Research Article Open Access
Mood Symptoms are Related to Psychotic Symptoms in Severe Alzheimer's Disease
Koji Hori1*, Kimiko Konishi2, Hiroi Tomioka1, Masayuki Tani3, Genshin Minegishi4, Hiroaki Tanaka4, Sachiko Yokoyama1, Tomonori Oshio1 and Mitsugu Hachisu51Department of Psychiatry, Showa University Northern Yokohama Hospital, Kanagawa, Japan
2Tokyo Metropolitan Tobu Medical Center for Persons with Developmental/Multiple Disabilities, Tokyo, Japan
3Department of Psychiatry, Showa University Karasuyama Hospital, Tokyo, Japan
4Department of Psychiatry, Showa University Fujigaoka Hospital, Kanagawa, Japan
5Department of Clinical Psychopharmacy, School of Pharmaceutical Sciences, Showa University, Tokyo, Japan
- *Corresponding Author:
- Koji Hori Department of Psychiatry
Showa University Northern Yokohama Hospital
35-1 Chigasakichuo, Tsuzukiku, Yokohama-City
Kanagawa, 224-8503, Japan
Tel: +81-45-949-7000
Fax: +81-45-949-7927
E-mail: kojihori@med.showa-u.ac.jp
Received October 27, 2011; Accepted January 04, 2012; Published January 08, 2012
Citation: Hori K, Konishi K, Tomioka H, Tani M, Minegishi G, et al. (2012) Mood Symptoms are Related to Psychotic Symptoms in Severe Alzheimer’s Disease. J Addict Res Ther S5:002. doi:10.4172/2155-6105.S5-002
Copyright: © 2012 Hori K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
The aim of the present study was to investigate the relationship between cognitive dysfunction and the behavioral and psychological symptoms of dementia (BPSD) in patients with Alzheimer’s disease (AD). The subjects were 79 consecutive AD patients referred because of BPSD. First, we investigated the correlation between cognitive function and the severity of dementia or each symptom domain of BPSD. Then the AD patients were divided into a group with higher performance (n=40, HP group) and a group with lower performance (n=39, LP group). Subsequently, we compared BPSD between these two groups and a factor analysis of BPSD was conducted in each group. We found that disturbance of activity and disturbance of diurnal rhythm were negatively correlated with cognitive function (p<0.05), while affective disturbance was positively correlated with cognitive function (p<0.05). Factor analysis showed that a mood cluster (affective disturbance plus anxieties and phobias) was associated with a psychiatric cluster (paranoid and delusional ideation plus hallucinations) and with aggressiveness in the LP group. These results indicate that disease progress of AD infl uences the BPSD of AD patients in two ways. That is, behavioral symptoms become more severe and the mood cluster conversely becomes milder but is connected to the psychiatric cluster and aggressiveness.
Keywords
Dementia; Alzheimer’s disease; Behavioral and psycho- logical symptoms of dementia; Cognitive dysfunction
Introduction
Th e life expectancy has become longer in developed countries including Japan [1] and the number of patients with dementia, especially Alzheimer’s disease (AD), has increased because aging is the one of the main risk factors for dementia [2]. AD patients develop behavioral and psychological symptoms (BPSD) along with cognitive dysfunction, which increase the burden on their caregivers and lead to early institutionalization [3]. BPSD are thought to be related to various factors, such as cognitive dysfunction, gender, aging, educational level, personality characteristics, and environmental factors [4,5]. Among these factors, cognitive dysfunction is strongly related to BPSD, i.e., disease progress of AD infl uences BPSD. Regarding the relationship of neuropsychiatric symptoms to cognitive dysfunction, there have been two main types of studies. In one type, the prevalence and severity of BPSD have been compared between the mild and severe stages in patients with dementia or AD [6-8]. In the other type, the factor structure of BPSD has been analyzed in all patients [9-12]. However, there have been few investigations into the qualitative diff erences of BPSD between the relatively mild stage and the relatively severe stage of AD. In this study, we therefore investigated the correlation between cognitive function and clinical factors (sex ratio, educational level, age at the onset of dementia, age at the time of testing, severity of dementia, and each symptom domain of BPSD) in patients with AD and we also carried out a factor analysis of BPSD that compared a higher performance group with a lower performance group. Th en we investigated the quantitative and qualitative diff erences of BPSD between these two groups. However, we did not investigate the infl uence of medications in this study because patients with the onset or changes of symptoms aft er starting medication were excluded, since we only enrolled patients who came to our hospital within one year aft er the onset of BPSD and whose symptoms were stable during this period. Moreover, the prescribed medications were not correctly recorded at study entry in many cases.
Materials and Methods
Recruitment
Among the patients consecutively referred to a psychogeriatric clinic at National Shimofusa Hospital (Chiba, Japan) because of BPSD between March 1, 2000 and March 31, 2005, AD was diagnosed in accordance with the criteria developed by a working group of the National Institute of Neurological and Communicative Disorders and Stroke in collaboration with the Alzheimer’s Disease and Related Disorders Association [13]. Patients who were diagnosed as having other disorders such as drug or alcohol abuse, those with a history of cerebral hemorrhage or cerebral infarction, and those with severe physical illnesses were excluded. Patients who were suff ering from severe adverse reactions to drugs (e.g. delirium) were also excluded. Adverse reactions were identifi ed as symptoms that occurred aft er medications were started or changed. One hundred and nine subjects were enrolled in accordance with these criteria.
BPSD can fl uctuate over time, and sometimes may even subside for a long period before returning [8,9]. Th erefore, we selected patients who came to our hospital within one year aft er the onset of BPSD whose symptoms were unchanged during that period. As a result, Therthere were 79 eligible subjects with AD. We did not assess the effects of medications because patients with the onset or changes of symptoms after starting medication were omitted from this study. Moreover, medications were not correctly recorded at study entry in many patients.
Variables investigated
Demographic data, cognitive function, and severity of dementia: We evaluated demographic data (sex ratio, educational level, age at the onset of dementia, age at testing, and severity of dementia), cognitive function, and BPSD at study entry. The severity of dementia was evaluated by Functional Assessment Staging (FAST) [14], which is a tool for measuring the severity of AD on a seven-point scale. Cognitive function was evaluated with the Mini-Mental State Examination (MMSE) [15].
Behavioral and psychological symptoms of dementia: BPSD were assessed by employing the Behavioral Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD) [16], which measures behavioral and psychological symptoms in seven symptom domains (paranoid and delusional ideation, hallucinations, activity disturbances, aggressiveness, diurnal rhythm disturbances, affective disturbance, and anxieties and phobias). The symptoms assessed by BEHAVE-AD are divided into three clusters, which are a psychiatric cluster (paranoid and delusional ideation, and hallucinations), a behavioral cluster (activity disturbances, aggressiveness, and diurnal rhythm disturbances), and a mood cluster (affective disturbance, and anxieties and phobias). We calculated the total score for each symptom domain of BEHAVE-AD.
Statistical analysis
First, we investigated correlations between the MMSE and clinical data (sex ratio, educational level, age at the onset of dementia, age at testing, FAST score, and each symptom domain of BEHAVE-AD) by Spearman’s correlation analysis.
Then we divided the AD patients into two groups, which were a higher performance (HP) group and a lower performance (LP) group. The HP group had an MMSE score of 12 or higher and the LP group had a score below 12 because the median MMSE score for our patients was 12 and this procedure resulted in a similar number of patients in both groups.
Subsequently, we compared demographic data, cognitive function, and each symptom domain of the BEHAVE-AD between these two groups. The sex ratio was compared between the HP and LP groups by using the chi-square test, while the other demographic data, cognitive function, and BPSD were compared with Student’s t-test. In all analyses, p<0.05 was accepted as statistically significant.
Third, we performed factor analysis of BEHAVE-AD items followed by an orthogonal rotational procedure (varimax rotation) to extract meaningful descriptions of BPSD for the HP and LP groups. Factors with eigen values greater than 1.0 were selected. Items with a loading of 0.40 or more were included as there was an inter-relationship among such items.
Data were analyzed by using the statistical software package SPSS-12.0J for Windows (Tokyo, Japan).
Informed consent was obtained from the legal representative or the patients themselves, if possible. This study was approved by the Ethical Committee of National Shimofusa Hospital (Chiba, Japan).
Results
Table 1 shows the mean values for demographic data, MMSE scores, and severity of dementia in all AD patients, the HP group, and the LP group. The sex ratio, educational level, age at onset of dementia, and age at testing were not different between the HP and LP groups. However, the LP group showed lower cognitive function and more severe symptoms than the HP group.
Table 2 shows the correlations identified between the MMSE score and clinical data (sex ratio, educational level, age at onset of dementia, age at testing, FAST score, and each symptom domain of BEHAVE-AD) by Spearman’s correlation analysis. There were negative correlations between the total MMSE score and activity disturbances or diurnal rhythm disturbances, while there was a positive correlation between the total MMSE score and affective disturbance (p<0.05). Accordingly, the FAST score was negatively correlated with the total MMSE score (p<0.0001).
Table 3 shows the scores for each symptom domain of BEHAVE-AD in the HP and LP groups. Scores for activity disturbances and diurnal rhythm disturbances were significantly higher in the LP group than the HP group (p<0.05), while the score for affective disturbance was significantly lower in the LP group than the HP group (p<0.05).
| ALL | HP group | LP group | p value | |
|---|---|---|---|---|
| Number | 79 | 40 | 39 | |
| (Male/female) | (35/44) | (16/24) | (19/20) | 0.9178 |
| Educational level (years) | 9.30 (4.07) | 10.13 (3.61) | 8.46 (4.37) | 0.0693 |
| Age at dementia onset (years) | 76.68 (9.47) | 75.35 (8.47) | 78.05 (10.37) | 0. 2068 |
| Age at testing (years) | 79.85 (9.07) | 78.30 (8.29) | 81.44 (9.65) | 0. 1251 |
| FAST score | 4.99 (1.07) | 4.50 (0.75) | 5.49 (1.12) | <0.0001* |
| MMSE score | 10.87 (7.80) | 17.35 (4.14) | 4.23 (4.24) | - |
Data are given as the mean (S.D.), except for the number of patients.
AD: AlzheimerâÂ?Â?s disease
HP group: AlzheimerâÂ?Â?s disease patients with an MMSE score=12.
LP group: AlzheimerâÂ?Â?s disease patients with an MMSE score<12.
FAST: Functional Assessment Staging
MMSE: Mini-Mental State Examination
The HP and LP groups were compared for sex ratio using the c2 test, while other demographic data, cognitive function, and BPSD were compared between these two groups using StudentâÂ?Â?s t-test.
Table 1: Demographic data of all AD patients, the HP group, and the LP group.
| s value | p value | |
|---|---|---|
| Sex ratio | -0.121 | 0.2866 |
| Educational level | 0.148 | 0.1901 |
| Age at dementia onset | -0.143 | 0.2066 |
| Age at testing | -0.221 | 0.0513 |
| FAST score | -0.588 | <0.0001* |
| Delusions | 0.136 | 0.2280 |
| Hallucinations | 0.111 | 0.3279 |
| Activity disturbance | -0.274 | 0.0157 |
| Aggressiveness | -0.181 | 0.1093 |
| Rhythm disturbance | -0.473 | <0.0001* |
| Affective disturbance | 0.264 | 0.0196* |
| Anxiety | 0.134 | 0.2262 |
AD: AlzheimerâÂ?Â?s disease
MMSE: Mini-Mental State Examination
FAST: Functional Assessment Staging
BEHAVE-AD: behavioral pathology in AlzheimerâÂ?Â?s disease rating scale
delusions: paranoid and delusional ideation, rhythm disturbance: diurnal rhythm disturbances, anxiety: anxieties and phobias
p<0.05*.
Table 2: Correlations between the MMSE score and clinical variables (demographic data and each BEHAVE-AD domain).
The results of factor analysis of BPSD are shown in Table 4 for the HP group and in Table 5 for the LP group. In the HP group, these factors explained 75.2% of the variance. Factor 1 represented “disruptive behaviors” (explaining 30.4% of the variance), which included activity disturbances, aggressiveness, and diurnal rhythm disturbances. Factor 2 represented “depression” (explaining 26.6%), including affective disturbances as well as anxieties and phobias. Factor 3 represented “psychosis” (explaining 18.2%), including paranoid and delusional ideation as well as hallucinations. Thus, the mood cluster, behavioral cluster, and psychiatric cluster were clearly separated in the HP group.
In the LP group, three meaningful factors were also extracted, but these were different from those in the HP group. These three factors explained 70.0% of the variance. Factor 1 represented “psychotic depression” (explaining 33.6%), which included paranoid and delusional ideation, hallucinations, aggressiveness, affective disturbances, and anxieties and phobias. Factor 2 represented “disruptive behaviors” (explaining 20.7%), including activity disturbances and aggressiveness. Factor 3 represented “diurnal rhythm disturbances” (explaining 15.7%) and only included diurnal rhythm disturbances. In the LP group, the mood cluster was connected with the psychiatric cluster and aggressiveness.
| LP group | HP group | p value | |
|---|---|---|---|
| Delusions | 1.83 (2.00) | 1.54 (2.39) | 0.5648 |
| Hallucinations | 1.00 (1.11) | 0.85 (1.20) | 0.5563 |
| Activity disturbance | 3.69 (2.57)* | 2.45 (2.32) | 0.0268 |
| Aggressiveness | 1.65 (1.93) | 2.49 (2.32) | 0.0897 |
| Rhythm disturbance | 1.62 (0.71)* | 0.90 (0.87) | 0.0001 |
| Affective disturbance | 0.67 (1.08) | 1.23 (1.17)* | 0.0306 |
| Anxiety | 0.94 (1.35) | 1.65 (2.06) | 0.0891 |
Data are given as the mean (SD)
HP group: AlzheimerâÂ?Â?s disease patients whose MMSE scores were =12.
LP group: AlzheimerâÂ?Â?s disease patients whose MMSE scores were <12.
AD: AlzheimerâÂ?Â?s disease
BEHAVE-AD: behavioral pathology in AlzheimerâÂ?Â?s disease rating scale
delusions: paranoid and delusional ideation, rhythm disturbance: diurnal rhythm disturbances, anxiety: anxieties and phobias
StudentâÂ?Â?s t-test: p<0.05*.
Table 3: Mean total scores for each BEHAVE-AD symptom domain in the HP and LP groups.
| Factor % variance |
1 | 2 | 3 |
|---|---|---|---|
| Disruptive behavior |
Depression | Psychosis | |
| 30.4% | 26.6% | 18.2% | |
| Delusions | -.073 | -.069 | #.826 |
| Hallucinations | .188 | .117 | #.852 |
| Activity disturbance | #.749 | -.301 | -.217 |
| Aggressiveness | #.854 | -.013 | .052 |
| Rhythm disturbance | #.733 | .085 | .060 |
| Affective disturbance | .003 | #.930 | .060 |
| Anxiety | -.133 | #.897 | .002 |
HP group: AlzheimerâÂ?Â?s disease patients whose MMSE scores were =12.
AD: AlzheimerâÂ?Â?s disease
BEHAVE-AD: behavioral pathology in AlzheimerâÂ?Â?s disease rating scale
delusions: paranoid and delusional ideation, rhythm disturbance: diurnal rhythm disturbance, anxiety: anxieties and phobias
#: symptoms with a factor loading=.400.
Table 4: Results of varimax rotation factor analysis of BEHAVE-AD items in the HP group.
| Factor % variance |
1 | 2 | 3 |
|---|---|---|---|
| Psychotic depression |
Disruptive behaviors |
Rhythm disturbance |
|
| 33.6% | 20.7% | 15.7% | |
| Delusions | #.812 | .223 | -.078 |
| Hallucinations | #.728 | .007 | .177 |
| Activity disturbance | -.108 | #.889 | -.120 |
| Aggressiveness | #.524 | #.665 | 268 |
| Rhythm disturbance | -.003 | -.005 | .940 |
| Affective disturbance | #.494 | -.002 | .370 |
| Anxiety | #.739 | -.412 | -.193 |
LP group: AlzheimerâÂ?Â?s disease patients whose MMSE scores were <12.
AD: AlzheimerâÂ?Â?s disease
BEHAVE-AD: behavioral pathology in AlzheimerâÂ?Â?s disease rating scale
delusions: paranoid and delusional ideation, rhythm disturbance: diurnal rhythm disturbances, anxiety: anxieties and phobias
#: symptoms with a factor loading=.400
.
Table 5: Results of varimax rotation factor analysis of BEHAVE-AD items in the LP group.
Discussion
In the present study of AD patients, we identified differences of BPSD between the HP group and the LP group.
We obtained a negative correlation between the total MMSE score and activity or diurnal rhythm disturbances, as well as a positive correlation between the total MMSE score and affective disturbance. Although there were no significant differences between the HP group and the LP group with respect to demographic data (sex ratio, educational level, age at the onset of dementia, and age at testing), the total scores for the BEHAVE-AD symptom domains of activity disturbances and diurnal rhythm disturbances were significantly higher (p<0.05) in the LP group than the HP group, and the score for affective disturbance was significantly lower (p<0.05) in the LP group. These results were compatible with previous reports. Teri et al. [6], Hope et al. [7], and Nagaratnam et al. [8] reported similar findings, with more frequent abnormal behavior being observed in patients who had severe dementia and depression being mainly noted at the mild stage. Such findings have been interpreted as follows: severe cognitive dysfunction reduces the recognition of a person’s own situation, so patients with severe AD tend to be less depressed.
It has been reported that the main factors of BPSD are “apathy”, “depression”, “psychosis”, and “motor agitation” according to factor analysis of patients with dementia or patients with AD [9-12]. These symptoms were independent of each other in previous studies, as was found for the HP group in our study, though Mirakhur et al. included irritability and/or agitation together with depression [12]. The mean MMSE scores of the patients studied by other authors were relatively high and were close to the scores of the patients in our HP group. Therefore, the results we obtained by factor analysis of BPSD in the HP group were also similar to those reported before. There have been no reports about factor analysis of BPSD at the severe stage of AD, to our knowledge, so this may be the first comparison of a factor analysis of BPSD between the severe and mild stages of AD. In the mild stage of AD, we found that the psychiatric cluster, behavioral cluster, and mood cluster of BPSD were independent, while the clusters were connected to each other after AD became severe. When the mood cluster was connected to the psychiatric cluster and aggressiveness, it became milder in the severe stage of AD, indicating that not only cognitive function but also BPSD are influenced by progression of AD [17,18].
These results suggest that disease progress of AD influences the BPSD of patients with AD in two ways, which are that behavioral symptoms become quantitatively more severe, and that while there is also a qualitative change, the mood cluster becomes connected to the psychiatric cluster and aggressiveness.
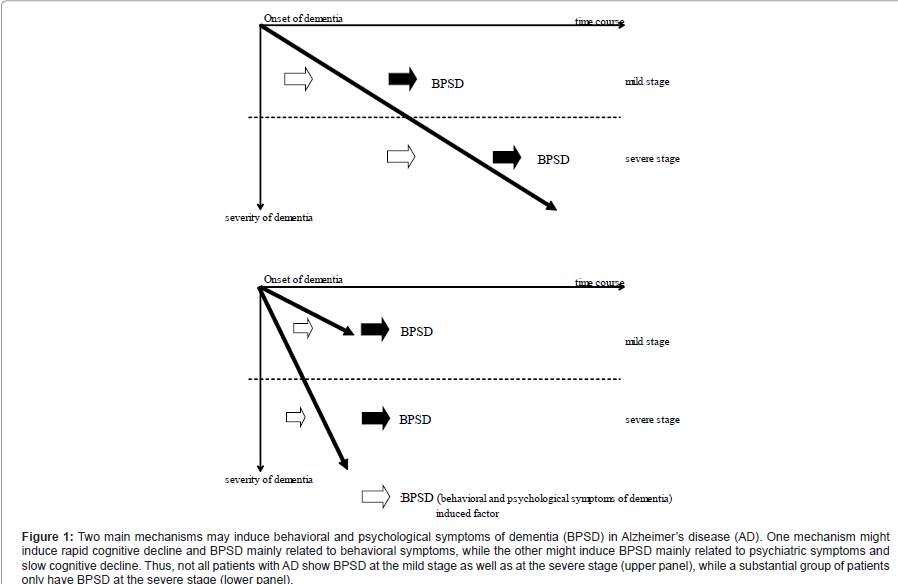
It is interesting that there were no differences of the age at the onset of dementia or age at testing between the LP group and the HP group. This means that the duration of AD was not a determinant of the performance of our patients. At least two possible explanations for this finding can be suggested. First, there may be two different causes of BPSD in AD patients. Bidzan et al. suggested that BPSD in AD patients are differently influenced by the progression of dementia and by cognitive dysfunction, and that aggressive behavior is associated with the degree of cognitive impairment [19]. Ito et al. reported that there are two independent pathophysiological processes contributing to BPSD in AD, one of which is associated with behavioral symptoms and overlaps with cognitive dysfunction, while the other is associated with psychological symptoms and is not related to cognitive dysfunction [20]. In our study, we detected two distinct psychotic factors and one behavioral factor in the HP group, whereas there was overlap of psychotic and behavioral factors in the LP group. In the LP group, the first factor consisted of paranoid and delusional ideation, hallucinations, aggressiveness, affective disturbances, and anxieties and phobias. From the observations of Bidzam et al. and Ito et al., as well as our own findings, we speculate that two main mechanisms may induce BPSD in AD patients, with one causing behavioral symptoms accompanied by rapid cognitive decline and the other causing psychotic symptoms along with slow cognitive decline. The patients in our LP group had worse symptoms of dementia and showed more advanced BPSD within one year of the onset of behavioral symptoms. Therefore, AD patients may develop BPSD at either the mild stage or the severe stage of cognitive dysfunction, and there is a subset of AD patients who show rapid decline of cognitive function and possibly only manifest BPSD when there is a severe decrease of their cognitive function (Figure 1). The other possibility is that the age at onset of dementia was not well defined and the accuracy of identifying the “onset” might have differed between the LP group and the HP group. The caregivers in the LP group noted that cognitive disturbance was prominent and that the patients generally did not recognize when they began to lose their memory. On the contrary, the caregivers in the HP group stated that patients noticed when their memory loss first occurred.
We previously investigated the effect of aging on BPSD in patients with AD and found that it also had two different influences, which were disturbance of the sleep-waking cycle and connection of the mood cluster to the psychiatric cluster and aggressiveness [21]. Based on the results of the present study and our previous work, there are similarities and differences between the effect of aging and the course of AD. Although there is an influence of disease progress on BPSD in AD patients just as there is an influence of aging, these two factors (aging and disease progress) affect BPSD in a different manner. Therefore, progression of AD occurs via a different process from that associated with aging. Th e similarity of the eff ect of disease progress on BPSD in AD and the eff ect of aging is that the mood cluster becomes connected to the psychiatric cluster and aggressiveness. We refer to this as “implicit depression and explicit psychosis”. However, activity disturbances are more easily infl uenced by disease progress. On the contrary, aging only has an infl uence on diurnal rhythm disturbances. Th us, disease progress and aging both aff ect BPSD, but these two factors have a diff erent infl uence.
Figure 1:Two main mechanisms may induce behavioral and psychological symptoms of dementia (BPSD) in AlzheimerâÂ?Â?s disease (AD). One mechanism might induce rapid cognitive decline and BPSD mainly related to behavioral symptoms, while the other might induce BPSD mainly related to psychiatric symptoms and slow cognitive decline. Thus, not all patients with AD show BPSD at the mild stage as well as at the severe stage (upper panel), while a substantial group of patients only have BPSD at the severe stage (lower panel).
Th ere were several limitations of the present study, including a small sample size, lack of a control group, and its cross-sectional design that meant not observing the longitudinal course of cognitive dysfunction. Moreover, MMSE is simple but not thorough and refi ned tool measuring cognitive function. Th erefore, we should have better to investigate various kinds of cognitive function. However, it is diffi cult to perform complex cognitive tests for elderly demented patients, especially severe demented patients. Further investigations will be required to fully delineate the relationship between cognitive dysfunction and the features of BPSD in AD patients, with the aim of developing better interventions (medical and non-medical) for BPSD associated with AD.
References
- Helmer C, Pérès K, Letenneur L, Guttiérez-z-Robledo LM, Ramaroson H, et al. (2006) Dementia in subjects aged 75 years or over with in the PAQUID cohort: prevalence and burden by severity. Dement Geriatr Cogn Disord 22: 87-94.
- Jorm AF, Korten AE, Henderson AS (1987) The prevalence of dementia a quantitative integration of the literature. Acta Psychiatr Scand 76: 465-479.
- Baumgarten M, Becker RJ, Gauther S (1990) Validity and reliability of the dementia behavior disturbance scale. J Am Geriatr Soc 38: 221-226.
- Finkel SI, Costa e Silva J, Cohen G, Miller S, Sartorius N (1996) Behavioral and Psychological Signs and Symptoms of Dementia: a consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr 8: 497-500.
- Shinosaki K, Nishikawa T, Takeda M (2000) Neurobiological basis of behavioral and psychological symptoms of dementia of the Alzheimer type. Psychiatr Clin Neurosci 254: 611-620.
- Teri L, Larson EB, Reifler BV (1988) Behavioral disturbance in dementia of the Alzheimer’s type. J Am Geriatr Soc 36: 1-6.
- Hope T, Keene J, Gedling K, Cooper S, Fairburn C, et al. (1997) Behavior changes in the dementia 1: point of entry data a prospective study. Int J Geriatr Psychiatry 12: 1062-1073.
- Nagaratnam N, Lewis-Jones M, Scott D, Palazzi L (1998) Behavioral and psychiatric manifestations in dementia patients in a community: caregiver burden and outcome. Alzheimer Dis Assoc Disord 12: 330-334.
- Frisoni GB, Rozzini L, Gozzetti A, Binetti G, Zanetti O, et al. (1999) Behavioral symptoms in Alzheimer’s disease: description and correlates. Dement Geriatr Cogn Disord 10: 130-138.
- Matsuoka K, Miyamoto Y, Ito H, Kurita H (2003) Relationship between behavioral disturbances and characteristics of patients in special for dementia. Psychiatr Clin Neurosci 57: 567-574.
- Aalten P, de Vugt ME, Lousberg R, Korten E, Jaspers N, et al. (2003) Behavioral problems in dementia: a factor analysis of the Neuropsychiatric Inventory. Dement Geriatr Cogn Disor 15: 99-105.
- Mirakhur A, Craig D, Hart DJ, McLlroy SP, Passmore AP (2004) Behavioral and psychological syndromes in Alzheimer’s disease. Int J Geriatr Psychiatry 19: 1035-1039.
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, et al. (1984) Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force in Alzheimer’s disease. Neurology 56: 939-944.
- Reisberg B (1988) Functional assessment staging (FAST). Psychopharmacol Bull 24: 653-659.
- Folstein MF, Folstein SE, McHugh RP (1975) “Mini-Mental State.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12: 95-112.
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, et al. (1987) Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry 48: 9-15.
- Levy ML, Cummings JL, Fairbanks LA, Bravi D, Calvani M, et al. (1996) Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer’s disease. Am J Psychiatry 153: 1438-1443.
- Chen P, Ganguli M, Mulsant BH, DeKosky ST (1999) The temporal relationship between depressive symptoms and dementia. Arch Gen Psychiatry 56: 261-266.
- Bidzan L, Pachalska M, Grochmal-Bach B, Bidzan M, Jastrzebowska G (2008) Behavioral and psychological symptoms of dementia of the Alzheimer type in nursing home residents. Med Sci Monit 14: CR559-CR567.
- Ito T, Meguro K, Akanuma K, Meguro M, Lee E, et al. (2007) Behavioral and psychological symptoms assessed with the BEHAVE-AD-FW are differently associated with cognitive dysfunction in Alzheimer’s disease. J Clin Neurosci 142: 850-855.
- Konishi K, Hori K, Oda T, Tominaga I, Asaoka T , et al. (2009) Effects of aging on behavioral symptoms in Alzheimer’s disease. Psychogeriatrics 9: 11-16.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14661
- [From(publication date):
specialissue-2013 - Apr 20, 2025] - Breakdown by view type
- HTML page views : 10089
- PDF downloads : 4572