Review Article Open Access
Neural Cancer Stem Cells: Focusing on Chromosome Ends
Fernando Pires Hartwig*
Postgraduate Program in Epidemiology, Federal University of Pelotas, Oncology Research Group, Technology Development Center (Biotechnology Unit), Federal University of Pelotas, Brazil
- Corresponding Author:
- Fernando Pires Hartwig
Postgraduate Program in Epidemiology
Federal University of Pelotas, Oncology Research Group
Technology Development Center (Biotechnology Unit)
Federal University of Pelotas, Brazil
Tel: (5553)81347172
E-mail: fernandophartwig@gmail.com
Received date: June 10, 2013; Accepted date: June 24, 2013; Published date: June 26, 2013
Citation: Hartwig FP (2013) Neural Cancer Stem Cells: Focusing on Chromosome Ends. J Alzheimers Dis Parkinsonism 3:115. doi: 10.4172/2161-0460.1000115
Copyright: © 2013 Hartwig FP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Telomeres are DNA tandem repeats associated with six proteins located at chromosome ends. Telomere shortening happens after each replication round in the majority of human cells, including adult stem cells, leading to telomere dysfunction and activation of senescence and/or apoptosis. In this commentary, key studies evidencing that telomerase overexpression (which is the most common telomere lengthening mechanism in cancer) confer stem-like properties to neural cells with tumorigenic potential, which is in accordance to previously proposed models of TERT overexpression as a mechanism for the emergence of neural cancer stem cells. In addition, the recent finding that telomerase inhibition is a promising anti-neural cancer stem cell therapy (at least in gliomas) is confronted with other studies, resulting in the identification of potential limitations: the presence of alternative lengthening of telomeres in neural cancer stem cells (already identified in glioma stem cells) and of the possibility that short telomeres is not an universal feature of neural cancer stem cells. Further investigation regarding telomere length measurement in neural cancer stem cells of different origins and development of clinically feasible and applicable methods to distinguish telomerase-positive from ALT-positive neural cancer stem cells are pointed as important perspectives to better understand the potential of the promising telomerase inhibition-based therapy to fight neural cancer stem cells.
Keywords
Telomere; Telomerase; Neural stem cell; Cancer stem cell; Telomerase inhibition
Abbreviations
ASC: Adult Stem Cell; CSC: Cancer Stem Cell; NSC: Neural Stem Cell; NCSC: Neural Cancer Stem Cell; ALT: Alternative Lengthening of Telomeres
Introduction
Telomeres and Telomerase, and Implications for Cancer and CSCs
Telomeres are 5´ TTAGGG 3´ DNA tandem repeats located at the ends of eukaryotic chromosomes, with a structure conformed by at least six associated proteins-commonly referred to as telomere shelterin [1]. Due to the end replication problem (briefly, the incapacity of the DNA replication machinery to replicate the very ends of linear chromosomes) [2], the telomeres are shortened after each cell division. Considering that critical telomere shortening results in the recognition of chromosome ends as DNA damage sites (activating senescence and/or apoptosis responses), telomere length has been considered a key factor in cellular lifespan [3]. Telomere shortening occurs in cells that do not have activity of a ribonucleoprotein complex called telomerase, which promotes telomere lengthening by reverse transcription (catalyzed by the telomere reverse transcriptase subunit, encoded by the TERT gene) based on a RNA template (named telomerase RNA component, encoded by the TERC gene) [4,5]. Physiologically, telomerase is active in cells with a low degree of differentiation, including embryonic stem cells, germline stem cells and adult stem cells (ASCs). In the last, however, telomerase levels are sufficient only to delay telomere shortening, ultimately leading to ASC senescence or death [6,7]. Considering the importance of these cells in organism homeostasis, specifically in tissue self-renewal during life, telomere biology in ASC is considered one of the fundamental aspects of physiological aging in models that consider the interplay among telomerase, tumor suppression and ASC viability [8].
These aspects of telomere biology have also been implicated in disease. There are well characterized syndromes (such as dyskeratosis congenita) caused by mutations in telomere-related genes, resulting in premature telomere shortening and, consequently, premature aging-related phenotypes and impairments characteristic of ASC failure (especially in high-turnover tissues, such as the skin and the blood) [9-11]. In addition, telomere biology has major implications for cancer. Telomerase activity is one of the most prevalent cancer markers, present (at detectable levels) in approximately 85-90% of human cancers [12,13]. This makes telomerase as important targets for anti-cancer therapies that aim to reduced or eliminate replicative immortality (which is considered a cancer hallmark) [14]. Another telomere-related cancer hallmark is genome instability: telomere shortening makes chromosome ends more prone to end-to-end fusions, which favors the accumulation of genomic abnormalities after each cell cycle, in a phenomenon known as breakage-fusion-bridge cycles. In this regard, critical telomere shortening is a common early event in carcinogenesis, which favors the accumulation of mutations and emergence of cells with proliferative advantages over the others, such as having telomerase active [14]. However, it is important to note that, since critical telomere shortening results in tumor suppression responses activation, telomere shortening can also be considered a tumor suppression mechanism that limits the lifespan of a given cell, thus reducing the chances of damage accumulation in a single cell. Whether telomere shortening act as a tumor suppression or a genome instability mechanisms depend on the capacity of sensing critically shortened telomeres, which can have important consequences for the health outcomes of telomere shortening in a given individual (in an over-simplified dichotomy, tumor suppression responses may influence the likelihood of developing cancer or loss of tissue self-renewal capacity [15]).
The roles of telomere biology in cancer have been receiving an extra degree of attention due to the emergence of the cancer stem cell (CSC) theory. According to this model, the capacity of tumor cells to acquire new characteristics (due to genomic instability) may favor the emergence of stem-like cancer cells. These cells are currently suggested to be a key aspect in therapy resistance (because CSCs lie in a protective microenvironment and are quiescent in well-established tumors) and, consequently, tumor recurrence (since CSCs may, when needed to maintain “tumor homeostasis”, proliferate at elevated rates) [16]. These characteristics are typical of ASCs, which depended (among other factors) on telomerase activity/telomere integrity to maintain tissue homeostasis. The importance of telomerase for ASCs and the common features between ASCs and CSCs suggest that telomerase activity is a potentially promising target for anti-cancer therapies also in the context of CSCs, highlighting the importance of understanding the roles of telomere biology in CSCs of different origins.
Roles of telomerase in NCSCs
Considering that telomere dysfunction is a consequence of the end-replication problem, it is a logical notion that telomere-related aging phenotypes are more pronounced in high-turnover tissues. In dyskeratosis congenita, for example, the classical clinical manifestation involves skin-related phenotypes, and the main cause of death is bone marrow failure [17,18]. Interestingly, telomere syndromes can also cause lung disease (where the cell turnover is very slow), mainly when combined with other factors such as cigarette smoking, indicating that telomere dysfunction also affects slow-turnover tissues as a component risk factor for disease [19]. Importantly, the importance of telomere dysfunction has also been observed on other slow-turnover tissues [9]. Indeed, there are key pieces of experimental evidence that points to a role of telomere biology in several organs. First, it is important to consider that ASCs have been identified in both high and slow-turnover tissues in humans and murines [20], indicating that tissue self-renewal is an important homeostasis mechanism. Second, both telomere length and TERT expression can be used to identify primitive cells in a given lineage [8,21]. Combining these evidences, one could hypothesize that telomere dysfunction causes aging-related phenotypes throughout the body by impairing ASCs. This has been investigated in an elegant in vivo strategy, which demonstrated that telomerase reactivation in genetically engineered TERT-deficient mice capable of somatic TERT reactivation eliminates degenerative phenotypes in several organs and tissues. Importantly, telomerase reactivation has also reversed neurodegeneration by restoring neural progenitor cell populations, resulting in alleviation of hyposmia and recovery of innate olfactory avoidance responses [22], thus showing the importance of telomere biology for neural stem cells (NSCs) function.
In fact, the importance of telomere integrity and telomerase activity for NSCs has been investigated in different studies, as previously reviewed [23]. Evidence also supports the notion that telomere biology is an important mechanism of neural cancer stem cells (NCSCs). It has been shown that TERT transduction in fetal neural progenitor cells results in loss of both diploid karyotype and contact inhibition, as well as anchorage independence and capacity to form neuroblastoma-like tumors in vivo, indicating that telomerase activation may favor the accumulation of oncogenic properties in primitive neural cells [24]. TERT transduction has also been shown to reduce the differentiation potential of NTera-2 (NT2) human teratocarcinoma cells following retinoic acid treatment in vitro [25], which is in accordance to the reduction of telomerase activity of NT2 and SK-N-SH neuroblastoma cells upon their differentiation in vitro [26] and loss of telomerase activity in NSCs upon differentiation into astrocytes when cultured with TGF-beta [27]. Moreover, TERT overexpression in P19 embryonal carcinoma cells retain markers of neuroepithelial precursors, do not complete neuronal differentiation and present an extended proliferation capacity [28], which are classical stem-like properties. However, there is evidence that supports that TERT overexpression in neural progenitor cell does not result in cancer [29]. It has been shown that immortalized lineage-restricted neural progenitors from human fetal spinal cord by retrovirus-mediated TERT overexpression cultured with basic fibroblast growth factor have been shown to give rise to functional phenotypically-restricted neural subpopulations, with no evident tumorigenesis [30]. In addition, there are defined protocols for generating lines of immortalized neural progenitor cells, whose neural progeny are phenotypically restricted, post-mitotic and functionally competent [31]. These results indicate that telomerase activity in neural progenitor cells may favor tumorigenesis (including the emergence of stem-like phenotypes, such as reduced differentiation) in cells that have a tumorigenic potential [25], in addition to the use of well-defined protocols that induce differentiation (this has been previously discussed in other contexts [32]).
The evidence discussed above suggests a role for telomerase in the immortalization of neural progenitor cells and in acquiring stemlike characteristics in neural cells with potential tumorigenic capacity, indicating that telomerase activation in neural cancer cells may be an important step for the emergence of NCSCs from a tumor mass. In this regard, it has been shown that TERT and NOTCH1upregulation associates with in vivo (engrafted in the central nervous system of immunosuppressed mice) tumorigenicity of clonally derived neural stem/progenitor cell cultures from the olfactory bulb that presented maintenance of growth factor dependence and multipotentiality at late passages in vitro, although tumor formation happened only in a subset of mice that received cells with these characteristics [33], indicating that telomerase activation may occur during neural tumorigenesis and that such cells are selected over telomerase-negative tumor cell populations. Other studies relating telomerase activity and NCSCs have been reviewed elsewhere [23], resulting in the proposal of a model in that TERT overexpression is required for NCSCs establishment. In addition, this review also suggested telomere as a promising target for anti- NCSCs therapies. However, studies more recent than this publication are of great relevance in this regard. For instance, telomerase inhibition has been shown to be specific to glioma NCSCs (which suffered proliferation arrest, cell maturation, DNA damage, loss of self-renewal and stem cell capabilities), since no telomerase activity was found in NSCs, which also had long telomeres when compared to glioma NCSCs, indicating that the exhaustion of these cells by telomerase inhibition may be an efficient and specific (given the telomerase independency of NSCs) therapeutic approach for neural tumors [34,35].
Telomerase inhibition to exhaust NCSCs: potential limitations and perspectives
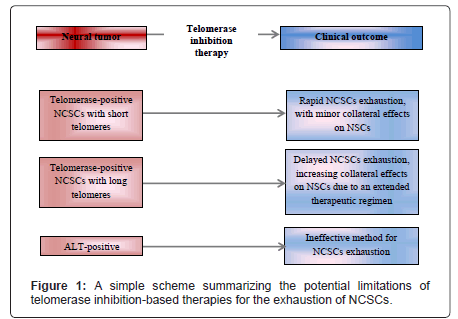
Although inhibiting telomerase as therapeutic approach anti-NCSCs is promising, there are some factors that must be considered, especially given the concern of the implications for cognitive function caused by collateral effects of cancer therapy on neural stem cells expressed in the modern literature [36]. First, it is important to recognize that telomerase activity levels in human adult stem/progenitor cells are sufficient to only delay rather than fully prevent telomere shortening in different stem cells compartments, such as hematopoietic, skin, intestinal crypt and pancreas [37-40], and neural [41]. This may seem unexpected since tissues such as the hematopoietic and the skin are known to be highly dependent on telomerase activity for their long-term homeostasis, which indicates that low levels of telomerase activity may still be of functional importance. However, given the longer telomeres of NSCs [34], it is plausible to assume that a short-term telomerase inhibition might not have significantly detrimental consequences for NSCs. Additionally, telomerase inhibition seems to be among the best alternatives to target telomere biology in glioma NCSCs, and since telomestatin treatment has been reported to have mild negative effects on neural progenitor cells [42]. The two possibly most important limitations of targeting telomerase to fight NCSCs are summarized in two key studies. First, the telomerase-independent recombination-based telomere lengthening mechanism termed alternative lengthening of telomeres (ALT) has been shown to confer to glioma NCSCs the capacity to sustain longterm proliferation and, importantly, that ALT-positive glioma NCSCs are more resistant (both in vitro and in vivo) to ionizing radiation than their telomerase-positive counterparts [43]. Another study showed that a specific subpopulation of adamantinomatouscraniopharyngioma cells presents overexpression of the Wnt/β-catenin pathway, and that such cells share properties with normal pituitary progenitor/stem cells, including relatively long telomeres [44], indicating that stem-like adamantinomatouscraniopharyngioma cells (plausibly CSCs) have long telomeres and, then, this would be a intracranial (although not literally from neuronal origin) tumor resistant to telomerase inhibition.
Since telomerase activity favors the acquisition of stem-like properties in neural cells with tumorigenic potential and is restricted to NCSCs (at least in gliomas) when compared to their normal stem cells counterparts, targeting telomerase as an anti-NCSC therapeutic approach seems to be promising. However (as illustrated in Figure 1), such therapy is likely to be less effective in ALT-positive NCSCs (since they-which have been evidenced to occur-do not depend on telomerase to lengthen their telomeres) and in the possibility that some telomerasepositive NCSCs might have long telomeres (consequently, NCSC telomere exhaustion would require more extended therapies, increasing the risk of side effects in NSCs telomere homeostasis). The notion that efficiency of telomerase inhibition depends on CSC telomere length indicates the importance of determining whether short telomeres is an universal feature of glioma NCSCs, and such investigation is required for other neural tumors, such as medulloblastomas (especially considering that each of these tumors can have different cells of origin, as well as some cells of origin in common [45]). In conclusion, further studies to establish which neural tumors are more likely to have their NCSCs exhausted by telomerase inhibition (telomerase-positive NCSCs with short telomeres) and develop methods clinically feasible and applicable to distinguish telomerase-positive from ALT-positive NCSCs are needed to accurately assess the potential and range of application of telomerase inhibition-based therapies to fight NCSCs and reduce risk of neural tumor recurrence.
References
- de Lange T (2005) Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev 19: 2100-2110.
- Olovnikov AM (1973) A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 41: 181-190.
- Deng Y, Chan SS, Chang S (2008) Telomere dysfunction and tumour suppression: the senescence connection. Nat Rev Cancer 8: 450-458.
- Greider CW, Blackburn EH (1987) The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell 51: 887-898.
- Blackburn EH (2005) Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 579: 859-862.
- Blasco MA (2005) Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet 6: 611-622.
- Flores I, Benetti R, Blasco MA (2006) Telomerase regulation and stem cell behaviour. Curr Opin Cell Biol 18: 254-260.
- Flores I, Blasco MA (2010) The role of telomeres and telomerase in stem cell aging. FEBS Lett 584: 3826-3830.
- Armanios M, Blackburn EH (2012) The telomere syndromes. Nat Rev Genet 13: 693-704.
- Kirwan M, Dokal I (2009) Dyskeratosis congenita, stem cells and telomeres. Biochim Biophys Acta 1792: 371-379.
- Buckingham EM, Klingelhutz AJ (2011) The role of telomeres in the ageing of human skin. Exp Dermatol 20: 297-302.
- Belair CD, Yeager TR, Lopez PM, Reznikoff CA (1997) Telomerase activity: a biomarker of cell proliferation, not malignant transformation. Proc Natl Acad Sci U S A 94: 13677-13682.
- Shay JW, Bacchetti S (1997) A survey of telomerase activity in human cancer. Eur J Cancer 33: 787-791.
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.
- Hartwig FP, Collares T (2013) Telomere dysfunction and tumor suppression responses in dyskeratosis congenita: Balancing cancer and tissue renewal impairment. Ageing Res Rev 12: 642-652.
- Visvader JE, Lindeman GJ (2008) Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 8: 755-768.
- Dokal I (2011) Dyskeratosis congenita. Hematology Am Soc Hematol Educ Program 2011: 480-486.
- Bessler M, Wilson DB, Mason PJ (2010) Dyskeratosis congenita. FEBS Lett 584: 3831-3838.
- Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, et al. (2011) Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med 184: 904-912.
- T├?┬?├?┬írnok A, Ulrich H, Bocsi J (2010) Phenotypes of stem cells from diverse origin. Cytometry A 77: 6-10.
- Breault DT, Min IM, Carlone DL, Farilla LG, Ambruzs DM, et al. (2008) Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci U S A 105: 10420-10425.
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, et al. (2011) Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469: 102-106.
- Rahman R, Heath R, Grundy R (2009) Cellular immortality in brain tumours: an integration of the cancer stem cell paradigm. Biochim Biophys Acta 1792: 280-288.
- Wang Y, Bai Y, Li X, Hu Q, Lin C, et al. (2004) Fetal human neural progenitors can be the target for tumor transformation. Neuroreport 15: 1907-1912.
- Richardson RM, Nguyen B, Holt SE, Broaddus WC, Fillmore HL (2007) Ectopic telomerase expression inhibits neuronal differentiation of NT2 neural progenitor cells. Neurosci Lett 421: 168-172.
- Jain P, Cerone MA, Leblanc AC, Autexier C (2007) Telomerase and neuronal marker status of differentiated NT2 and SK-N-SH human neuronal cells and primary human neurons. J Neurosci Res 85: 83-89.
- Miura T, Katakura Y, Yamamoto K, Uehara N, Tsuchiya T, et al. (2001) Neural stem cells lose telomerase activity upon differentiating into astrocytes. Cytotechnology 36: 137-144.
- Schwob AE, Nguyen LJ, Meiri KF (2008) Immortalization of neural precursors when telomerase is overexpressed in embryonal carcinomas and stem cells. Mol Biol Cell 19: 1548-1560.
- Natesan S (2005) Telomerase extends a helping hand to progenitor cells. Trends Biotechnol 23: 1-3.
- Roy NS, Nakano T, Keyoung HM, Windrem M, Rashbaum WK, et al. (2004) Telomerase immortalization of neuronally restricted progenitor cells derived from the human fetal spinal cord. Nat Biotechnol 22: 297-305.
- Roy NS, Chandler-Militello D, Lu G, Wang S, Goldman SA (2007) Retrovirally mediated telomerase immortalization of neural progenitor cells. Nat Protoc 2: 2815-2825.
- Hartwig FP, Nedel F, Collares TV, Tarquinio SB, N├?┬?├?┬Âr JE, et al. (2012) Telomeres and tissue engineering: the potential roles of TERT in VEGF-mediated angiogenesis. Stem Cell Rev 8: 1275-1281.
- Casalbore P, Budoni M, Ricci-Vitiani L, Cenciarelli C, Petrucci G, et al. (2009) Tumorigenic potential of olfactory bulb-derived human adult neural stem cells associates with activation of TERT and NOTCH1. PLoS One 4: e4434.
- Castelo-Branco P, Zhang C, Lipman T, Fujitani M, Hansford L, et al. (2011) Neural tumor-initiating cells have distinct telomere maintenance and can be safely targeted for telomerase inhibition. Clin Cancer Res 17: 111-121.
- Hjelmeland AB, Rich JN (2011) Molecular targeting of neural cancer stem cells: TTAGGG, you're it! Clin Cancer Res 17: 3-5.
- Gibson E, Monje M (2012) Effect of cancer therapy on neural stem cells: implications for cognitive function. Curr Opin Oncol 24: 672-678.
- Br├?┬?├?┬╝mmendorf TH, Balabanov S (2006) Telomere length dynamics in normal hematopoiesis and in disease states characterized by increased stem cell turnover. Leukemia 20: 1706-1716.
- Hiyama E, Gollahon L, Kataoka T, Kuroi K, Yokoyama T, et al. (1996) Telomerase activity in human breast tumors. J Natl Cancer Inst 88: 116-122.
- Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, et al. (2005) Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells 23: 594-603.
- Sugihara M, Ohshima K, Nakamura H, Suzumiya J, Nakayama Y, et al. (1999) Decreased expression of telomerase-associated RNAs in the proliferation of stem cells in comparison with continuous expression in malignant tumors. Int J Oncol 15: 1075-1080.
- Wright LS, Prowse KR, Wallace K, Linskens MH, Svendsen CN (2006) Human progenitor cells isolated from the developing cortex undergo decreased neurogenesis and eventual senescence following expansion in vitro. Exp Cell Res 312: 2107-2120.
- Miyazaki T, Pan Y, Joshi K, Purohit D, Hu B, et al. (2012) Telomestatin impairs glioma stem cell survival and growth through the disruption of telomeric G-quadruplex and inhibition of the proto-oncogene, c-Myb. Clin Cancer Res 18: 1268-1280.
- Silvestre DC, Pineda JR, Hoffschir F, Studler JM, Mouthon MA, et al. (2011) Alternative lengthening of telomeres in human glioma stem cells. Stem Cells 29: 440-451.
- Andoniadou CL, Gaston-Massuet C, Reddy R, Schneider RP, Blasco MA, et al. (2012) Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol 124: 259-271.
- Visvader JE (2011) Cells of origin in cancer. Nature 469: 314-322.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14316
- [From(publication date):
July-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 9745
- PDF downloads : 4571