniform biocompatible α-amino acid-based polymer nanoparticles containing hydrophobic dipeptides in the polymer side chains were also synthesized. The effect of these nanoparticles on amyloid fibril formation kinetics was elucidated. These engineered nanoparticles are effective in the study and control of the process of amyloid fibril formation, and as selective biomarkers of amyloid plaques for multimodal imaging. This study may contribute to the mechanistic understanding of the protein aggregation processes, leading to development of new diagnostic and therapeutic strategies against amyloid-related diseases.
Keywords
Neurodegenerative diseases; Fluorescent γ-Fe2O3 nanoparticles; Poly(N-acryloyl amino acid) nanoparticles; Amyloid β peptide; Protein folding
Introduction
Amyloid aggregate formation is a pathological process that occurs in many diseases, including Parkinson’s, Huntington’s, prion diseases and Alzheimer’s disease (AD) [1-3]. The proteins undergo transition from the normally soluble form into amyloid fibrils organized mainly into cross β-sheets, which accumulate in the extracellular space of various tissues [4-6]. The extracellular plaques that form in AD are composed of fibrils of the Aβ peptide, a small peptide with 39-43 amino acids [7]. Aβ is continuously secreted by normal cells in culture, and is a component of plasma and cerebrospinal fluid of healthy individuals in the soluble form [8,9]. In AD, this Aβ self-assembles to form neurological toxic aggregates with various morphologies such as soluble oligomers and insoluble protofibrils and fibrils. According to the literature, the soluble oligomers are the most toxic species that cause the death of brain cells [10,11]. Currently, there is no cure for these diseases and treatment options are extremely limited [12]. A pharmacological approach for preventing amyloid aggregation is interfering with the pre-fibrillar species, and/or destabilizing the β-sheet conformations by agents that specifically stabilize the soluble form of the protein [13].
Recent literature indicates increasing interest in developing nanoparticles to detect, prevent and treat protein-misfolding diseases [14-16]. Their potential influence on protein fibrillation is a function of the nanoparticle surface physical and chemical properties. The large surface area to volume ratio of nanoparticles allows modification of surface properties, so as to control the adsorption and interaction processes. Increased local protein concentration on the nanoparticle surface, and/or changes in protein conformation upon binding could promote aggregation, while trapping of early intermediates may inhibit further aggregation [17]. Moreover, in vitro and in vivo studies have demonstrated that nanoparticles can be exploited for overcoming the difficulty of crossing the blood brain barrier (BBB), and have greater in vivo stability [18,19].
The neuronal damage of AD is irreversible, and eventually leads to dementia and ultimately death. Therefore, there is an urgent need for early diagnosis by in vivo imaging agents that specifically detect the location and density of amyloid plaques in the living human brain [20-22]. Compounds such as Thioflavin T (ThT) derivatives and Congo red (CR) have been evaluated as potential probes for fluorescence imaging of Aβ plaques [20-22]. Peptides such as radiolabeled Aβ derivatives and tritium-LPFFD have also been investigated as Aβ plaque-selective imaging agents. The core structures of these reagents contribute to their high binding affinity to Aβ aggregates [23,24].
In this review, we describe the development of a new, pioneering nanotechnology-based approach for potential diagnostics and therapy of neurodegenerative diseases, particularly AD. We describe the design, synthesis and use of various new engineered magnetic and non-magnetic nanoparticles for controlling (enhance or delay) the conformational changes of proteins that form amyloid fibrils, e.g. Aβ40 protein involved in AD, and for specific marking and removal of precursor protofibrils and amyloid fibrils via a magnetic field [25-29].
Selective Marking of Amyloid Fibrils by Dualmodal Maghemite Nanoparticles
Biocompatible, non-toxic and biodegradable magnetic iron oxide nanoparticles have been developed, and their use has been demonstrated in various biomedical applications, such as hyperthermia, diagnosis, cell-labeling and sorting, DNA separation, MRI and drug delivery [30-36]. The addition of fluorescent moieties may also provide new multimodal nanomaterials, with a broad range of both potential diagnostic and therapeutic applications [37].
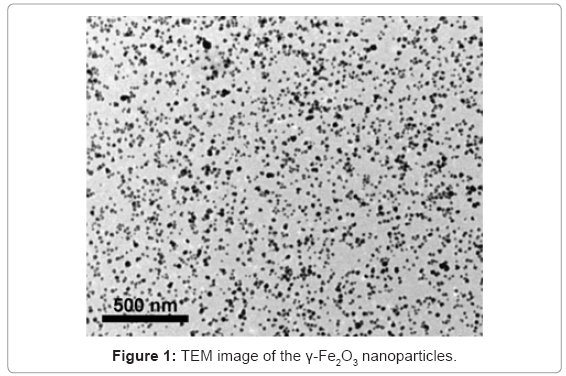
In our study, we developed a novel method for selective marking of amyloid fibrils, e.g. insulin and Aβ40, by both fluorescent and nonfluorecent γ-Fe2O3 nanoparticles [25,27]. We first chose insulin as a model amyloidogenic protein. Insulin amyloid fibril deposits had been observed in patients with type II diabetes, and after insulin infusion and repeated injection. Then, we extended our study to Aβ40 protein involved in AD. γ-Fe2O3 nanoparticles of narrow size distribution were synthesized by nucleation, followed by controlled growth of maghemite thin films onto gelatin-iron oxide nuclei [25,34]. The diameter of the γ-Fe2O3 nanoparticles was measured to be 15.0 ± 1.2 nm, and their stability against agglomeration was observed by transmission electron microscopy (TEM) (Figure 1). The γ-Fe2O3 nanoparticles containing the fluorescent probe rhodamine (R-γ-Fe2O3) or fluorescein (F-γ-Fe2O3) were prepared similar to the non-fluorescent γ-Fe2O3 nanoparticles, substituting the gelatin for gelatin covalently bound to rhodamine isothiocyanate (RITC), or fluorescein isothiocyanate (FITC), respectively [27,28]. While many fluorescent nanoparticles have their fluorescent moieties bound to the surface, our nanoparticles contain fluorescent dye, covalently encapsulated within the nanoparticles. There is, therefore, retention of the surface properties, including the zeta potential and surface bound ligand capacity. The encapsulation also increases the photostability of the dye [38].
Magnetic human amyloid fibril/γ-Fe2O3 nanoparticle assemblies were prepared by interaction of the γ-Fe2O3 nanoparticles with the insulin or Aβ40 amyloid fibrils, during or after their formation [34]. The nanoparticles selectively labeled the insulin and Aβ40 amyloid fibrils at both stages, even under competitive conditions, e.g. in the presence of 4% HSA.
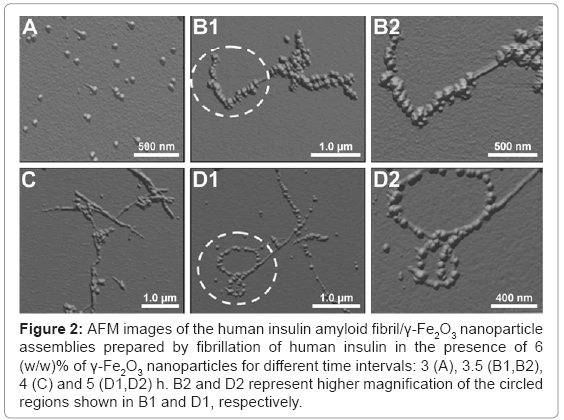
Different stages of growth of the human insulin fibril/γ-Fe2O3 nanoparticle assemblies during the insulin fibrillation process are depicted in the atomic force microscopy (AFM) images (Figure 2). The samples were prepared by dispersing fresh monomeric insulin in an aqueous continuous phase, in the presence of 6 (w/w)% of the γ-Fe2O3 nanoparticles. 3 h after the initiation of the fibrillation process (Figure 2A) the appearance of small spherical-like aggregate structures is revealed, indicating the onset of the fibril growth. Figures 2B-2D illustrates the gradual growth of the insulin fibrils. The initial fibrils are small and linear, while the more developed fibrils are longer and partially twisted. The selective binding of the γ-Fe2O3 nanoparticles onto the insulin fibrils, with almost no unassociated nanoparticles in the background, is also shown in Figure 2.
Figure 2: AFM images of the human insulin amyloid fibril/γ-Fe2O3 nanoparticle assemblies prepared by fibrillation of human insulin in the presence of 6 (w/w)% of γ-Fe2O3 nanoparticles for different time intervals: 3 (A), 3.5 (B1,B2), 4 (C) and 5 (D1,D2) h. B2 and D2 represent higher magnification of the circled regions shown in B1 and D1, respectively.
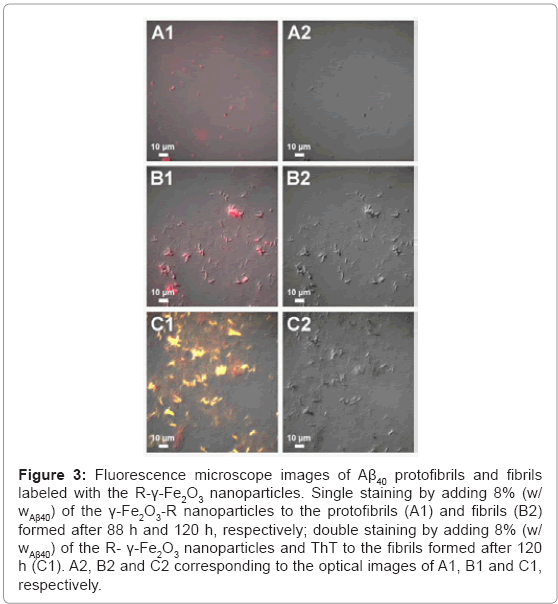
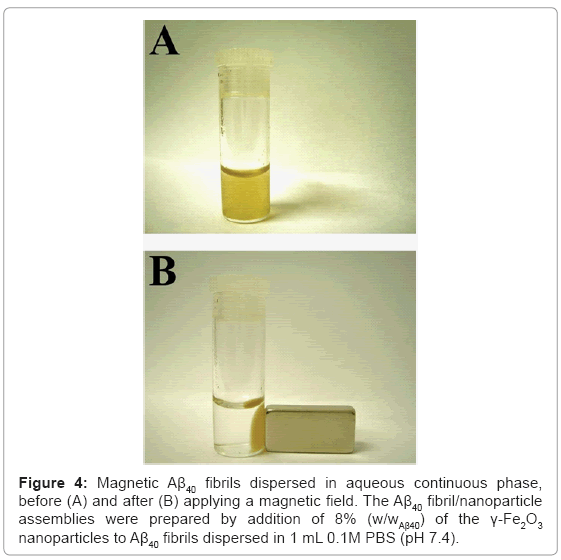
Selective fluorescent marking of Aβ40 protofibrils and fibrils with 8% (w/wAβ40) of the R-γ-Fe2O3 nanoparticles was also illustrated (Figure 3). By adding the nanoparticles before or after the completion of the fibrillation process, the resulting Aβ40 protofibrils (Figures 3A1 and 3A2) and fibrils (Figures 3B1 and 3B2) are single-stained with red fluorescence. As mentioned above, ThT is a fluorescent dye that binds specifically to amyloid fibrils. Adding ThT to the Aβ40 fibril/R-γ-Fe2O3 nanoparticle assemblies, followed by removal of excess ThT, resulted in double-stained Aβ40 fibrils, with red and yellow fluorescence (Figures 3C1 and 3C2). This result confirms the selective marking of the Aβ40 fibrils by the R-γ-Fe2O3 nanoparticles. The magnetic properties of the γ-Fe2O3 nanoparticles bound to the insulin or Aβ40 amyloid fibrils are also advantageous. The obtained magnetic amyloid fibril assemblies can easily be completely removed from the aqueous phase using a simple magnet (Figure 4).
Figure 3: Fluorescence microscope images of Aβ40 protofibrils and fibrils labeled with the R-γ-Fe2O3 nanoparticles. Single staining by adding 8% (w/wAβ40) of the γ-Fe2O3-R nanoparticles to the protofibrils (A1) and fibrils (B2) formed after 88 h and 120 h, respectively; double staining by adding 8% (w/wAβ40) of the R- γ-Fe2O3 nanoparticles and ThT to the fibrils formed after 120 h (C1). A2, B2 and C2 corresponding to the optical images of A1, B1 and C1, respectively.
Effect of Fluorinated Magnetic Core-Shell Nanoparticles on the Kinetics of Insulin Amyloid Fibril Formation
Fluorinated alcohols such as trifluoroethanol and hexafluoroisopropanol have been reported to induce α-helical conformation in fibril-forming peptides [39,40]. Their strong hydrophobic character probably causes alterations of the hydration shell of the amyloidic sequence. Based on these results, Rocha et al. [40] demonstrated the utilization of fluorinated nanoparticles, composed of a complex of polyampholytes and dodecanoic and perfluorododecanoic acid, in the induction of an α-helix-rich structure in the B18 peptide (a short peptide sequence with a strong tendency to self assemble and form amyloid fibrils). Their alkylated analogues, in absence of fluorinecontaining groups, however, do not induce α-helix formation, and may even enhance the rate of fibril growth. Fluorinated nanoparticles are therefore, potential candidates for the inhibition and reverse of conformational changes of amyloid proteins [40,41].
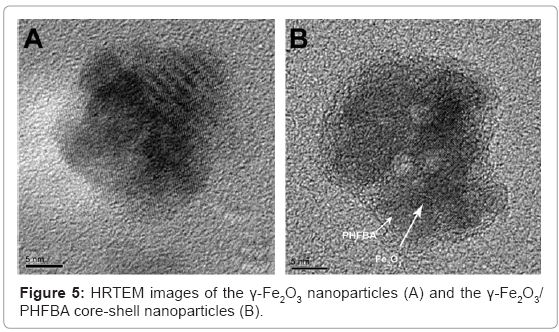
In our study, newly designed fluorinated γ-Fe2O3 core-shell magnetic nanoparticles were shown to induce a significant direct slow transition from α-helix to β-sheets that occurs during insulin fibril formation [26]. These uniform magnetic γ-Fe2O3/Poly(2,2,3,3,4,4,4-heptafluorobutyl acrylate) (γ-Fe2O3/PHFBA) core-shell nanoparticles were prepared by emulsion polymerization of the fluorinated monomer 2,2,3,3,4,4,4-heptafluorobutyl acrylate (HFBA), in the presence of the γ-Fe2O3 nanoparticles. The high-resolution TEM (HRTEM) images (Figure 5) show that the core comprised crystalline iron oxide, with perfect arrangement of the atomic layers, while the 3-4 nm PHFBA shell was amorphous.
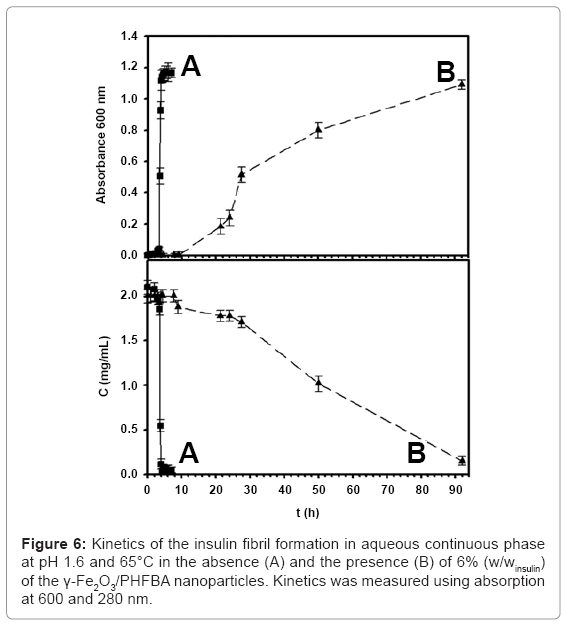
The effect of γ-Fe2O3/PHFBA nanoparticles on the kinetics of the insulin fibril formation was investigated. Without the presence of nanoparticles (Figure 6A), the main growth of the insulin fibrils occurred approximately 3 h after initiation of the fibrillation process, and completed after approximately 5 h. Similar behavior was also observed in the presence of 6% (w/winsulin) of the uncoated γ-Fe2O3 nanoparticles [25]. However, in the presence of 6% (w/winsulin) γ-Fe2O3/ PHFBA nanoparticles (Figure 6B), insulin fibril growth was significantly delayed, and initiation occured only after 22 h. The presence of the perfluorinated carbon groups (-CF2 and -CF3) on the surface of the γ-Fe2O3 nanoparticles probably stabilizes the α-helix structure by hydrophobic interactions, delaying the α-helix to β-sheet transition during the insulin fibril formation [39,40]. The loss of the ordered water coating around the peptide, due to the perfluorinated carbon groups, causes the –NH and –C=O groups of the backbone to interact on short distances with each other, favoring the α-helix conformation. Short-range interactions between nearby amino-acid residues stabilize the α-helix structure, in contrast to long-range interactions stabilizing the β-sheet structure [39]. An opposite correlation between the insulin fibril formation and the insulin monomeric concentration is obtained, as observed by the increase and decrease of the measured absorbances of fibrils (600 nm) and monomeric insulin (280 nm), respectively. In the absence of nanoparticles, complete fibril formation is observed after 5 h, while the concentration of the insulin is almost zero. This indicates that after 5 h, all the monomeric insulin had been converted to insulin fibrils. This correlation was also observed in the presence of the γ-Fe2O3/PHFBA nanoparticles, but only after 92 h.
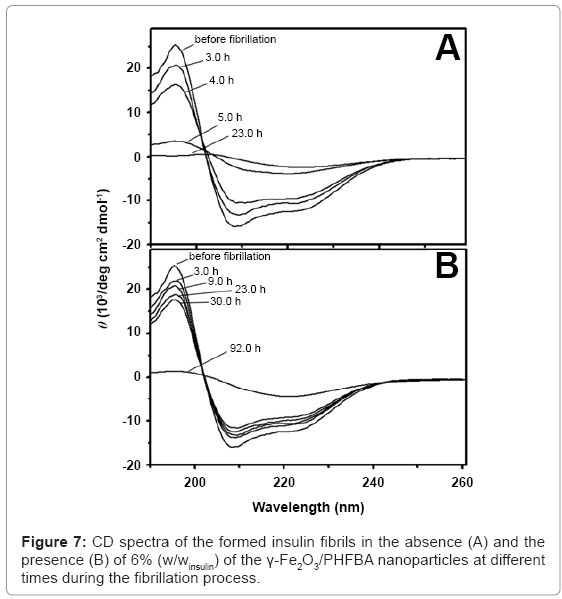
Circular dichroism (CD) analyses were performed to determine the secondary structure changes during the insulin fibril formation, in the absence and the presence of the core-shell nanoparticles (Figure 7). Without the presence of nanoparticles (Figure 7A), before the initiation of the fibrillation process, the CD spectrum of the insulin aqueous solution shows a band at 195 nm and a double minimum at 208 and 222 nm, indicating native protein dominated by α-helix conformation. By heating the insulin solution for 5 h, the CD spectrum of the insulin shows the disappearance of the 195 and 222 nm bands, as well as appearance of a minimum ellipticity at 216 nm, indicating the presence of extensive β-sheet structures. Similar CD spectra were also observed in the presence of 6% (w/winsulin) of the non-coated γ-Fe2O3 nanoparticles. In the presence of 6% (w/winsulin) of the γ-Fe2O3/PHFBA nanoparticles (Figure 7B), the α-helical structure remains almost constant for up to 22 h of heating, beyond which transformation to β-sheet structure begins.
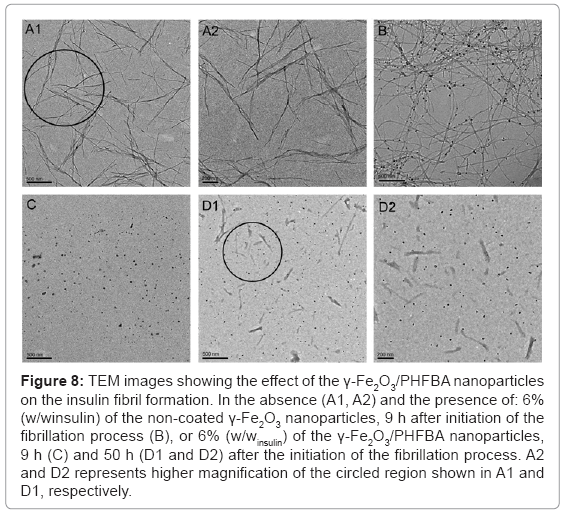
The inhibition of the insulin fibril formation, in the presence of the γ-Fe2O3/PHFBA nanoparticles, was also confirmed by TEM images (Figure 8). After 9 h of the fibrillation process, in the absence (Figures 8A1 and 8A2) or presence of 6% (w/winsulin) of the non-coated γ-Fe2O3 nanoparticles (Figure 8B), the length of the fibrils was up to several micrometers. The non-coated γ-Fe2O3 nanoparticles also appear to selectively bind with the axial external surface of the fibrils, with almost no unassociated nanoparticles (Figure 8B). However, at the same time point, different behavior was observed in the presence of 6% (w/winsulin) of the γ-Fe2O3/PHFBA core-shell nanoparticles, revealing only free nanoparticles, indicating that the fibrils were not yet formed (Figure 8C). Figures 8D1 and 8D2 show that 50 h after the initiation of the fibrillation process, the obtained fibrils were still much shorter than those observed in the presence of the non-coated γ-Fe2O3, and were not marked at all by the core-shell nanoparticles.
Figure 8: TEM images showing the effect of the γ-Fe2O3/PHFBA nanoparticles on the insulin fibril formation. In the absence (A1, A2) and the presence of: 6% (w/winsulin) of the non-coated γ-Fe2O3 nanoparticles, 9 h after initiation of the fibrillation process (B), or 6% (w/winsulin) of the γ-Fe2O3/PHFBA nanoparticles, 9 h (C) and 50 h (D1 and D2) after the initiation of the fibrillation process. A2 and D2 represents higher magnification of the circled region shown in A1 and D1, respectively.
Effect of Peptide-Conjugated Fluorescent-Maghemite Nanoparticles on the Kinetics of Aβ40 Fibrillation Process
Several reserch groups have been involved in the identification of the specific peptide sequence that is critical in amyloid aggregate formation [42-46]. The FF residues, the hydrophobic core of residues 19-20 of the Aβ protein, are well known as crucial sequences for the β-sheet formation that trigger the fibrillation process [42-46]. It is possible that the Aβ fibrillation process is partially driven by hydrophobic interactions, via recognition between the FF pairs [42-46]. Short peptides homologous to the hydrophobic core of Aβ, for example LPFFD, were designed to bind specifically to the entire Aβ, and used as inhibitors of its fibrillation process via the FF recognition motif [47-49]. Although peptide or protein analogues with specific FF binding sites may be of primary importance for studying neurodegenerative disorders, no therapeutic agents using this strategy have been developed. This is because of difficulties with penetrating the BBB, the complexity of their synthesis, and that their stability and efficacy has been found to be low in vivo [50].
Various nanoparticles have been reported to promote the protein nucleation process leading amyloid fibril formation in vitro, for example, copolymer particles of N-isopropylacrylamide/N-tertbutylacrylamide, cerium oxide, quantum dots (QDs), carbon nanotubes and titanium oxide [51,52]. Only few studies have reported the inhibitory effect of nanoparticles on the Aβ fibrillation process. Very recently, Cabaleiro-Lago et al. [53] reported the inhibition of the Aβ40 fibril formation by copolymer nanoparticles of variable hydrophobicity, and also demonstrated the dual effect of commercial polystyrene (PS) nanoparticles, with amino modification toward the Aβ40 and Aβ42 fibril formation [54]. Yoo et al. [55] have shown an inhibition effect of CdTe QDs on Aβ40 fibrillation. Fluorinated nanoparticles [41] and sulfonated and sulfated PS nanoparticles [56], have also been reported as potential candidates for the inhibition of Aβ fibril formation.
Our study was extended by surface modification of the fluorescent- γ-Fe2O3 nanoparticles with different peptides (Aβ40 and LPFFD), which led to a significant increase in their binding affinity towards the Aβ40 fibrils, and affected the kinetics of the Aβ40 fibrillation process [28]. For this purpose, F-γ-Fe2O3 nanoparticles were coated with human serum albumin (HSA), via a precipitation process. A succinimidyl polyethylene glycol succinimidyl ester (NHS-PEG-NHS) spacer arm was covalently conjugated to the F-γ-Fe2O3~HSA nanoparticles. This was used for the covalent conjugation of Aβ40 or LPFFD to their surface [28]. The effect of these conjugated proteins on the kinetics of the Aβ40 fibrillation process was elucidated. The Aβ40-conjugated nanoparticles (F-γ-Fe2O3~HSA-PEG-Aβ40) were found to accelerate the Aβ40 fibrillation process, while the LPFFD-conjugated nanoparticles (F γ-Fe2O3~HSA-PEG-LPFFD) caused inhibition.
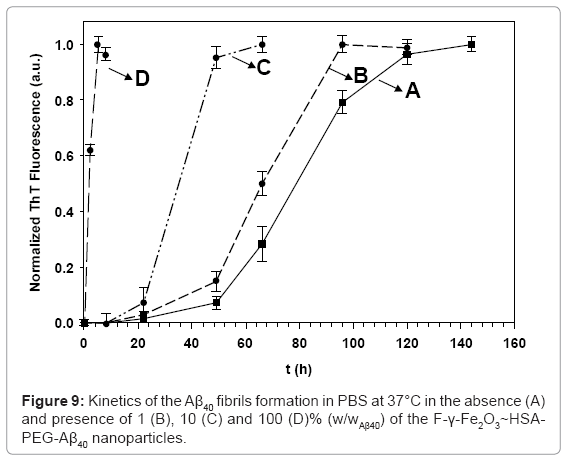
Fibril formation kinetics of the Aβ40 was monitored by the temporal development of ThT binding [57], at different concentrations of the F-γ- Fe2O3~HSA-PEG-Aβ40 nanoparticles (Figure 9). In the absence of the nanoparticles (Figure 9A), the main growth of the Aβ40 fibrils occurred approximately 60 h after initiation of the fibrillation process, and completed after approximately 120 h. This sigmoidal behavior was also observed in the presence of different concentrations of the control F-γ- Fe2O3~HSA nanoparticles [28]. However, the presence of increasing concentrations of the F-γ-Fe2O3~HSA-PEG-Aβ40 nanoparticles gradually accelerates the kinetics of the Aβ40 fibril growth. Instead of initiating the fibrillation process in the absence of the F-γ-Fe2O3~HSAPEG- Aβ40 nanoparticles after 60 h, the addition of increasing concentrations of the F-γ-Fe2O3~HSA-PEG-Aβ40 nanoparticles of 1 (B), 10 (C), and 100 (D)% (w/wAβ40), accelerates the initiation of the fibril formation to be after 51, 26, and 1.5 h, respectively. This indicates that the Aβ40 conjugated nanoparticles substantially decrease the fibrillation lag time, and thereby, enhance the kinetics the Aβ40 fibril formation. The promoting effect probably results from the presence of the surface bound Aβ40, which is known for its high affinity towards Aβ40 prefibril monomers and oligomers [58-60]. This high binding affinity leads to the enhanced adsorption of the Aβ40 prefibril aggregates to the surface of the nanoparticles, resulting in their rapid association to form fibrils [61].
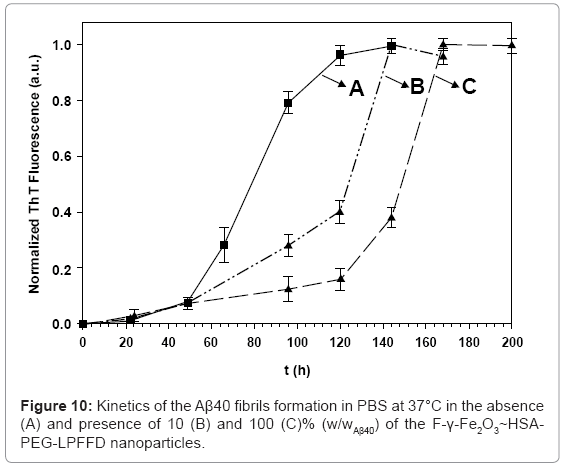
Fibril formation kinetics of Aβ40 in the absence (A) and the presence (B,C) of the F-γ-Fe2O3~HSA-PEG-LPFFD nanoparticles was also investigated (Figure 10). The observed behavior was opposite to the behavior obtained in the presence of similar concentrations of the F-γ-Fe2O3~HSA-PEG-Aβ40 nanoparticles. The Aβ40 fibril growth kinetics were delayed in the presence of 10 and 100% (w/wAβ40) of the γ-Fe2O3-F~HSA-PEG-LPFFD nanoparticles, and initiated after only 78 and 124 h, respectively. Despite this contrasting behavior, the same selective marking of the fibrils with the Aβ40-conjugated nanoparticles or LPFFD-conjugated nanoparticles was observed [28].
Effect of Amino Acid-Based Polymer Nanoparticles on the Kinetics of Aβ40 Fibrillation Process
Poly (amino acid) nanoparticles have recently attracted great attention due to their potential non-toxicity, biocompatibility, nonimmunogenicity and biofunctionality. These nanoparticles containing amino acid moieties are potentially useful at various biomedical applications, such as drug or gene delivery agents, tissue engineering scaffolds and chiral recognition [62-65]. The synthesis and radical polymerization of different acryl monomers that their side chain composed of amino acid moieties have been reported [66]. Their corresponding polymers, poly (N-acryloyl amino acids), are expected to be functional materials, and studies were mainly focused on their unique polymerization behavior, structures and properties [67-69]. Yet, there was no report describing the preparation of polymeric nanoparticles composed of N-acryloyl amino acid monomers.
In our study, we designed for the first time new uniform biocompatible amino acid-based polymer nanoparticles containing hydrophobic dipeptides in the polymer side chains [29]. The dipeptide residues were designed similarly to the hydrophobic core sequence of Aβ. The rational of our design is to engineer polymer nanoparticles containing extremely high concentrations of the FF motif. This motif in the nanoparticles, therefore, should act as a recognition motif within Aβ and interferes in its fibril formation.
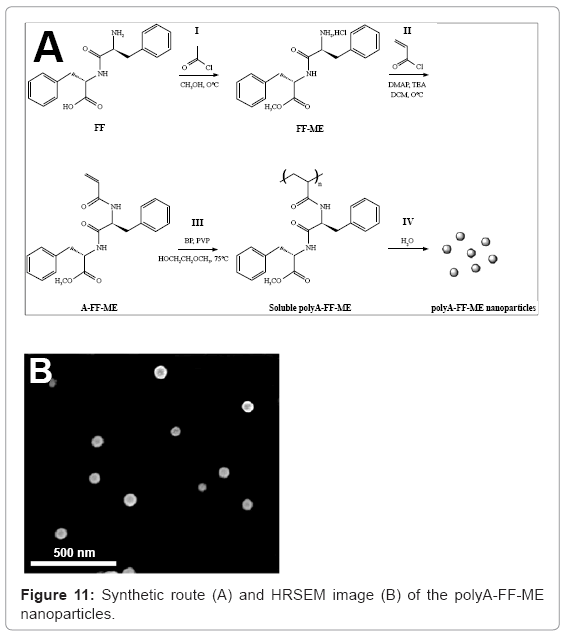
Poly (N-acryloyl-L-phenylalanyl-L-phenylalanine methyl ester) (polyA-FF-ME) nanoparticles of 57 ± 6 nm were synthesized by dispersion polymerization of the monomer A-FF-ME in 2-methoxy ethanol, followed by precipitation of the obtained polymer in aqueous solution [29], as illustrated in figure 11. Cell viability assay confirmed that no significant cytotoxic effect of the polyA-FF-ME nanoparticles on different human cell lines, e.g. PC-12 and SH-SY5Y, was observed. In the presence of these nanoparticles, a significant slow secondary structure transition from random coil to β-sheets during Aβ40 fibril formation was observed, resulting in significant inhibition of Aβ40 fibrillation kinetics. However, the polyA-FF-ME analogous nanoparticles containing the L-alanyl-L-alanine (AA) dipeptide in the polymer side groups, polyAAA- ME nanoparticles, accelerate the Aβ40 fibrillation kinetics.
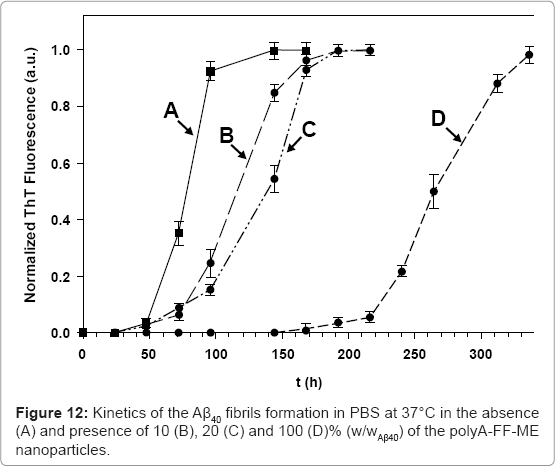
Kinetics of the Aβ40 fibril formation in the absence and presence of increasing concentrations of the polyA-FF-ME nanoparticles are shown in figure 12. In the absence of the nanoparticles (Figure 12A), the main growth of the Aβ40 fibrils occurred approximately 60 h after initiation of the fibrillation process, and was completed after approximately 120 h. However, the kinetics of the Aβ40 fibril growth, in the presence of increasing concentrations of the polyA-FF-ME nanoparticles, was significantly delayed (Figures 12B-12D). The presence of 10 (B), 20 (C), and 100 (D)% (w/wAβ40) of the polyA-FFME nanoparticles inhibits the initiation of the fibrils formation to be only after 85, 99, and 233 h, respectively. This indicates that the polyA-FF-ME nanoparticles substantially increase the fibrillation lag time, and thereby, inhibit the kinetics of the Aβ40 fibril formation. This inhibitory effect might be explained by the presence of the pairs of FF residues of these nanoparticles, which are known for their high affinity to the corresponding residues of Aβ40 prefibril aggregates through hydrophobic interactions. This high binding affinity disturbs the monomer-critical nuclei equilibrium by trapping the monomers, and/or blocking the growing oligomers ends on the surface of the nanoparticles, thereby decreasing their solution concentration and interfering with their elongation to form fibrils. However, the ability to distinguish whether these nanoparticles adsorbed the monomers, or oligomers, or both, remains to be investigated.
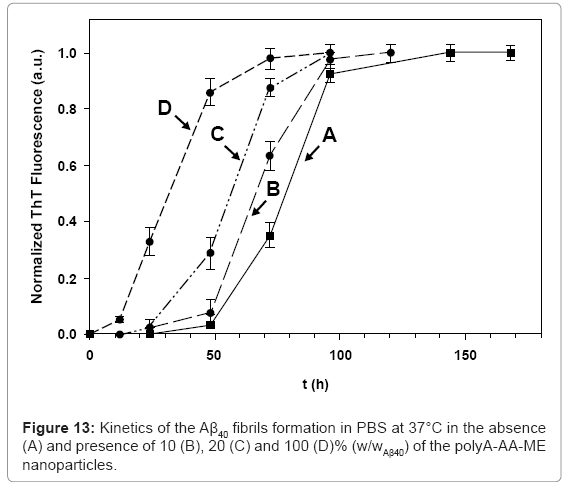
Our assumption that the observed inhibitory effect, in the presence of the polyA-FF-ME nanoparticles, is derived from specific hydrophobic interactions between the nanoparticles and the Aβ prefibril aggregates, via the FF recognition motif, was examined by replacing the polyAFF- ME nanoparticles by polyA-AA-ME nanoparticles. An opposite behavior was observed compared the presence of similar concentrations of the polyA-FF-ME nanoparticles. The kinetics of the Aβ40 fibril growth in the presence of 10, 20, and 100 % (w/wAβ40) were accelerated, and initiated after 53, 38, and 18 h, respectively (Figure 13). This promoting effect is probably due to the absence of the FF interfering moieties in these nanoparticles. Therefore, the increased local concentration of the Aβ40 prefibrils adsorbed on the polyA-AA-ME nanoparticle surface promoted their growth into fibrils. The promoting effect achieved in the presence of the polyA-AA-ME nanoparticles may also be considered as a desired process. This is because that the polyA-AA-ME nanoparticles decrease the fibrillation lag time, thereby shorting the half life time of the Aβ40 prefibrils, thereby decreasing their toxicity in solution.
Summary and Conclusions
This review describes the design and synthesis of new uniform functional magnetic iron oxide nanoparticles and amino acid-based polymer nanoparticles. These engineered nanoparticles affect the kinetics of the amyloid fibrillation process differentially. The fluorinated maghemite core-shell nanoparticles, γ-Fe2O3/PHFBA, inhibit the insulin amyloid fibrillation process. The presence of the perfluorinated carbon groups on the nanoparticles’ surface probably stabilizes the α-helix structure via hydrophobic interactions, delaying the α-helix to β-sheet transition during the insulin fibril formation. The LPFFD-conjugated maghemite nanoparticles and the polyA-FF-ME nanoparticles inhibit the Aβ40 fibrillation process. This inhibition probably results from the intermolecular hydrophobic interactions between the pairs of FF residues of the nanoparticles, with the corresponding residues of the Aβ40 prefibrillar aggregates, which disrupt the self-assembly of Aβ40 into fibrils. This observation was confirmed by the promoting effect achieved in the presence of the Aβ40-conjugated maghemite nanoparticles and the polyA-AA-ME nanoparticles. Further studies should be performed to investigate whether these engineered nanoparticles are effective as therapeutics in vivo. Still, our studies intend to provide a new mechanistic insight into amyloidogenic peptide interaction with nanoparticles, leading to the development of a new therapeutic strategy against amyloid-related diseases.
The maghemite magnetic nanoparticles may also be encapsulated with a fluorescent dye. These engineered fluorescent magnetic dualmodal nanoparticles have a great advantage due to the combination of the magnetic and fluorescence imaging into one nanostructured material. These nanoparticles, which also selectively marks Aβ40 and insulin amyloid fibrils, might enable the early detection of plaques using both MRI and fluorescence microscopy, and therefore, may be applied in in vivo AD diagnosis studies.
Acknowledgements
These studies were partially supported by a BSF (Israel-USA Binational Science Foundation) grant, and by a Minerva Grant (Microscale and Nanoscale Particles and Films).
References
- Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75: 333-366.
- Sipe JD, Benson MD, Buxbaum JN, Ikeda S, Merlini G, et al. (2010) Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid 17: 101-104.
- Selkoe DJ (2003) Folding proteins in fatal ways. Nature 426: 900-904.
- Sunde M, Blake CC (1998) From the globular to the fibrous state: protein structure and structural conversion in amyloid formation. Q Rev Biophys 31: 1-39.
- Ohnishi S, Takano K (2004) Amyloid fibrils from the viewpoint of protein folding. Cell Mol Life Sci 61: 511-524.
- Kagan BL, Thundimadathil J (2010) Amyloid peptide pores and the beta sheet conformation. Adv Exp Med Biol 677: 150-167.
- Price DL, Sisodia SS, Gandy SE (1995) Amyloid beta amyloidosis in Alzheimer's disease. Curr Opin Neurol 8: 268-274.
- Haass C, Schlossmacher MG, Hung AY, Vigo-Pelfrey C, Mellon A, et al. (1992) Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature 359: 322-325.
- Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, et al. (1992) Isolation and quantification of soluble Alzheimer's beta-peptide from biological fluids. Nature 359: 325-327.
- Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 8: 101-112.
- Kumar S, Udgaonkar JB (2010) Mechanisms of amyloid fibril formation by proteins. Curr Sci 98: 639-656.
- Karran E, Mercken M, De Strooper B (2011) The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov 10: 698-712.
- Härd T, Lendel C (2012) Inhibition of amyloid formation. J Mol Biol 421: 441-465.
- Spuch C, Saida O, Navarro C (2012) Advances in the treatment of neurodegenerative disorders employing nanoparticles. Recent Pat Drug Deliv Formul 6: 2-18.
- Sahni JK, Doggui S, Ali J, Baboota S, Dao L, et al. (2011) Neurotherapeutic applications of nanoparticles in Alzheimer's disease. J Control Release 152: 208-231.
- Brambilla D, Le Droumaguet B, Nicolas J, Hashemi SH, Wu LP, et al. (2011) Nanotechnologies for Alzheimer's disease: diagnosis, therapy, and safety issues. Nanomedicine 7: 521-540.
- Cabaleiro-Lago C, Szczepankiewicz O, Linse S (2012) The effect of nanoparticles on amyloid aggregation depends on the protein stability and intrinsic aggregation rate. Langmuir 28: 1852-1857.
- Silva GA (2006) Neuroscience nanotechnology: progress, opportunities and challenges. Nat Rev Neurosci 7: 65-74.
- Kabanov AV, Gendelman HE (2007) Nanomedicine in the diagnosis and therapy of neurodegenerative disorders. Prog Polym Sci 32: 1054-1082.
- Mathis CA, Mason NS, Lopresti BJ, Klunk WE (2012) Development of positron emission tomography ß-amyloid plaque imaging agents. Semin Nucl Med 42: 423-432.
- Ono M, Saji H (2011) SPECT Imaging Agents for Detecting Cerebral ß-Amyloid Plaques. Int J Mol Imaging 2011: 543267.
- Sair HI, Doraiswamy PM, Petrella JR (2004) In vivo amyloid imaging in Alzheimer's disease. Neuroradiology 46: 93-104.
- Sumbria RK, Boado RJ, Pardridge WM (2012) Imaging amyloid plaque in alzheimer's disease brain with a biotinylated Aß peptide radiopharmaceutical conjugated to an IgG-avidin fusion protein. Bioconjug Chem.
- Adessi C, Frossard MJ, Boissard C, Fraga S, Bieler S, et al. (2003) Pharmacological profiles of peptide drug candidates for the treatment of Alzheimer's disease. J Biol Chem 278: 13905-13911.
- Skaat H, Sorci M, Belfort G, Margel S (2009) Effect of maghemite nanoparticles on insulin amyloid fibril formation: selective labeling, kinetics, and fibril removal by a magnetic field. J Biomed Mater Res A 91: 342-351.
- Skaat H, Belfort G, Margel S (2009) Synthesis and characterization of fluorinated magnetic core-shell nanoparticles for inhibition of insulin amyloid fibril formation. Nanotechnology 20: 225106.
- Skaat H, Margel S (2009) Synthesis of fluorescent-maghemite nanoparticles as multimodal imaging agents for amyloid-beta fibrils detection and removal by a magnetic field. Biochem Biophys Res Commun 386: 645-649.
- Skaat H, Shafir G, Margel S (2011) Acceleration and inhibition of amyloid-ß fibril formation by peptide conjugated fluorescent-maghemite nanoparticles. J Nanopart Res 13: 3521-3534.
- Skaat H, Chen R, Grinberg I, Margel S (2012) Engineered polymer nanoparticles containing hydrophobic dipeptide for inhibition of amyloid-ß fibrillation. Biomacromolecules 13: 2662-2670.
- de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, et al. (2005) Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 23: 1407-1413.
- Hergt R, Hiergeist R, Hilger I, Kaiser WA, Lapatnikov Y, et al. (2004) Maghemite nanoparticles with very high AC-losses for application in RF-magnetic hyperthermia. J Magn Magn Mater 270: 345-357.
- Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, et al. (2002) Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther 9: 102-109.
- Rudge SR, Kurtz TL, Vessely CR, Catterall LG, Williamson DL (2000) Preparation, characterization, and performance of magnetic iron-carbon composite microparticles for chemotherapy. Biomaterials 21: 1411-1420.
- Margel S, Gura S (2006) Nucleation and growth magnetic metal oxide nanoparticles and its use. Israel Patent No. WO9962079.
- Perlstein B, Ram Z, Daniels D, Ocherashvilli A, Roth Y, et al. (2008) Convection-enhanced delivery of maghemite nanoparticles: Increased efficacy and MRI monitoring. Neuro Oncol 10: 153-161.
- Shi X, Wang SH, Swanson SD, Ge S, Cao Z, et al. (2008) Dendrimer-functionalized shell-cross linked iron oxide nanoparticles for in vivo magnetic resonance imaging of tumors. Adv Mater 20: 1671-1678.
- Quarta A, Di Corato R, Manna L, Ragusa A, Pellegrino T (2007) Fluorescent-magnetic hybrid nanostructures: preparation, properties, and applications in biology. IEEE Trans Nanobioscience 6: 298-308.
- Perlstein B, Lublin-Tennenbaum T, Marom I, Margel S (2010) Synthesis and characterization of functionalized magnetic maghemite nanoparticles with fluorescent probe capabilities for biological applications. J Biomed Mater Res B Appl Biomater 92: 353-360.
- Vieira EP, Hermel H, Möhwald H (2003) Change and stabilization of the amyloid-beta(1-40) secondary structure by fluorocompounds. Biochim Biophys Acta 1645: 6-14.
- Rocha S, Thünemann AF, Pereira MC, Coelho MA, Möhwald H, et al. (2005) The conformation of B18 peptide in the presence of fluorinated and alkylated nanoparticles. Chembiochem 6: 280-283.
- Rocha S, Thünemann AF, Pereira Mdo C, Coelho M, Möhwald H, et al. (2008) Influence of fluorinated and hydrogenated nanoparticles on the structure and fibrillogenesis of amyloid beta-peptide. Biophys Chem 137: 35-42.
- Hilbich C, Kisters-Woike B, Reed J, Masters CL, Beyreuther K (1992) Substitutions of hydrophobic amino acids reduce the amyloidogenicity of Alzheimer's disease beta A4 peptides. J Mol Biol 228: 460-473.
- Gazit E (2005) Mechanisms of amyloid fibril self-assembly and inhibition. Model short peptides as a key research tool. FEBS J 272: 5971-5978.
- Soto C, Sigurdsson EM, Morelli L, Kumar RA, Castaño EM, et al. (1998) Beta-sheet breaker peptides inhibit fibrillogenesis in a rat brain model of amyloidosis: implications for Alzheimer's therapy. Nat Med 4: 822-826.
- Takahashi T, Mihara H (2008) Peptide and protein mimetics inhibiting amyloid beta-peptide aggregation. Acc Chem Res 41: 1309-1318.
- Marshall KE, Morris KL, Charlton D, O'Reilly N, Lewis L, et al. (2011) Hydrophobic, aromatic, and electrostatic interactions play a central role in amyloid fibril formation and stability. Biochemistry 50: 2061-2071.
- Viet MH, Ngo ST, Lam NS, Li MS (2011) Inhibition of aggregation of amyloid peptides by beta-sheet breaker peptides and their binding affinity. J Phys Chem B 115: 7433-7446.
- Rocha S, Cardoso I, Börner H, Pereira MC, Saraiva MJ, et al. (2009) Design and biological activity of beta-sheet breaker peptide conjugates. Biochem Biophys Res Commun 380: 397-401.
- Lowe TL, Strzelec A, Kiessling LL, Murphy RM (2001) Structure-function relationships for inhibitors of beta-amyloid toxicity containing the recognition sequence KLVFF. Biochemistry 40: 7882-7889.
- Estrada LD, Soto C (2007) Disrupting beta-amyloid aggregation for Alzheimer disease treatment. Curr Top Med Chem 7: 115-126.
- Linse S, Cabaleiro-Lago C, Xue WF, Lynch I, Lindman S, et al. (2007) Nucleation of protein fibrillation by nanoparticles. Proc Natl Acad Sci U S A 104: 8691-8696.
- Wu WH, Sun X, Yu YP, Hu J, Zhao L, et al. (2008) TiO2 nanoparticles promote beta-amyloid fibrillation in vitro. Biochem Biophys Res Commun 373: 315-318.
- Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, Lindman S, Minogue AM, et al. (2008) Inhibition of amyloid beta protein fibrillation by polymeric nanoparticles. J Am Chem Soc 130: 15437-15443.
- Cabaleiro-Lago C, Quinlan-Pluck F, Lynch I, Dawson KA, Linse S (2010) Dual effect of amino modified polystyrene nanoparticles on amyloid ß protein fibrillation. ACS Chem Neurosci 1: 279-287.
- Yoo SI, Yang M, Brender JR, Subramanian V, Sun K, et al. (2011) Mechanism of fibrillation inhibition of amyloid peptides by inorganic nanoparticles reveal functional similarities with proteins. Angew Chem Int Ed 50: 5110-5115.
- Saraiva AM, Cardoso I, Saraiva MJ, Tauer K, Pereira MC, et al. (2010) Randomization of amyloid-ß-peptide(1-42) conformation by sulfonated and sulfated nanoparticles reduces aggregation and cytotoxicity. Macromol Biosci 10: 1152-1163.
- Levine H (1995) Thioflavine T interaction with amyloid ß-sheet structures. Amyloid 2: 1-6.
- Maggio JE, Stimson ER, Ghilardi JR, Allen CJ, Dahl CE, et al. (1992) Reversible in vitro growth of Alzheimer disease beta-amyloid plaques by deposition of labeled amyloid peptide. Proc Natl Acad Sci U S A 89: 5462-5466.
- Wengenack TM, Curran GL, Poduslo JF (2000) Targeting alzheimer amyloid plaques in vivo. Nat Biotechnol 18: 868-872.
- Lee HJ, Zhang Y, Zhu C, Duff K, Pardridge WM (2002) Imaging brain amyloid of Alzheimer disease in vivo in transgenic mice with an Abeta peptide radiopharmaceutical. J Cereb Blood Flow Metab 22: 223-231.
- Fei L, Perrett S (2009) Effect of nanoparticles on protein folding and fibrillogenesis. Int J Mol Sci 10: 646-655.
- Dutta P, Dey J (2011) Drug solubilization by amino acid based polymeric nanoparticles: Characterization and biocompatibility studies. Int J Pharm 421: 353-363.
- Yang HM, Lee HJ, Park CW, Yoon SR, Lim S, et al. (2011) Endosome-escapable magnetic poly(amino acid) nanoparticles for cancer diagnosis and therapy. Chem Commun (Camb) 47: 5322-5324.
- Akagi T, Shima F, Akashi M (2011) Intracellular degradation and distribution of protein-encapsulated amphiphilic poly(amino acid) nanoparticles. Biomaterials 32: 4959-4967.
- Skey J, O’Reilly RK (2008) Synthesis of chiral micelles and nanoparticles from amino acid based monomers using RAFT polymerization. J Polym Sci A Polym Chem 46: 3690-3702.
- O’Reilly RK (2010) Using controlled radical polymerisation techniques for the synthesis of functional polymers containing amino acid moieties. Polym Int 59: 568-573.
- Bentolila A, Vlodavsky I, Ishai-Michaeli R, Kovalchuk O, Haloun C, et al. (2000) Poly(N-acryl amino acids): a new class of biologically active polyanions. J Med Chem 43: 2591-2600.
- Mori H, Matsuyama M, Sutoh K, Endo T (2006) RAFT polymerization of acrylamide derivatives containing L-phenylalanine moiety. Macromolecules 39: 4351-4360.
- Sanda F, Endo T (1999) Syntheses and functions of polymers based on amino acids. Macromol Chem Phys 200: 2651-2661.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16206
- [From(publication date):
June-2013 - Nov 24, 2025] - Breakdown by view type
- HTML page views : 11362
- PDF downloads : 4844