Research Article Open Access
New Direct Competition Free but Not Label Free Immunosensor Method for IgG Determination
Elisabetta Martini, Mauro Tomassetti* and Luigi Campanella
Department of Chemistry, University of Rome “ Sapienza”, P.le Aldo Moro 5, 00185, Rome, Italy
- *Corresponding Author:
- Mauro Tomassetti
Department of Chemistry
University of Rome “Sapienza”
P.le Aldo Moro 5, 00185 Rome, Italy
Tel: +39 0649913722
Fax: +39 06490631
E-mail: mauro.tomassetti@uniroma1.it
Received date: March 14, 2013; Accepted date: April 03, 2013; Published date: April 05, 2013
Citation: Martini E, Tomassetti M, Campanella L (2013) New Direct Competition Free but Not Label Free Immunosensor Method for IgG Determination. J Anal Bioanal Techniques S7:008. doi: 10.4172/2155-9872.S7-008
Copyright: © 2013 Martini E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
In the present study, to reduce the excessively long time required for each “competition” measurement performed using the customary competitive immunosensors, an innovative “direct”, i.e. competition free, immunosensor method for IgG determination, was developed. Unlike what is usually reported in the literature for the latter type of immunosensor devices, which are usually “label free”, that is, which do not use a marker since the signal is often obtained directly as a result of immunocomplex formation, in the present competition free method, an enzymatic marker was again used to perform robust electro-enzyme measurement, while the transducer used was of the classic gas diffusion potentiometric type. The new immunosensor affords a considerable reduction in analysis time, but maintains good repeatability and selectivity and so IgG analysis in human milk and serum was successfully performed using it. For the immunosensor developed, a systematic study was also performed to ensure its complete analytical characterisation using standard solutions of IgG and a comparison involving a classical “competitive” ELISA type immunosensor was also performed. Lastly a short excursus on label free immunosensor methods reported in the literature is included to justify the choice made in the present research.
Keywords
IgG analysis; Human serum; Human milk; Direct and competition methods
Introduction
In the last few years we fabricated several competitive immunosensors for the analysis of IgG proteins [1-7]. In the present study, to reduce the excessively long time required for each competition measurement, we developed an innovative “direct” immunosensor method for IgG determination. Of course other immunosensors for the direct measurement of several different antigens have already been described in the literature [8-13]; these devices usually do not involve any “competition” procedure and generally do not make use of a marker, because in these probes the signal is often obtained directly as a result of immunocomplex formation (which may, for example, be a cause of potential variation in the membrane). These devices are usually unsuccessful for various reasons (low signal, high noise and scarce selectivity) even though they afford a considerable reduction in analysis time. Nevertheless, we recently resumed studies on direct immunosensor types as part of research conducted on new electrochemical “competition free” immunosensors developed for the analysis of other proteins [14,15] and designed to perform measurements using different operating schemes. In the latter case, however, unlike what is usually reported in the literature for this kind of immunosensor, an enzymatic marker was again used to perform the electrochemical measurement while the transducer used was of the classic amperometric type [14,15]. In the present research a slightly different approach, although of the same type, was used for the measurement of IgG, while the electrochemical transducer was of the potentiometric type. The new geometry of the direct method selected by us was also discussed on the basis of several different label free immunosensor methods reported in the literature.
Materials and Methods
Materials
The Ny+ Immobilon Affinity membrane (porosity 0.65 μm) was from Merck Millipore Headquarters, Billerica, MA, USA; Monoclonal anti-human immunoglobulin G, purified human immunoglobulin G from human serum (purity ≥ 95%), and anti-human immunoglobulin G-urease conjugate were from Sigma Immunochemicals, (Sigma Aldrich srl, Milan, Italy); Albumin (from bovine serum) (BSA), urea, lactalbumin, casein and TRIS (hydroxymethyl-aminomethane) and TWEEN® 20 were from Sigma Aldrich srl, Milan, Italy; magnesium chloride, potassium phosphate monobasic, potassium phosphate bibasic and all other solvents or reagents of the highest purity were from Carlo Erba, Milan, Italy. All antigens, antibodies and proteins were used without further purification.
Apparatus
Potentiometric measurements were carried out in a 25 ml glass cell, thermostated at 25°C, under continuous stirring. An ammonia potentiometric gas-diffusion electrode (NH3 analysis, mod. 6.0506.010) and the NH3 gas-permeable membrane (6.1249.000) were supplied by Metrohm, Switzerland. Measurements were carried out using a potentiometer (Orion model SA 720) connected to a recorder (AMEL mod. 868).
Samples analyzed
50 ml of human serum (aseptically filled) were purchased from Sigma Aldrich srl, Milan, Italy; human milk samples were taken from one healthy mother in the eighth month after giving birth.
IgG immobilization on Immobilon membrane
The Immobilon Ny+ Membrane (a positively charged nylon membrane, with polyester reinforcement optimized for reliable and reproducible transfer, immobilization, hybridization, and subsequent reprobing) was cut into 1.0 cm2 squares and 25.0 μL of a 50 mg mL-1 immunoglobulin G solution was deposited directly on the membrane surface. The Immobilon membrane was then dried at room temperature for about 3 h and stored at 4°C.
Potentiometric immunosensor assembly
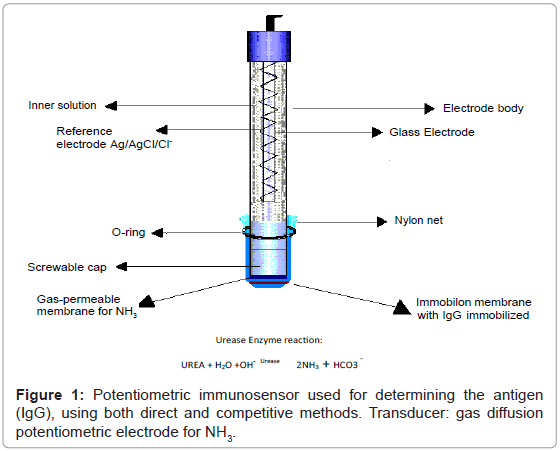
The transducer consisted of a gaseous diffusion potentiometric electrode for ammonia comprising a pH sensitive “combined” glass membrane (Metrohm) and a Ag/AgCl/Cl- reference electrode, an internal solution of NH4C1 0.01 M, in which glass and reference electrodes were immersed, contained in a plastic cap (which was screwed onto the body of the electrode), sealed at the lower end with a gas permeable (Metrohm Italiana Srl, Formello, Italy) membrane; a dialysis membrane overlapped the gas permeable membrane to prevent the surfactant used from altering the permeability of the gas permeable membrane. Lastly the Immobilon membrane with the immobilized IgG was superimposed on the first two membranes. Finally, nylon net was placed over the latter membrane. The three membranes and the net were secured by a rubber O-ring to the plastic cap, as shown in figure 1. The entire plastic cap supporting the membranes was stored at +4°C during the periods in which the immunosensor was not being used in order to avoid having to dismantle the membranes.
Direct measurement of IgG
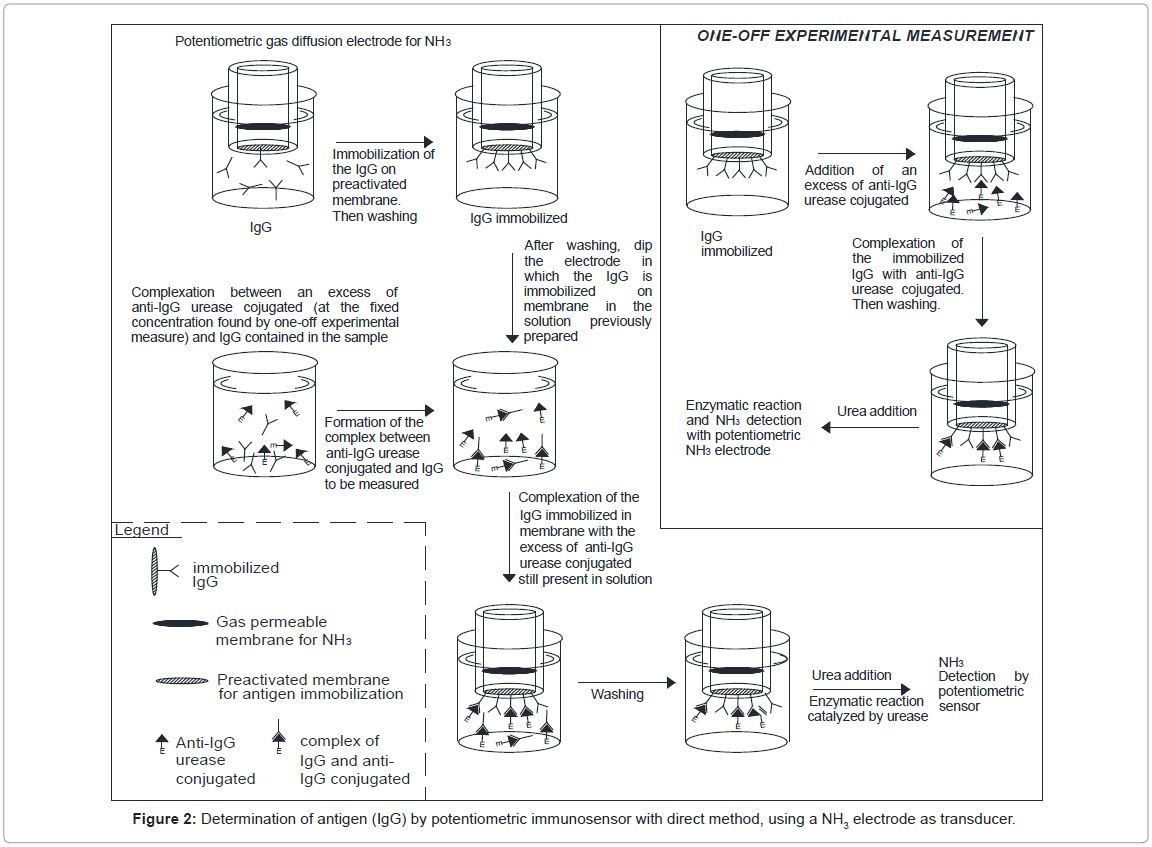
For the purpose of directly measuring a real sample, an innovative procedure was therefore developed. First of all a one-off experimental measure was performed of the concentration of the labelled antibody required to form the full complex with the respective antigen, which was immobilized on an ad hoc membrane (Immobilon). Before each measurement, the potentiometric sensor with the Immobilon membrane (on which Immunoglobulins G (IgG) had been immobilized on the head) was immersed in 5 mL of Tris-HCl buffer solution 0.1 M containing 0.05% Tween 20 and 2.5% by weight BSA (in order to minimize non-specific absorption on the membranes). Each measurement was then performed after washing and renewing the 5 mL of Tris-HCl buffer solution by complexing the antigen to be measured contained in the sample with the fixed excess concentration of labelled antibody solution (determined in a one-off experimental measurement). Lastly, the immunosensor treated as described above was immersed in the latter solution under stirring and the complexation between the antigen immobilized on the membrane and the labelled antibody left free in solution was “completed”. The final enzymatic measurement was then performed, after probe separation from the solution and washing, by adding the specific substrate (50 μL of 0.01 M urea solution) to the renewed buffer solution. Lastly, after measuring the difference between the signals recorded in the one-off measurement and that obtained each time by the procedure described above, the signal obtained was entered in the calibration curve. A gaseous diffusion potentiometric transducer for NH3 was always used to assemble the immunosensors for IgG (immunoglobulins), while the enzyme marker used was always urease, the conjugate of which with the antigen is available on the market. The sequence of events occurring during the IgG assay using the potentiometric immunosensor is outlined in figure 2. All the values used to construct the calibration curve were obtained using the same procedure.
Competitive classical measurement of IgG
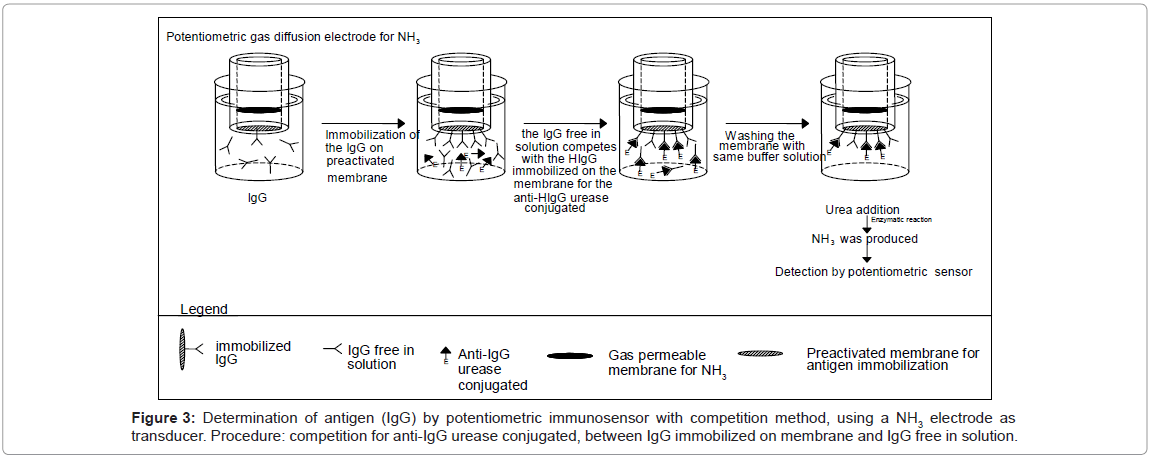
The Immobilon membrane, on which the Immunoglobulins IgG was immobilized (see section IgG immobilization on Immobilon membrane), was fixed to the head of the potentiometric electrode for NH3 as described in section Potentiometric immunosensor assembly. Then the immunoglobulins G free in solution and to be determined, were allowed to compete with the same antigen but immobilized on the membrane Immobilon in order to produce the antibody reaction with the fixed supply of antibody, free in solution and labelled with urease. In detail: before measurement, the potentiometric sensor with the Immobilon membrane (on which IgG were immobilized) fixed to the head was immersed in 5 mL of Tris-HCl buffer solution 0.1 M containing 0.05% Tween 20 and 2.5% by weight BSA (in order to minimize nonspecific absorption on the membranes). Then the immunosensor, after washing, was dipped in a renewed 5 mL of Tris-HCl buffer solution. Lastly the IgG to be determined, together with the fixed concentration of the enzyme-labelled anti-IgG (i.e. anti-IgG-urease conjugate), were allowed to incubate in this solution at 25°C for 1 “effective” hour. The antigen (IgG) free in solution competes with the IgG immobilized on the membrane in bonding the labelled anti-IgG. After washing with the same buffer to remove all the labelled anti-IgG not complexed with the IgG on the membrane and adding the enzyme substrate (50 μL of 0.01 M urea solution) to the solution in the measurement cell the signal measured was correlated with the quantity of labelled immunocomplex present on the membrane, itself correlated with the IgG concentration to be measured. This signal was then used to determine the concentration of IgG of the sample. The calibration curve was obtained after the addition to the 5 mL of buffer solution contained in the cell of 50 μL volumes of IgG solutions, at a concentration such that the final concentration lay in the range between 10-8 and 10-5 M and recording the potential variation; lastly each signal value found was plotted versus the final IgG concentration. In practice the sequence of events occurring during the IgG assay using potentiometric measurements is outlined in figure 3.
IgG measurements and “recovery” test in real samples of serum and milk
The optimized immunosensor was used to determine the IgG concentration in two different samples of human serum and milk. The potentiometric immunosensor was in all cases employed using both the direct and the competition methods (see the two procedures reported in figures 2 and 3); this was possible because, by using two measurement methods (competition and direct), a wide linear range, a low detection limit and high sensitivity were actually found in both cases. Both methods were therefore applied for the purpose of comparison. To measure the IgG protein in serum sample, a fixed quantity (1 g) of each product was dissolved in 10 mL of phosphate buffer (pH 8.0; 0.1 M). For IgG determination by the direct method 2.5 mL of this solution were added to the measuring cell containing 2.5 mL of phosphate buffer 0.2 M, while for the competitive method 100 μL of this solution were added to the measuring cell containing 5.0 mL of phosphate buffer 0.1 M. To measure the IgG protein in the milk sample 2.5 mL of human milk were added directly to the measuring cell containing 2.5 mL of phosphate buffer 0.2 M, while for the competitive method 1.0 mL of milk sample was added to the measuring cell containing 4.0 mL of phosphate buffer 0.12 M. The measurement was then carried out using both the competition and the direct methods as described in sections Direct measurement of IgG and 2.7, respectively. Account was of course taken of the various different sample dilutions in the various measures performed.
Recovery tests were also performed on the two diluted 1:10 milk samples using the competition method, while prior dilution was not necessary in the direct method. For the recovery tests the human serum and milk samples were spiked with known volumes of standard IgG solution so as to obtain a final concentration in the measuring cell respectively of about 10-8 M of IgG in the phosphate buffer solution (0.1 M, pH 8.0) for competition methods, and of about 10-7 M for the direct method. To this end, a solution of IgG was prepared by dissolving 1.5 mg of IgG in 100 mL of phosphate buffer. 10 mL of this solution was again diluted twice when using the competitive procedure. The IgG concentration in the samples was determined before and after the addition in both the direct and the competition methods.
Results and Discussion
Short excursus on direct immunosensor methods
The need to speed up the analysis time of immunological methods has always been in the forefront of the main aims of researchers operating in this sector. In particular, in the case of immunosensors it has been attempted to develop immunodevices for which no ‘competitive’ step is provided, which is necessary in the case of competitive and separatory methods but which ensures the repeatability and robustness characteristics of the latter. Several different approaches were followed to achieve this, many of which are highly ingenious. It is clear from the outset that the introduction of highly modern transducers that are alternative to the more traditional electrochemical devices certainly encouraged the development of direct immunosensor methods. One example consists of piezoelectric immunosensors like those investigated by Roederer and Bastiaans [16], or Muramatsu et al. [17], and those proposed by Kosslinger et al. [18], or by Guilbault et al. [19], in which one of the two proteins giving rise to the immunocomplex was immobilized directly on a piezocrystal using silanizing reagents. It is interesting to note however that the latter two works already suggest the possibility that additional unspecific bindings may take place at the same time as the antibody reaction and show how the authors strive to overcome these drawbacks by operating in differential mode, that is, with two piezoelectric sensors, i.e. using due piezoelectric crystals, one of which operating as referee crystal. It is immediately apparent that the main drawback encountered by all direct immunosensors is the possible occurrence of non specific interactions with other proteins, which may simply be adsorbed on the sensitive element of the transducer. The same drawback may be found also in very recent immunosensors using SPR (Surface Plasmon Resonance) transduction. This transduction technique almost naturally favours the direct and unlabeled methods, as has been pointed out by many workers, such as Indyk and Filonzi [20], or Dong et al. [21]. The latter developed a multichannel microfluidic chip using poly (dimethylsiloxane) to selectively functionalize the surface and deliver the analyte solution.
According to the latter author, for instance, by using a PDMS pretreatment (microfluidic delivery system) to generate the sensing surface, the problems arising from non-specific adsorption on the matrix surface (non functionalized areas), are minimized. In addition, the use of extravidin and streptavidin instead of avidin as a blocking layer helps to decrease the non specific adsorption. On the other hand, the first of the two authors cited, operating in flow mode, endeavoured to measure the extent of non specific binding to the surface probe using samples to be analysed at various dilution levels injected over a non immobilized reference flow cell. Moreover, also the present authors recently compared a direct SPR method for IgG measurement [6,7] with traditional competition methods [3-5] and, in order to reduce the non specific adsorption on the SAM (self-assembled gold monolayer), activated with a mixture containing (EDC) carbodiimmide and (NHS) succinimmide, the nonreacted activated groups were deactivated by treatment with ethanolamine solution. This essentially shows how, also in these direct methods using the latest transduction methods, the danger of non specific interference is always present and is considered a risk by all researchers. Lastly, it may be taken for granted that this type of problem also affects several very interesting, but very complicated, optical immunosensors such as the one proposed by Ingenhoff et al. [22]: practically a label-free homogeneous immunoassay, in which the evanescent field of the guide mode penetrates the adjacent vicinity of the waveguide, i.e. the biochemical layer.
The effective refractive index of the waveguide itself during an antigen-antibody interaction is thus changed and such small changes can be transformed into intensity modulations using an integrated optical Mach-Zehnder interferometer; or the one described by Tatsu et al. [23], i.e. a fluorescent fiber-optic immunosensing system based on liposome containing carboxyfluorescein; even though the authors of these articles tend to gloss over the above problem, perhaps because of the complexity of the methods described, it is nevertheless interesting to note that Tatsu Y deems it very advantageous to amplify the intensity of the fluorescent signal produced as a result of the immunological binding onto the liposome and which causes the lysis of the liposome itself in order to minimize the effect of possible non specific interferences. Even though the immunosensors based on these new types of transducer are very interesting they are generally found to be rather complex and to require above all very expensive and delicate measuring apparatus and are thus still relatively rare. Conversely, the immunosensors based on electrochemical (potentiometric, amperometric, voltammetric) transducers are instead very economical as the required measuring instruments are not only relatively cheap but are generally available in ordinary laboratories.
These immunosensors are therefore more common and the most frequently studied. Many of them of course operate on the basis of ELISA type competitive procedures and generally produce good results [1-5,24-29]. However, also for these sensors the need to shorten analysis time by developing direct methods has often been felt. Of course, to achieve this, numerous approaches have been suggested, for instance, the most obvious one immediately seemed that of constructing an immunosensor that could generate a potentiometric signal as a direct consequence of the immunocomplex (antigen-antibody) formation. To this end potentiometric electrodes on the surface of which the antibody was immobilized one way or another (when it was desired to determine the respective antigen), the potential of which would vary precisely as a result of the formation of the antibody complex, appeared to be the most simple to implement. One, I should say classical, example of this type of immunosensor is that proposed by Feng et al. [30], who developed a direct immunosensor by means of covalent immobilization of the antibody on the silver (Ag) electrode using a silanizing agent and glutaraldehyde. The antigen detection was based on the change in the electric potential of the sensor before and after the antigen-antibody reaction. One variant often used more recently is that of coating the sensor with sensitive film, often using nanoparticles, a classical example of which is the one described by Li et al. [31]: in this case, cysteine was bonded on to the nano-Fe3O4 particles surface. The cysteine functionalized magnetic nanoparticles were coated on to a solid paraffin carbon paste electrode surface to covalently immobilize the antibody by employing a covalent glutaraldehyde crosslinking method. However, in this kind of sensor, the greatest difficulties are due to the fact that, when applied to real samples, other proteins present are adsorbed on to the electrode surface, and thus also contribute to varying the membrane potential. This kind of problem was evident right from the outset, even to researchers tackling this problem over several years. For instance, Yamamoto et al. [32], addressed this type of problem by suggesting a double layer formation for the interpretation of potential changes in antigen-antibody reactions in the case of an antibody modified electrode. In their research, in which they developed a potentiometric immunoelectrode made of titanium wire on which an antibody was chemically fixed, they used a special reference electrode, prepared in the same manner as the sensing immunoelectrode. Of course, no sensitization of the wire with the antibody protein was performed, so that the surface of the reference electrode was only chemically covered with urea. Clearly, the efforts already made by these authors to make the response of this type of immunodevice more selective are clearly indicative of the difficulties encountered in ensuring that these immunosensors function correctly. It is no coincidence that other authors sought to work around this problem in various ways, for instance, by attempting to substantially amplify the response of the ion-selective potentiometric transducer by modifying its membrane (in a very specific and highly ingenious way). For example this is true in the case of the so called PIMIA (potentiometric ionophore modulated immunoassay) biosensors [33]. This nevertheless introduces a new drawback as in the case of measurements performed on real samples, before being subjected to measurement the latter must first be treated to eliminate the ion responsible for the amplification of the potentiometric signal (e.g. the K+ ion). Furthermore, in recent years a good number of label-free immunosensors have been proposed which have many characteristics in common and are also conceptually very similar, although of course differing in the way the antibody membrane is assembled. This type of immunosensor is usually based on the modulation of the electrochemistry of the surface bound redox FC species, i.e. K3Fe(CN)6. Such immunosensors generally use a glassy carbon (GC) or gold electrode as support and the measurements are of the voltammetric, or amperometric type. For instance Shi and Ma [34] proposed a label-free immunosensor based on a GC electrode, the surface of which was first drop-coated with a mixture of (FC) and chitosan (CHIT), then covered with a nafion (NF) membrane containing gold nanoparticles that act as conducting wires between the electrolyte solution and the redox membrane. After binding with polyethylenimine (PEI), glutaraldehyde (GA) was used to link PEI and the antibody. When the antigen came into contact with the electrode surface thus modified, an increase in antigen on the biosensor blocked the electron transfer and was detected as a reduction in electrochemical redox response. Measurements were carried out using square wave voltammetry. A label-free immunosensor based on modified nanoporous gold (NPG) film electrode was proposed by Wei et al. [35]. Due to its high conductivity, large surface area and good biocompatibility, a modified NPG film electrode was used for the adsorption of the antibody. Also in this case, the sensing signal was based on the monitoring of the electrode’s current response to K3Fe(CN)6, which was extremely sensitive to the formation of immunocomplex within the nanoporous film. Tian et al. [36] described an electrochemical immunosensor based on gold nanoparticles supported on cross-linked starch functionalized multi-walled carbon nanotubes in a nanocomposite film (AuNPs/CAS-MWCNTs). The modified electrode was prepared by a simply casting method: the whole compound after treatment with nafion solution was casted onto a pretreated GC electrode surface; lastly the electrode was immersed in the antibody solution. The formation of the antibody-antigen complex decreases the CV peak current of the hexacyanoferrate redox, increasing the antigen concentration. Another label-free immunosensor of the same kind was studied by Wang et al. [37], modifying a GC electrode. The redox membrane was prepared in this case using CHIT and the thionine (THI) redox reaction, instead of the hexacyanoferrate one. Nafion was used to attach the redox species to the membrane. The strong binding capacity between (NF) and (PFI) was utilized to attach the antibody to the electrode by cross-linking. The silver nanoparticles deposited on SiO2 nanoparticles embedded in the NF membrane acted as a conducting wire between the electrolyte solution and redox membrane. DPV (Differential Pulse Voltammetry) and CV (Cyclic Voltammetry) were used for the measurements. Lastly a direct label-free immunosensor device was developed by Hu et al. [38] using an Ag@BSA microsphere, as this Ag and BSA (Bovine Serum albumin) composite can be considered as an effective and versatile platform for the immobilization of biomolecules. An Au electrode was used as the support electrode to be modified with Ag@BSA microspheres; the monoclonal antibody was immobilized on this microsphere by cross-linking, using glutaraldehyde. Also in this case the redox species used was hexacyanoferrate, while the measurements were performed using DPV (Differential Pulse Voltammetry) and EIS (Electrochemical Impedance Spectroscopy). Of course in all these works the authors addressed the problem of limiting as far as possible any interference caused by the presence of other proteins possibly adhering to the electrode surface by simple adsorption or also due to the formation of electrostatic or affinity bonds. To this end, after immobilizing the antibody on the electrode, the modified electrode was treated before use with BSA solutions in order to block any possible remaining active sites and avoid non specific adsorption. Other authors attempted to develop direct immunosensors, free of any competition step, using an enzyme, which is not however used in this case as a marker. Fonong and Rechnitz [39] for instance developed a homogeneous immunoassay method which utilizes a potentiometric CO2 gas-diffusion sensing membrane electrode based on the enzyme inhibition by antibody of CO2 production in the enzymatic reaction, as the chloroperoxidase enzyme is conjugated to the antibody and β-ketoadipic acid is the enzymatic substrate. Along these lines, the authors of the present article, taking into account the foregoing, but also the many positive results obtained by themselves [1-5] and by other authoritative authors [24-29] using classical competition immunosensors, have recently been investigating the possibility of developing direct immunosensor methods that can do without the competitive step, which is the factor largely responsible for the lengthy analysis times. At the same time it is possible to retain the positive factors that have made competitive immunological methods so “robust” and reliable, and generally ensure an amperometric or potentiometric signal that is sufficiently strong, and therefore easily reproducible, and at the same time highly specific and thus generally free of any possible interference. However, in this way it is also possible to retain the heterogeneous-separatory type test which makes it possible to retain also the classical rinsing of the immunosensor which in any case guarantees the absence of any additional unspecific binding due to adsorbed proteins which may therefore be completely removed by the specific washing operations. Clearly also this type of procedure may be implemented in different ways even though the basic assay concept and the principal operating steps essentially remain the same. For this purpose we recently published a preliminary investigation in which an immunosensor was constructed to determine the antigen lactoferrin [14,15]. The device used an amperometric transducer for hydrogen peroxide or one for oxygen (Clark type). The enzyme peroxidase (HRP) was used as enzymatic marker in which the antibody of the analyte to be determined, namely anti-lactoferrin, was immobilized in a polymeric membrane. Using this type of “competition free” but not “label free” immunosensor it was possible to practically halve analysis time compared with the same competitive immunosensor of the classical ELISA type, passing from about 1 h and 30 min to about ½ hour, while the main analytical characteristics of precision, “robustness”, and so on remained unchanged. The present investigation therefore follows the same line of research; in this case, a direct, i.e. “competition free” immunosensor IgG analysis was constructed, although with several modifications compared with the preceding one. In this case, the transducer was a gaseous diffusion potentiometer for NH3, in itself highly selective, as well as having a sufficiently rapid response time; the enzyme urease was used as enzymatic marker of the antibody, free in solution, while a constant amount of the antigen was immobilized on the polymeric membrane. This was therefore a direct method similar to the previous one, i.e. with a non competition operating geometry but slightly different from the one used in the preceding works [14,15].
Results obtained using our direct method
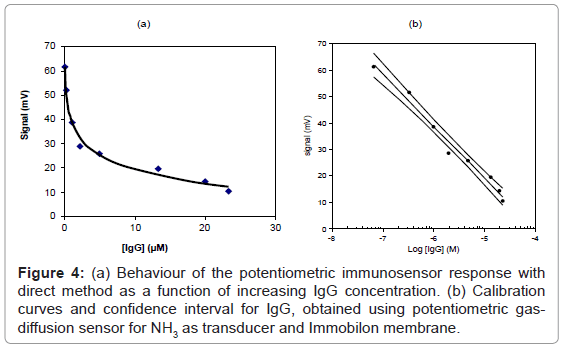
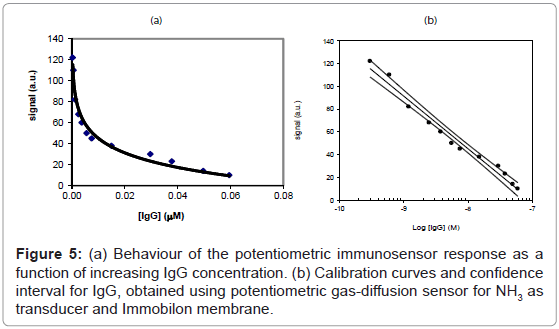
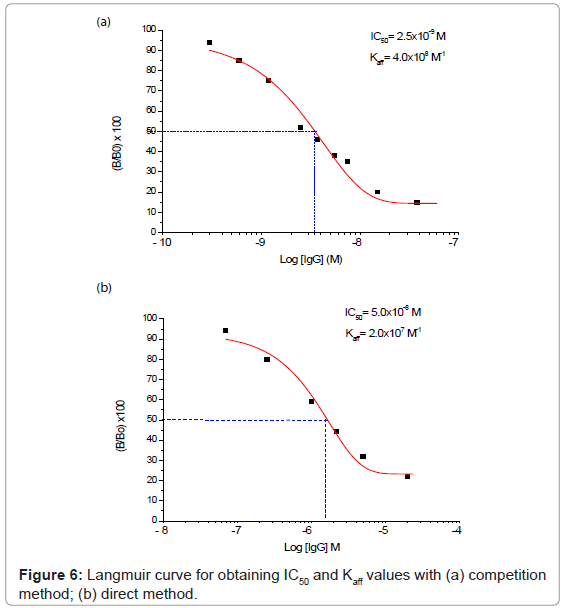
The two “direct” and “competition” methods used in the present paper for the measurement of IgG also in real samples are shown respectively in figures 2 and 3, while the behaviour of two immunosensor responses and the relative calibration curves for IgG determination are shown in figures 4(a) and 5(a), and figures 4(b) and 5(b), respectively. Lastly, the main analytical data for the determination of IgG proteins are reported in table 1, respectively. The values of IgG concentration in milk and serum, found by direct immunosensor and competitive methods, are set out in table 2. Lastly, the Langmuir curves and values of IC50 and Kaff using both direct and competition methods are set out in figures 6(a) and 6(b). The percent selectivity values for IgG immunosensor with direct procedure versus several common proteins contained in milk, such as lactalbumin and casein, are shown in table 3. Finally, the results of recovery tests performed by the standard addition method, both in serum and human milk, are reported in table 4.
| Methods | DIRECT METHOD Geometry of the test: conjugation between IgG, immobilized on the membrane of immunosensor and the excess of urease conjugated anti-IgG, free in solution. (a) | COMPETITION METHOD Geometry of the test: competition between IgG, immobilized on the membrane of immunosensor, and IgG free, for the urease conjugated anti-IgG, free in solution (b) |
|---|---|---|
| Regression equation (Y= a.u., X= M) Confidence level (1-α) = 0.95; | Y = -19.4 (± 3.1) × -77.9 (± 13.4) (n–v) = 6 ; (t=2.45) | Y = -41.3 (± 4.3) log × -323.9 (± 18.4) (n–v) = 9 ; (t=2.26) |
| Linear range (M) | 2×10-5 - 7×10-8 | 6.0×10-8 ÷ 3×10-10 |
| Correlation coefficient | 0.9813 | 0.9801 |
| Repeatability of the measurement (as pooled SD%) | 6.8 | 8.6 |
| Low detection limit (LOD) (M) | 4×10-8 | 1.7×10-10 |
| Instrumental response time (minutes) | @ 10 | @ 15 |
(a.u.=mV), Employed transducer: potentiometric electrode for NH3; Employed Membrane: Immobilon; Buffer solution: Tris (0.1 M), pH 8.0; Conjugation temperature 25°C; Conjugation time: 15 min.
Table 1: Analytical characterization of potentiometric immunosensor for determining IgG using direct method (a) and competition method (b) respectively.
| Matrix | Found IgG concentration in the undiluted sample by direct method n= 5; SD% ≤ 5.5 | Found IgG concentration in the undiluted sample by competitive methods n= 5; SD% ≤ 5.5 | Values reported in literature |
|||
| μM | mg L-1 | μM | mg L-1 | mg L-1 | Ref | |
| Human Milk (freeze conservation) |
0.182 | 27.3 | 0.152 | 22.8 | 10-76 | [62] |
| Human Milk | 0.203 | 30.5 | 0.198 | 29.7 | 10-76 | [62] |
| Human serum | 19.5 | 2,925 | 25.7 | 3,855 | 5,810-10,500 | [48] |
Table 2: IgG determination by immunosensors using direct and competition methods in human milk and serum.
| Matrix | Order of magnitude of protein molecular weight (kDa) | Percent selectivity values for IgG immunosensor n= 5; RSD% ≤ 5 |
|---|---|---|
| IgG | 150.0 | 100.0 |
| Lactalbumin | 14.2 | 5.8 |
| Casein | 24.0 | 30.2 |
Table 3: Percent selectivity values for IgG immunosensor with direct procedure vs. several common proteins contained in milk. % response of immunosensor vs. several different milk proteins taking as 100 the response to the IgG at the same concentrations.
| Sample n° | Nominal concentration of samples (M) RSD% ≤ 5.5 | Found experimental concentration (M) RSD% ≤ 5.5 | % Recovery RSD% ≤ 5.5 | |||
|---|---|---|---|---|---|---|
| Direct | Competitive | Direct | Competitive | Direct | Competitive | |
| Human milk | 2.00×10-7 (undiluted) | 1.0×10-8 (≈ diluted 1:10) | 1.99×10-7 | 0.988×10-8 | 99.5% | 98.8% |
| Human serum | 1.0×10-7 (undiluted) | 1.0×10-8 (≈ diluted 1:10) | 0.965×10-7 | 1.04×10-8 | 96.5% | 104% |
Table 4: Recovery tests for IgG in human serum and milk using direct and competitive immunosensor methods. Samples were suitably diluted before analysis.
Figures 2 and 3 illustrate both the direct and the competitive procedures. In the present research the “competitive procedure” used for IgG measurement is of the ELISA type, although it is not one of the more commonly used ELISA procedures. The method used involved “competition” between IgG free in solution to be measured and IgG immobilized on the immunosensor membrane, for bonded with the enzyme-labelled anti-IgG free in solution (Figure 3). This procedure was chosen because, in this way, both competition and direct methods shared the fact of using the urease labeled antibody free in solution and the same quantity of antigen immobilized in the membrane. Efforts were initially focused on optimizing the operating conditions, several of which, such as the pH and the buffer solution, were the same as those used in previous work [14]; also other conditions were experimentally optimized, i.e. the concentration of the anti-IgG enzyme conjugate, free in solution, was found to be 10-5 M [14]. As regards the working temperature, it was found that above about 25°C the increase in immunosensor response was practically negligible, so the measurement solution was kept in a thermostated cell at 25°C (room temperature).
Moreover, the actual incubation time used for the competition method was one “effective” hour under constant stirring and the total analysis time about 1 hour and a half, while the total analysis time for the direct measurement method was about ½ hour. Lastly, monoclonal antibodies were used (see Section Materials). However, optimization was also performed in both the transducer (sensitive, above all thanks to the larger surface area available for the gaseous exchange gas permeable membrane) and in the way the final enzymatic-potentiometric measurement was made.
It was also attempted to optimize the method in order to allow the reutilization (after each routine immunosensor measurement) of the Immobilon membrane, on the surface of which the IgG were immobilized, albeit for only a limited number of tests. The best results so far were obtained by gently washing with Glycine-HCl buffer, 0.1 M, pH 2.0, containing 2.5 M MgCl2 [3] and the number of times the membrane can be reused is a maximum of 3.
As can be observed by comparing the data in table 1, the analytical results obtained varied considerably according to whether the direct or the competition method was used: indeed, using the first method, the LOD was found to be of the order of 4×10-8 M and the linearity range was between about 2×10-5 and 7×10-8 M, while using the second method the LOD was found to be of the order of 10-10 M with a linearity range of about 6×10-8 to 3×10-10 M. For IgG determination the two procedures thus allowed two comparatively different concentration intervals to be covered in which it is possible to perform the measurements. On the other hand, the linearity range obtained for the direct method is still well suited to measuring the IgG in human milk and serum, (Table 2) with a good reproducibility (RSD% ≤ 5.5). As reported in literature [40-53], in some sera or milk samples, the values of immunoglobulin G generally do not exceed a concentration of 10-7 M. Furthermore, it must be considered that the competition procedure also involves a further dilution of the sample, which in practice often attains final concentrations of the order of 5×10-8 M, while also for the direct procedure this value lay in the middle of the linearity range. Lastly, the Kaff values obtained using both methods are set out in figure 6. The value obtained is seen to be of the order of 108 M-1 for the competitive immunosensor and 107 M-1 for the direct method. These values were found to be in good agreement with the scanty data available on the topic in the literature [54,55] and are both in any case sufficiently high to allow an efficient immunological method to be developed. Table 2 shows the IgG values, expressed both in mg L-1 and μM, found in human serum and two samples of human milk. The values found for frozen milk and fresh milk do not differ appreciably, as confirmed by literature data [56-60], in which no significant alterations of IgG concentration due to thermal treatment are reported. Lastly, it was also ascertained that the immunosensor method, as for the majority of immunological tests involving proteins, is not subject to any interference from the other minor electrolytes present in the solution [61,62]. Tests were also run to assess selectivity towards other proteins (see selectivity values reported in table 3). It emerged that lactalbumin displays practically no interference in immunoglobulin G measures. Casein could actually act as a possible non negligible interferent, above all because the percentage of this protein is higher than the other proteins contained in milk [51,61,62]. Moreover, casein can easily be separated out by precipitation simply by lowering the sample pH to about 4.5 [51].
Lastly, both the direct and competition methods were used to determine IgG in two samples of milk. To this end, table 4 shows the values of the “recoveries” obtained using the standard addition method applied to the human serum and milk samples tested. As can be seen, whether performing the measures with the direct method or the competition method the recovery values are always close to 100%. Even if this is not sufficient in itself to demonstrate the accuracy of these immunosensor methods, it may in any case be considered a necessary condition for validating the method’s accuracy. Moreover, the precision may be considered practically the same for both methods (direct or competition) and in any case always acceptable (RSD ≤ 5.5). Furthermore, these immunosensor methods have proved to be relatively robust as, when operating in a buffered environment for example, they are not subject to any variation in pH which might be induced by the addition of the test sample. Lastly, it has been shown that even small variations in the temperature of the measuring cell, which is thermostated to ambient temperature, or of the time of incubation or competition, are not critical factors.
Conclusion
The main result of this investigation of two different operating procedures (i.e. direct or competition) involves above all very different measurement times. Indeed, in the competition method, the competition step lasts about one hour [1-6], which considerably prolongs the total analysis time to about 1.5 hours (minimum 1 h and a quarter), while in the direct method the conjugation steps leading to the formation of the immuno-complex each last about 15 minutes, for a total time of about 30 minutes. It might be objected that having reduced the analysis time by means of the method we propose to less than half the previous time compared with competition methods is not a completely satisfactory result. On closer scrutiny this natural objection appears somewhat unjustified in view of the fact that, even in numerous other direct methods mentioned by us in the above section Results and Discussion, the measurement times are sometimes about ½ h [18,19], including the SPR methods [20] and that in some label-free direct methods, in order to obtain a good response, a reaction time of about 1 h [23,32,35] was in some cases necessary. Indeed a somewhat lengthy time is often required to perform those treatments with BSA which must be carried out before measuring the antigen in order to block the remaining active sites and avoid non specific adsorption. It is true that in two recent articles by Crosson and Rossi [63], Crosson et al. [64], carried out respectively using a quartz crystal microbalance and an SPR binding rate method, the assay method actually seems to be as low as 5 and 4 min, respectively. The question is, however, as in other cases, should the antigen immobilization time required for each measurement (respectively, 33 and 20 min, in the last two works cited) and in some case also the time required to prepare the support needed for each measurement be included in the analysis time or not?
Finally the linearity range is different for each method described by the authors of the present paper: about 10-8-10-10 M for the competition method and 10-5-10-8 M for the direct method. Also the latter linearity interval has however a sufficiently low lower limit to allow IgG determination to be carried out with ease in milk and serum. The conclusion may thus be drawn that direct immunosensor methods of this kind, in which no competitive step is thus envisaged, but which however still use an enzymatic marker, are as precise, robust and reliable as the corresponding competition methods. Moreover, they have the additional advantage of taking half the analysis time and furthermore do not have the drawbacks of poor selectivity and precision often found in direct potentiometric methods which do not use an enzymatic marker as the signal is produced directly as a result of the antibody complex formation.
Acknowledgements
This work was funded by “Sapienza University of Rome”, “University Project”.
References
- Campanella L, Attioli R, Colapicchioni C, Tomassetti M (1999) New amperometric and potentiometric immunosensors for anti-human immunoglobulin G determinations. Sens Act B-Chem 55: 23-31.
- Campanella L, Martini E, Tomassetti M (2007) Immunochemical potentiometric method for HIgG and anti-HIgG determination, using a NH3 probe and immunoprecipitation as preconcentration procedure. Anal Lett 40: 113-125.
- Campanella L, Martini E, Tomassetti M (2008) Determination of HIgG and anti-HIgG using a single potentiometric immunosensor and two different “competitive methods” : Application to the analysis of globulin G in human serum. Sens Act B-Chem 130: 520-530.
- Campanella L, Lelo D, Martini E, Tomassetti M (2008) Immunoglobulin G determination in human serum and milk using an immunosensor of new conception fitted with an enzyme probe as transducer. Sensors 8: 6727-6746.
- Campanella L, Martini E, Pintore M, Tomassetti M (2009) Determination of lactoferrin and immunoglobulin g in animal milks by new immunosensors. Sensors (Basel) 9: 2202-2221.
- Tomassetti M, Martini E, Campanella L, Carlucci L, Favero G, et al. (2012) Determination of immunoglobulins G in human serum and cow milk using a direct immunological method based on surface plasmon resonance. Sensors and Microsystems Lecture Notes in Electrical Engineering 109: 3-7.
- Tomassetti M, Martini E, Campanella L, Favero G, Carlucci L, et al. (2013) Comparison of three immunosensor methods (surface plasmon resonance, screen-printed and classical amperometric immunosensors) for immunoglobulin G determination in human serum and animal or powdered milks. J Pharm Biomed Anal 73: 90-98.
- Nakamura N, Hashimoto K, Matsunaga T (1991) Immunoassay method for the determination of immunoglobulin G using bacterial magnetic particles. Anal Chem 63: 268-272.
- Ribone ME, Belluzo MS, Pagani D, Marcipar IS, Lagier CM (2006) Amperometric bioelectrode for specific human immunoglobulin G determination: optimization of the method to diagnose American trypanosomiasis. Anal Biochem 350: 61-70.
- Engvall E, Jonsson K, Perlmann P (1971) Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim Biophys Acta 251: 427-434.
- Arthington JD, Cattell MB, Quigley JD 3rd (2000) Effect of dietary IgG source (colostrum, serum, or milk-derived supplement) on the efficiency of Ig absorption in newborn Holstein calves. J Dairy Sci 83: 1463-1467.
- Okochi M, Ohta H, Tanaka T, Matsunaga T (2005) Electrochemical probe for on-chip type flow immunoassay: immunoglobulin G labeled with ferrocenecarboaldehyde. Biotechnol Bioeng 90: 14-19.
- Nakanishi K, Muguruma H, Karube I (1996) A novel method of immobilizing antibodies on a quartz crystal microbalance using plasma-polymerized films for immunosensors. Anal Chem 68: 1695-1700.
- Campanella L, Martini E, Tomassetti M (2010) Further development of lactoferrin immunosensor (part III). J Pharm Biomed Anal 53: 186-193.
- Campanella L, Martini E, Tomassetti M (2010) Immunosensors for the Direct Determination of Proteins: Lactoferrin and HIgG. Sensors and Microsystems Lecture Notes in Electrical Engineering 54: 215-218.
- Roederer JE, Bastiaans GJ (1983) Microgravimetric immunoassay with piezoelectric crystals. Anal Chem 55: 2333-2336.
- Muramatsu H, Dicks JM, Tamiya E, Karube I (1987) Piezoelectric crystal biosensor modified with protein A for determination of immunoglobulins. Anal Chem 59: 2760-2763.
- Kösslinger C, Drost S, Aberl F, Wolf H, Koch S, et al. (1992) A quartz crystal biosensor for measurement in liquids. Biosens Bioelectron 7: 397-404.
- Guilbault GG, Hock B, Schmid R (1992) A piezoelectric immunobiosensor for atrazine in drinking water. Biosens Bioelectron 7: 411-419.
- Indyk HE, Filonzi EL (2003) Determination of immunoglobulin G in bovine colostrum and milk by direct biosensor SPR-immunoassay. J AOAC Int 86: 386-393.
- Dong Y, Wilkop T, Xu D, Wang Z, Cheng Q (2008) Microchannel chips for the multiplexed analysis of human immunoglobulin G-antibody interactions by surface plasmon resonance imaging. Anal Bioanal Chem 390: 1575-1583.
- Ingenhoff J, Drapp B, Gauglitz G (1993) Biosensors using integrated optical devices. Fresenius J Anal Chem 346: 580-583.
- Tatsu Y, Yamamura S, Yoshikawa S (1992) Fluorescent fibre-optic immunosensing system based on complement lysis of liposome containing carboxyfluorescein. Biosens Bioelectron 7: 741-745.
- Jie G, Zhang J, Wang D, Cheng C, Chen HY, et al. (2008) Electrochemiluminescence immunosensor based on CdSe nanocomposites. Anal Chem 80: 4033-4039.
- Darain F, Park SU, Shim YB (2003) Disposable amperometric immunosensor system for rabbit IgG using a conducting polymer modified screen-printed electrode. Biosens Bioelectron 18: 773-780.
- Zhong Z, Li M, Xiang D, Dai N, Qing Y, et al. (2009) Signal amplification of electrochemical immunosensor for the detection of human serum IgG using double-codified nanosilica particles as labels. Biosens Bioelectron 24: 2246-2249.
- Wang Z, Yang Y, Li J, Gong J, Shen G, et al. (2006) Organic-inorganic matrix for electrochemical immunoassay: Detection of human IgG based on ZnO/chitosan composite. Talanta 69: 686-690.
- Marco MP, Gee S, Hammock BD (1995) Immunochemical techniques for environmental analysis I. Immunosensors. TrAC Trends Anal Chem 14: 341-350.
- Jia XC, Raya R, Zhang L, Foord O, Walker WL, et al. (2004) A novel method of Multiplexed Competitive Antibody Binning for the characterization of monoclonal antibodies. J Immunol Methods 288: 91-98.
- Feng CL, Xu YH, Song LM (2000) Study on highly sensitive potentiometric IgG immunosensor. Sens Act B-Chem 66: 190-192.
- Li J, Gao H (2008) A Renewable Potentiometric Immunosensor Based on Fe3O4 Nanoparticles Immobilized Anti-IgG. Electroanalysis 20: 881-887.
- Yamamoto N, Nagasawa Y, Sawai M, Sudo T, Tsubomura H (1978) Potentiometric investigations of antigen-antibody and enzyme-enzyme inhibitor reactions using chemically modified metal electrodes. J Immunol Methods 22: 309-317.
- Keating MY, Rechnitz GA (1984) Potentiometric digoxin antibody measurements with antigen-ionophore based membrane electrodes. Anal Chem 56: 801-806.
- Shi W, Ma Z (2011) A novel label-free amperometric immunosensor for carcinoembryonic antigen based on redox membrane. Biosens Bioelectron 26: 3068-3071.
- Wei Q, Zhao Y, Xu C, Wu D, Cai Y, et al. (2011) Nanoporous gold film based immunosensor for label-free detection of cancer biomarker. Biosens Bioelectron 26: 3714-3718.
- Tian J, Huang J, Zhao Y, Zhao S (2012) Electrochemical immunosensor for prostate-specific antigen using a glassy carbon electrode modified with a nanocomposite containing gold nanoparticles supported with starch-functionalized multi-walled carbon nanotubes. Microchim Acta 178: 81-88.
- Wang R, Chen X, Ma J, Ma Z (2013) Ultrasensitive detection of carcinoembryonic antigen by a simple label-free immunosensor. Sens Act B-Chem 176: 1044-1050.
- Hu C, Yang DP, Xu K, Cao H, Wu B, et al. (2012) Ag@BSA core/shell microspheres as an electrochemical interface for sensitive detection of urinary retinal-binding protein. Anal Chem 84: 10324-10331.
- Fonong T, Rechnitz GA (1984) Homogeneous potentiometric enzyme immunoassay for human immunoglobulin G. Anal Chem 56: 2586-2590.
- French M (1986) Serum IgG subclasses in normal adults. Monogr Allergy 19: 100-107.
- Litwin SD, Zehr BD, Insel RA (1990) Selective concentration of IgD class-specific antibodies in human milk. Clin Exp Immunol 80: 263-267.
- Gasparoni A, Avanzini A, Ravagni Probizer F, Chirico G, Rondini G, et al. (1992) IgG subclasses compared in maternal and cord serum and breast milk. Arch Dis Child 67: 41-43.
- Yap PL, Mirtle CL, Harvie A, McClelland DB (1980) Milk protein concentrations in neonatal milk (witch's milk). Clin Exp Immunol 39: 695-697.
- Mickleson KN, Moriarty KM (1982) Immunoglobulin levels in human colostrum and milk. J Pediatr Gastroenterol Nutr 1: 381-384.
- Ogra SS, Ogra PL (1978) Immunologic aspects of human colostrum and milk. I. Distribution characteristics and concentrations of immunoglobulins at different times after the onset of lactation. J Pediatr 92: 546-549.
- Meulenbroek AJ (1996) Human IgG Subclasses: Useful Diagnostic Markers for Immunocompetence. 2nd Edition, CLB, Plesmanlaan, Amsterdam.
- Stelwagen K, Carpenter E, Haigh B, Hodgkinson A, Wheeler TT (2009) Immune components of bovine colostrum and milk. J Anim Sci 87: 3-9.
- Stoop JW, Zegers BJ, Sander PC, Ballieux RE (1969) Serum immunoglobulin levels in healthy children and adults. Clin Exp Immunol 4: 101-112.
- Guidry J, Butler JE, Pearson RE, Weinland BT (1980) IgA, igG1, IgG2, IgM, and BSA in serum and mammary secretion throughout lactation. Vet Immunol Immunopathol 1: 329-341.
- Lachenauer CS, Baker CJ, Baron MJ, Kasper DL, Gravekamp C, et al. (2002) Quantitative determination of immunoglobulin G specific for group B streptococcal beta C protein in human maternal serum. J Infect Dis 185: 368-374.
- Abernethy G, Otter D, Arnold K, Austad J, Christiansen S, et al. (2010) Determination of immunoglobulin G in bovine colostrum and milk powders, and in dietary supplements of bovine origin by protein G affinity liquid chromatography: collaborative study. J AOAC Int 93: 622-627.
- Holland PT, Cargill A, Selwood AI, Arnold K, Krammer JL, et al. (2011) Determination of soluble immunoglobulin G in bovine colostrum products by protein G affinity chromatography-turbidity correction and method validation. J Agric Food Chem 59: 5248-5258.
- Fahey JL, McKelvey EM (1965) Quantitative Determination of Serum Immunoglobulins in Antibody-Agar Plates. J Immunol 94: 84-90.
- Krüger U, Wickert L, Wagener C (1989) Determination of epitope specificities and affinities of monoclonal antibodies in solution phase using biotin-labeled carcinoembryonic antigen and avidin as precipitating agent. J Immunol Methods 117: 25-32.
- Mi JB, Yan J, Ding XJ, Guo ZQ, Zhao MP, et al. (2007) Production and characterization of monoclonal antibody against recombinant human erythropoietin. Biomed Environ Sci 20: 184-188.
- Li-Chan E, Kummer A, Losso JN, Kitts DD, Nakai S (1995) Stability of bovine immunoglobulins to thermal treatment and processing. Food Res Int 28: 9-16.
- Lawrence RA, Lawrence RM (2011) The Collection and Storage of Human Milk and Human Milk Banking. Breastfeeding (7thedn), chapter 21, 689-717.
- Björkstén B, Burman LG, De Château P, Fredrikzon B, Gothefors L, et al. (1980) Collecting and banking human milk: to heat or not to heat? Br Med J 281: 765-769.
- Gapper LW, Copestake DE, Otter DE, Indyk HE (2007) Analysis of bovine immunoglobulin G in milk, colostrum and dietary supplements: a review. Anal Bioanal Chem 389: 93-109.
- Klobasa F, Goel MC, Werhahn E (1998) Comparison of freezing and lyophilizing for preservation of colostrum as a source of immunoglobulins for calves. J Anim Sci 76: 923-926.
- Fleenor WA, Stott GH (1981) Single radial immunodiffusion analysis for quantitation of colostral immunoglobulin concentration. J Dairy Sci 64: 740-747.
- Koenig A, de Albuquerque Diniz EM, Barbosa SF, Vaz FA (2005) Immunologic factors in human milk: the effects of gestational age and pasteurization. J Hum Lact 21: 439-443.
- Crosson C, Rossi C (2013) Quartz crystal microbalance immunosensor for the quantification of immunoglobulin G in bovine milk. Biosens Bioelectron 42: 453-459.
- Crosson C, Thomas D, Rossi C (2010) Quantification of immunoglobulin g in bovine and caprine milk using a surface plasmon resonance-based immunosensor. J Agric Food Chem 58: 3259-3264.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14650
- [From(publication date):
specialissue-2013 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10077
- PDF downloads : 4573