Editorial Open Access
Nanovehicles for Intracellular Protein Delivery
Juan L Vivero-Escoto*Department of Chemistry, University of North Carolina at Charlotte, Charlotte, NC 28223, USA The Center for Biomedical Engineering and Science, University of North Carolina at Charlotte, Charlotte, NC 28223, USA
- Corresponding Author:
- Juan L. Vivero-Escoto
Department of Chemistry
University of North Carolina at Charlotte
North Carolina 28223-0001, USA
E-mail: jviveroe@uncc.edu
Received date: December 27, 2012; Accepted date: December 28, 2012; Published date: December 30, 2012
Citation: Vivero-Escoto JL (2013) Nanovehicles for Intracellular Protein Delivery. J Biotechnol Biomater 3:e117. doi:10.4172/2155-952X.1000e117
Copyright: © 2013 Vivero-Escoto JL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Protein therapeutics holds significant promise for improving human health [1,2]. Our organism contains thousands of proteins, which perform essential functions in growth, development and metabolism regulation. Many diseases arise from the alterations in the functions of intracellular proteins [1]. Therefore, the administration of therapeutic proteins has shown great potential in the treatment of many diseases, including cancer and diabetes. Protein therapeutics has emerged since the 1980s and represents currently a significant part of biopharmaceuticals [2]. For example, Lantus®, an engineered protein (insulin) was one of the top ten selling biopharmaceuticals in 2009 [1]. Moreover, protein drugs with much better therapeutic performance are developed every year. The pharmaceutical research and manufacturers of America (PHRMA) listed 78 therapeutic proteins in 813 new biotechnology medicines related to more than 100 diseases in 2011, including virus infectious, cancer and autoimmune diseases [3]. The high intracellular activity and specificity of proteins compared to more conventional, low molecular weight drugs often allows for a better treatment of diseases. Moreover, protein drugs may be safer than gene therapy because no random or permanent genetic changes are involved [4]. Despite their potential medical applications, effective delivery of the proteins to a target site remains a challenge due to rapid clearance from the body. To achieve effective intracellular protein release, delivery platforms that overcome various biological barriers - from the system level, to the organ level, to the cellular level – are needed [4,5]. For example, the protein delivery carrier in the bloodstream needs to avoid kidney filtration, uptake by phagocytes, aggregation with serum proteins, and degradation by endogenous nucleases. Also, the delivery vehicle needs to transport the protein from the bloodstream through the vascular endothelial barriers. Moreover, once the carrier has been uptaken by the targeted cell, it must escape during early stages of the endolysosomal pathway, to avoid degradation by low pH and various hydrolytic enzymes in the lysosomes. Therefore, the clinical success of many therapeutic proteins is intimately dependent on the development of safe and efficient targeted protein delivery technologies.
The application of nanotechnology in the field of drug delivery systems has attracted much attention in the latest decades [6,7]. Recent breakthroughs have demonstrated that nanoparticle-based drug delivery platforms are an excellent alternative for the efficient delivery of, not only low molecular weight therapeutic agents, but for the release of genes and proteins as well [4,5,8,9]. In the case of intracellular protein delivery, these nanovehicles can accomplish critical functions to achieve the optimal therapeutic effect of protein drugs. For example, one of the roles of nanoparticles is to protect proteins from premature degradation within the biological environment. In principle, these nanocarriers can also avoid the uptake by the macrophagocytic system (MPS) by attenuating the receptor-mediated pathway. Moreover, the high surface to volume ratio of nanoparticles also leads to improved pharmacokinetics and biodistribution of the therapeutic proteins. A crucial feature of this delivery approach is the flexibility of tailoring the chemical and physical properties of the nanovehicle through controlled synthesis, assembly and chemical modifications. In summary, nanocarriers show several advantages for the effective and safe intracellular delivery of therapeutic proteins [4,5,9].
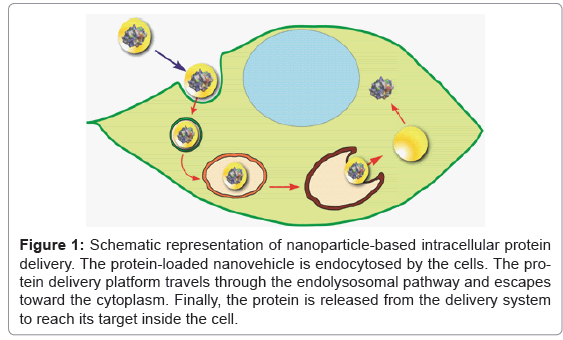
To date, various nanovehicles have been explored to facilitate intracellular delivery of proteins, such as liposomes, polymers, gold nanoparticles, mesoporous silica nanoparticles, quantum dots and carbon nanotubes [4,5,9,10]. Different approaches have been reported on the delivery of protein drugs using polymeric and liposomal carriers [11,12]. Unfortunately, no single formulation has been considered ideal for all types of protein drugs and approaches. Some of the main issues associated with these strategies are protein denaturation and deactivation due to the use of organic solvents to prepare some of the polymeric formulations. Moreover, liposomes usually display low protein loading, have inadequate stability, and exhibit sluggish protein release inside the cells. On the contrary, inorganic nanomaterials offer particular advantages for protein delivery. For example, quantum dots based protein delivery carriers can serve as both delivery and imaging agents. Gold nanoparticles can exhibit hyperthermia properties. However, these inorganic nanomaterials cannot be degraded by intrinsic mechanism in human body, bringing up huge safety concerns for the clinical administration [4]. Mesoporous silica nanoparticles have attracted a great deal of attention in last few decades for their application as delivery systems [10,13]. Mesoporous silica materials can be used as efficient multifunctional nanocarrier for drug delivery, imaging agents, and stimuli-responsive platforms. Nevertheless, the pore size can be a limitation for loading proteins into the preformed mesoporous silica scaffolds [10,14]. New strategies are being explored to expand the scope of mesostructured materials toward protein delivery [15]. Recent breakthroughs in the synthesis of core-shell nanostructured materials have shown their potential application as carriers for imaging agents [16]. We are currently exploring the development of a “ship-in-a-bottle” approach using core-shell nanostructured particles for the intracellular delivery of therapeutic proteins (research in progress). We are expecting to synthesize core-shell nanostructures, with tunable size, pore size and surface chemistry, able to carry and deliver protein drugs in a stimuliresponsive and spatio-temporal fashion. We envision that this strategy will move forward the application of protein therapeutics to clinical settings (Figure 1).
Figure 1: Schematic representation of nanoparticle-based intracellular protein delivery. The protein-loaded nanovehicle is endocytosed by the cells. The protein delivery platform travels through the endolysosomal pathway and escapes toward the cytoplasm. Finally, the protein is released from the delivery system to reach its target inside the cell.
References
- Carter P J (2011) Introduction to current and future protein therapeutics: A protein engineering perspective. Exp Cell Res 317: 1261-1269.
- Leader B, Baca QJ, Golan DE (2008) Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov 7: 21-39.
- (PhRMA), TPR a. M. o. A. s (2011) Biotechnology Medicines in Development, Report.
- Gu Z, Biswas A, Zhao M, Tang Y (2011) Tailoring nanocarriers for intracellular protein delivery. Chem Soc Rev 40: 3638-3655.
- Du J, Jin J, Yan M, Lu Y (2012) Synthetic nanocarriers for intracellular protein delivery. Curr Drug Metab 13: 82-92.
- Petros RA, De Simone JM (2010) Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov 9: 615-627.
- Taylor-Pashow KML, Della Rocca J, Huxford RC, Lin W (2010) Hybrid nanomaterials for biomedical applications. Chem Commun 46: 5832-5849.
- Meares CF, Yokoyama M (2012) Introduction to Gene Silencing and Delivery. Acc Chem Res 45: 959-960.
- Xu D, Hu Z, Su J, Wu F, Yuan W (2012) Micro and nanotechnology for intracellular delivery therapy protein. Nano-Micro Letters 4: 118-123.
- Vivero-Escoto JL, Slowing II, Trewyn BG, Lin VS (2010) Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 6: 1952-1967.
- Lee KY, Yuk SH (2007) Polymeric protein delivery systems. Progress in Polymer Science 32: 669-697.
- Crommelin DJA, Daemen T, Scherphof GL, Vingerhoeds MH, Heeremans JLM, et al. (1997) Liposomes: vehicles for the targeted and controlled delivery of peptides and proteins. Journal of Controlled Release 46: 165-175.
- Slowing II, Vivero-Escoto JL, Wu CW, Lin VS (2008) Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev 60: 1278-1288.
- Hudson S, Cooney J, Magner E (2008) Proteins in mesoporous silicates. Angew Chem Int Ed engl 47: 8582-8594.
- Lim JS, Lee K, Choi JN, Hwang YK, Yun MY et al. (2012) Intracellular protein delivery by hollow mesoporous silica capsules with a large surface hole. Nanotechnology 23: 085101.
- Qiao ZA, Huo Q, Chi M, Veith GM, Binder AJ, Dai S (2012) A "Ship-In-A-Bottle" Approach to Synthesis of Polymer Dots@Silica or Polymer Dots@Carbon Core-Shell Nanospheres. Adv Mater 24: 6017-6021.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 13849
- [From(publication date):
May-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 9266
- PDF downloads : 4583