Research Article Open Access
Midbrain Neural Stem Cells Show Unique Cell Survival, Neuronal Commitment and Neurotrophic Properties with Therapeutic Potential for Parkinson's Disease
Yin-Xiu Ding1, Li-Chun Wei2*, Yong-Hong Liu3, Li Duan1, Xi-Ying Jiao1, Yi Xia1and Liang-Wei Chen1*
1Institute of Neurosciences, The Fourth Military Medical University, Xi’an, 710032, China
2Department of Radiation Oncology, Xijing Hospital, The Fourth Military Medical University, China
3Department of Neurology, Xijing Hospital, The Fourth Military Medical University, Xi’an, China
- Corresponding Authors:
- Liang-Wei Chen
Institute of Neurosciences
The Fourth Military Medical University
Xi’an, 710032, China
Tel: +86-29-84776840
Fax: +86-29-3246270
E-mail: lwchen@fmmu.edu.cn - Li-Chun Wei
Department of Radiation Oncology
Xijing Hospital
The Fourth Military Medical University, China
E-mail: weilichun@fmmu.edu.cn
Received date: November 18, 2011; Accepted date: February 14, 2012; Published date: February 17, 2012
Citation: Ding YX, Wei LC, Liu YH, Duan L, Jiao XY, et al. (2012) Midbrain Neural Stem Cells Show Unique Cell Survival, Neuronal Commitment and Neurotrophic Properties with Therapeutic Potential for Parkinson’s Disease. J Alzheimers Dis S10:001. doi:10.4172/2161-0460.S10-001
Copyright: © 2012 Ding YX, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Parkinson’s disease (PD) is one severe debilitating neurological disease that results from massive and progressive degenerative loss of dopaminergic neurons in the substantial nigra, and new cell therapy appeal hopeful functional recovery of injured dopaminergic neuronal system and cure of PD. We are interested in therapeutic potential of neural stem cell in transplantation treatment against PD, the midbrain-derived neural stem cells (mNSCs) were studied by in vitro culture in focusing on their proliferative, differentiation and neurotrophic properties and in comparing with hippocampus-derived NSCs (hNSCs). The results revealed that: 1) The mNSCs showed lower BrdU incorporation ratio or lower proliferative rate than that of hippocampus-derived ones but had higher cell survival capacity in serum-free culture; 2) The mNSCs exhibited similar Tuj-1+ immature neuronal differentiation, but higher Nurr1+ and tyrosine hydroxylase (TH)+ cell fate commitment in comparison with that of hNSCs in at d1-d7 differentiation culture; 3) The mNSCs expressed several neurotrophic factors, i.e. brainderived neurotrophic factor(BDNF), glial-derived neurotrophic factor (GDNF), cerebral dopamine neurotrophic factor (CDNF) and DJ-1, that actively function in dopaminergic neuronal maintenance or neuroprotection, with their slightly different or similar levels in comparison to that of hNSCs. Taken together, this study has provided new evidence that mNSCs show the unique cell proliferation, cell survival, dopaminergic neuronal differentiation and neurotrophic properties, suggesting that midbrain-derived NSCs may present an ideal cell source or reliable tissue candidate for therapeutic cell transplantation or neuroprotective treatment of PD in human beings.
Keywords
Neural stem cell; Neurotrophic factors; Neuroprotection; Cell therapy; Parkinson’s disease
Abbreviations
BDNF: Brain-derived Neurotrophic Factor; CDNF: Cerebral Dopamine Neurotrophic Factor; GDNF: Glial-derived Neurotrophic Factor; NSC: Neural Stem Cells; PD: Parkinson’s Disease
Introduction
Parkinson’s disease (PD) is a common, debilitating, neurological disease in humans that results from massive and progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta. It is characterized by severe motor symptoms of tremor, bradykinesia, rigidity and postural instability, and pharmacological intervention such as levodopa to elevate dopamine level can alleviate the patient symptoms but cannot stop progression of the disease [1]. Neuroprotection of dopaminergic neurons from the progressive death and cell replacement therapy are hopeful strategies in treatment of PD [2,3]. It is well documented that various neurotrophic factors play important roles in developing and adult brain by supporting neuronal survival, neurite outgrowth and regeneration [4,5]. These neurotrophic factors, i.e. brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), cerebral dopamine neurotrophic factor (CDNF) and DJ-1 are actively involved in protection of adult catecholaminergic or dopaminergic neurons in mammalian brains, while down-regulation of their expression occurred in the aging brains and PD condition [6-9]. Increasing evidence also suggested deficiency in neurotrophic factor production or abnormality in neurotrophic support might constitute one prominent cause in degeneration of dopaminergic neurons, pathogenesis and disease progression of PD in human beings [10].
Moreover, the neural stem cells (NSCs) or dopaminergic progenitors are potently proposed for cell therapeutic application against PD, based on their potential neurotrophic and neuronal fate commitment properties [2,3,11]. In our previous studies, the neurotrophic factors BDNF and GDNF were identified in nestin-expressing neural progenitor cells in animal models of PD [12,13], and their binding receptors Trk B and tyrosine kinase product of c-Ret were also observed in the nigral neurons of normal and injury state [14,15]. Besides, PD animal studies showed that external administration of neurotrophic factors exhibited certain effect in prevention of dopaminergic neuronal degeneration and improvement of animal behavioral recovery [16,17]. These studies strongly suggest that a continual supplement of NSCderived neurotrophic factors may rescue dopaminergic neurons and show therapeutic outcome by halting or reversing PD pathological progression resulted from reduced neurotrophic support or neurotrophin deficiency [5]. The adult NSCs and induced pluoripotent stem cells-derived functional dopaminergic neurons shall further appeal a therapeutic recruitment of injured nigrostriatal dopaminergic neural system [18-20]. Interestingly, NSCs or dopaminergic progenitors were identified in brain regions such as subventricular zone (SVZ), hippocampus and midbrains of adults, and these NSCs in SVZ and hippocampus were defined for the neuronal differentiation and plasticity functions [21-26]. However, biological properties of midbrain-derived NSCs were largely unrevealed regarding their therapeutic potential for the neurodegenerative diseases, in particular PD. In present study, the unique properties of mouse midbrain-derived NSCs in cell proliferation, cell survival, neuronal cell commitment, neurotrophic factor generation and migration abilities were studied in comparison with hippocampus-derived NSCs and by in vitro cell culture of neurospheres, Bromodeoxyuridine (BrdU) incorporation, Flowcytometry, Western blotting, immunocytochemistry and laser scanning confocal microscopy.
Materials and Methods
Animals
A total of twenty newborn Kunming mice were used in the present study and supplied from the Animal Center of the Fourth Military Medical University of China. All animal experiments followed were carried out in according with the National Institute of Health guide for the care and use of Laboratory animals (NIH Publications No. 80-23) revised 1996, approved by the Committee of Animal Use for Research and Education of the Fourth Military Medical University, and all efforts were made to minimize animal suffering and reduce the number of animals used.
Cell culture
Neural stem cells were isolated from the midbrain and hippocampus of newborn mice at postnatal 0-3 days in ice-cold D1-hanks buffers. The midbrains and hippocampus were respectively dissected and chopped into small pieces and fresh tissue was incubated with 1-2ml accutase (Gibco, A11105-01) for 10min at 37°C. After gentle repeated beating with a polished Pasteur pipette, cell suspension was centrifugated for 5 min at 1000r. The supernatant was abandoned, cell pellet was re-suspended and seeded in cell density 0.5-1×106 in T25 tissue culture flasks (Corning) in the DMEM/F12 medium (Gibco, 11330- 032) supplemented with 2% B27 (Invitrogen), 20ng/ml recombinant human epidermal growth factor (EGF, Peprotech, 450-02), 20ng/ ml basic fibroblast growth factor (bFGF, Peprotech, 100-18B) After culture in a humidified 5% CO2/95% air incubator at 37°C about 5-7 days, the cells gradually grew in the floating neurospheres. They were dissociated using accutase digestion and subjected to 2-3 passages each about 3-5 days. These neurospheres in proper uniform size within 3-6 passages were used for analysis of cell proliferation, differentiation and neurotrophic properties.
BrdU incorporation
For detecting cell proliferative activity of neural stem cells, BrdU incorporation and visualization were performed in cultured neurospheres. The BrdU (Sigma, St Louis, MO) dissolved in culture medium was added at 10μM and continued incubation for 24hs. The neurospheres were seeded on coverslips coated with poly-L-lysine (25μg/ml, P6282, Sigma) in 24-well plate and incubation for 4hs in a humidified incubator with 5% CO2/95% air at 37°C. Cell culture of neurospheres was terminated by fixation with 4% paraformaldehyde for 40 min. After complete rinse with PBS, neurospheres was pretreated with 2mol/L HCl for 1h at 37°C and boric acid buffer (pH 8.5) for 10min at room temperature. BrdU-incorporated cells were visualized by immunostaining protocol. Briefly, cells were incubated with blocking solution containing 10% donkey serum and 0.3%Triton X-100 for 30min, sequentially incubated with primary antibody mixture of mouse anti-BrdU (1:200, Chemicon, MAB3262F) and rabbit anti-nestin (1:100, N5413, sigma) for 24h at 4°C, and secondary antibody mixture of fluorescein conjugated donkey anti-mouse IgG and rodamine conjugated donkey anti-rabbit IgG for 4hs at room temperature. After three rinses in PBS, coverslips were mounted with Fluorescence-preserving VECTASHIELD Mounting medium (Vector, H-1000) and examined under a laser scanning confocal microscope (LSCM, Olympus,FV-1000). The interested images were captured at optical slice of 0.134 μm thickness for further analysis and demonstration.
Cell differentiation
For identifying multiple differentiation capacity of neural stem cells, these neurospheres were collected, seeded on poly-L-lysine coated coverslips in 24 well plates or directly in the 24 well plates, and allowed to grow in DMEM/F12 medium supplemented with 10% fetal bovine serum (FBS, Gibco, 10099141) for cell differentiation. During prolonging culture period of d1-d7, increasing cells grew larger in size and many cells migrated from cultured neurospheres. At timepoints of d3 and d7, the cultured cells were terminated and subjected to immunostaining of nestin (a neural stem cell marker), Tuj-1 (an immature neuronal marker), GFAP (an astrocyte marker) and CNPase (an oligodendrocyte marker) for demonstrating cell differentiation abilities. The cells directly seeded and grew in 24 wells for 3 days were collected in fresh tissue for flowcytometry analysis.
Immunocytochemistry and confocal imaging
Immunofluorescent staining was performed to demonstrate various cell marker and neurotrophic factors in these neurospheres or migrating cells. After growing for 3-7days on glass coverslips, the cells were fixed and processed for immunocytochemical staining procedure. Briefly, the cells were incubated with 10% donkey serum-containing blocking solution for 30min at room temperature, and followed by incubation of primary antibody solution containing 10% donkey serum, 0.3% triton X-100 in PBS at 4°C for 24h, i.e. rabbit anti-BDNF (1:100, sc-20981, Santa Cruz), rabbit anti GDNF(D-20)(1:400, sc-328, Santa Cruz), rabbit anti CDNF (1:400, PRS4343, Sigma), rabbit anti DJ-1 (1:800, P9373, Sigma), rabbit anti Nurr1 (1:1000, AB5778, Chemicon), rabbit anti Tuj-1(1:500,T2200, Sigma), mouse anti tyrosine hydroxylase (1:40000, T1299, Sigma), mouse anti GFAP (1:4000, ), rabbit anti- GFAP (1:4000), rabbit anti-CNPase (Sigma, C9743, 1:500 dilution), or rabbit anti Doublecortion (DCX, 1:800, D9693, sigma) respectively. After three washes with PBS, the cells were further incubated with Alexa Fluor-488, or Alexa Fluor-594 conjugated donkey anti-mouse, rabbit or goat IgG (1:500, Molecular Probes) for 4hs at room temperature. After Hoechst nuclear counterstaining, the coverslips were mounted with Fluorescence-preserving VECTASHIELD Mounting medium (Vector, H-1000) and examined under LSCM. In addition, for immunostaining control experiments, primary antibody was substituted with normal mouse serum, or rabbit serum, or goat serum. The immunoreactive or positive cells were not found in these control staining samples (data not shown). In addition, the specificity of antibodies used was also indicated in the manufacture’s data-sheet.
Western blotting
Western blotting was performed to quantify protein expression levels of neurotrophic factors in cell culture in a standard protocol. Briefly, protein extracts were prepared from the culture cells of midbrainand hippocampus-derived NSCs. Fresh samples were homogenized at 4°C in 5 volumes of extraction buffer, and the centrifugation of homogenates for 10 min (12000g) was performed. After measurement of total protein amount, supernatant was mixed with four volumes of protein loading buffer, boiled for 5min at 99°C and stored at 4°C. Total protein (20-30μg per loading passage) was loaded for electrophoresis on 10% denaturing PAGE gels and transferred to the nitrocellulose membrane (Bio-Rad). This membrane were blocked with 5% skimmed milk in Tris-buffered saline containing 0.05% Tween 20, followed with BDNF, GDNF, CDNF and DJ-1 primary and secondary antibody incubation and final visualization of bands (Bio-Rad). By using β-actin as internal control, the quantitative analysis of immunoblotting bands was carried out and ratio data was presented.
Flowcytometry
Flowcytometry was performed to detect cell viability and cell cycle of NSCs in standard protocol. Cell samples were collected and centrifuged for 5min and supernatants were discarded. Cell pellets were suspended and incubated with 1-2ml accutase for 10 min at 37°C. After repeated beating, cell suspension was re-centrifugated for 5 min at 1000r, and supernatant was abandoned. The cells were re-suspended in 1ml PBS supplemented with 2ml dehydrated alcohol, shaken for a moment, sealed and kept at 4°C overnight. The midbrain-derived NSCs (mNSCs) and hippocampus-derived NSCs (hNSCs) were measured under flowcytometer for cell survival, necrosis and apoptosis.
Statistic analysis
For quantitative data analysis, BrdU, Tuj-1, TH, Nurr-1, GFAP, BDNF, GDNF, CDNF, DJ-1, and DCX-positive cells were counted in unit field of coverslips with growing mNSCs and hNSCs. The positive cell numbers, percentages of total Hoechst-stained cells, and density of immunoblotting bands in ratio were given as mean±S.E.M. (n=3, independent experiments). The differences between means were analyzed by one-way ANOVA (SPSS 18.0) with different cell markers as independent factors. When ANOVA test showed significant difference among means, the pair-wise comparisons between means were also performed by post hoc testing if required. The significance level was set at a P value of 0.05 for all analyses.
Results
Identification of mNSCs and neurospheres in cell culture
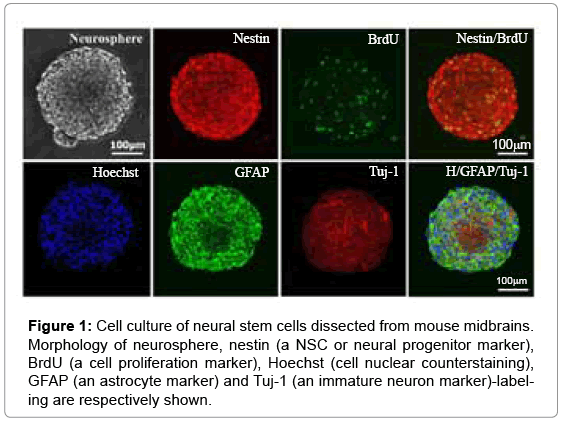
Many typical neurospheres appeared in cell culture of serumfree neurobasal medium for 5-7days and grew in uniform mediumsized ones with hundred of cells inside after 2-3 passages. After that, these neurospheres were seeded on glass coverslips and processed for detecting cell proliferation and multiple differentiation abilities. The BrdU incorporation experiment showed that in a population of mNSCs exhibited BrdU+ labeling or cell division sate. The neuronal, astrocytic and oligodendrocytic differentiation was identified by Tuj- 1+ (an immature neuron marker), GFAP+ (an astrocytic marker) and CNSase+ (an oligodendrocytic marker) labeling. These neurospheres with nestin (a NSC marker), BrdU (cell proliferative marker), GFAP and Tuj-1 labeling were clearly identified (Figure 1). In addition, CNSase labeling was also observed in the cultured neurospheres (data not shown).
Figure 1: Cell culture of neural stem cells dissected from mouse midbrains. Morphology of neurosphere, nestin (a NSC or neural progenitor marker), BrdU (a cell proliferation marker), Hoechst (cell nuclear counterstaining), GFAP (an astrocyte marker) and Tuj-1 (an immature neuron marker)-labeling are respectively shown.
The mNSCs showed lower BrdU incorporation but higher cell survival rate
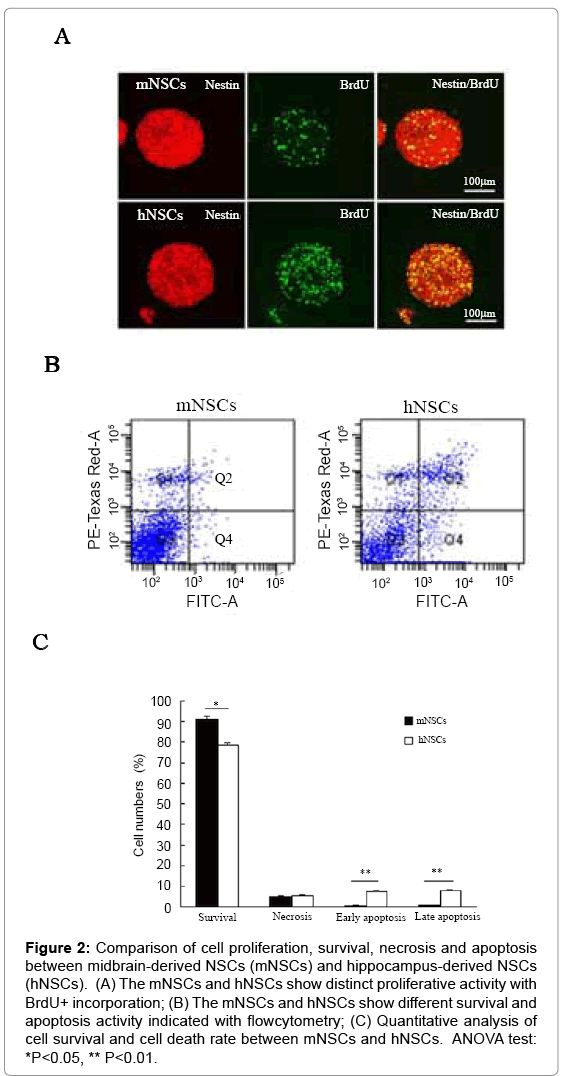
The cell proliferation, cell survival, necrosis and apoptosis were compared between mNSCs and hNSCs in cell culture of neurospheres by BrdU incorporation and flowcytometry analysis. BrdU/nestin double labeling experiment revealed that mNSCs had lower BrdU+ ratio than that of hNSCs (Figure 2A). Flowcytometry further indicated that mNSCs and hNSCs exhibited different cell viability and cell death patterns. The mNSCs showed higher rate of cell survival and lower rate of cell apoptosis in comparison with that of hNSCs. The different patterns of cell survival, necrosis, apoptosis of mNSCs and hNSCs were representatively shown by flowcytometry (Figure 2B,C).
Figure 2: Comparison of cell proliferation, survival, necrosis and apoptosis between midbrain-derived NSCs (mNSCs) and hippocampus-derived NSCs (hNSCs). (A) The mNSCs and hNSCs show distinct proliferative activity with BrdU+ incorporation; (B) The mNSCs and hNSCs show different survival and apoptosis activity indicated with flowcytometry; (C) Quantitative analysis of cell survival and cell death rate between mNSCs and hNSCs. ANOVA test: *P<0.05, ** P<0.01.
The mNSCs exhibited high TH+ and Nurr-1+cell commitment
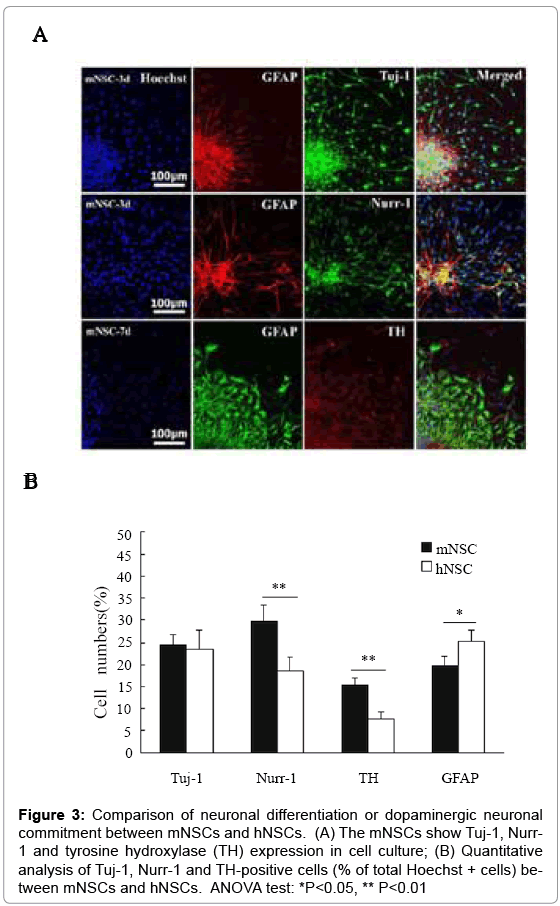
Immunocytochemistry was performed to detect Tuj-1, tyrosine hydroxylase (TH, a dopaminergic cell marker) and Nurr-1 (an orphan nuclear receptor that is necessary for induction of the dopaminergic phenotype) expression to demonstrate neuronal differentiation or dopaminergic neuronal commitment of mNSCs and hNSCs. The Tuj-1, TH, Nurr-1 and GFAP-positive cells were detected the in differentiation culture at d3 and d7 with serum-containing culture medium (Figure 3A). Quantitative analysis indicated that mNSCs showed Tuj-1+ commitment rate similar to that of hNSCs, but higher rate of Nurr-1+ and TH+ cells than that of hNSCs (Figure 3B). In contrast, however, hNSCs exhibited higher rate of GFAP+ cell differentiation than that of mNSCs.
Figure 3: Comparison of neuronal differentiation or dopaminergic neuronal commitment between mNSCs and hNSCs. (A) The mNSCs show Tuj-1, Nurr- 1 and tyrosine hydroxylase (TH) expression in cell culture; (B) Quantitative analysis of Tuj-1, Nurr-1 and TH-positive cells (% of total Hoechst + cells) between mNSCs and hNSCs. ANOVA test: *P<0.05, ** P<0.01
The mNSCs strongly expressed neurotrophic factor BDNF, GDNF, CDNF and DJ-1
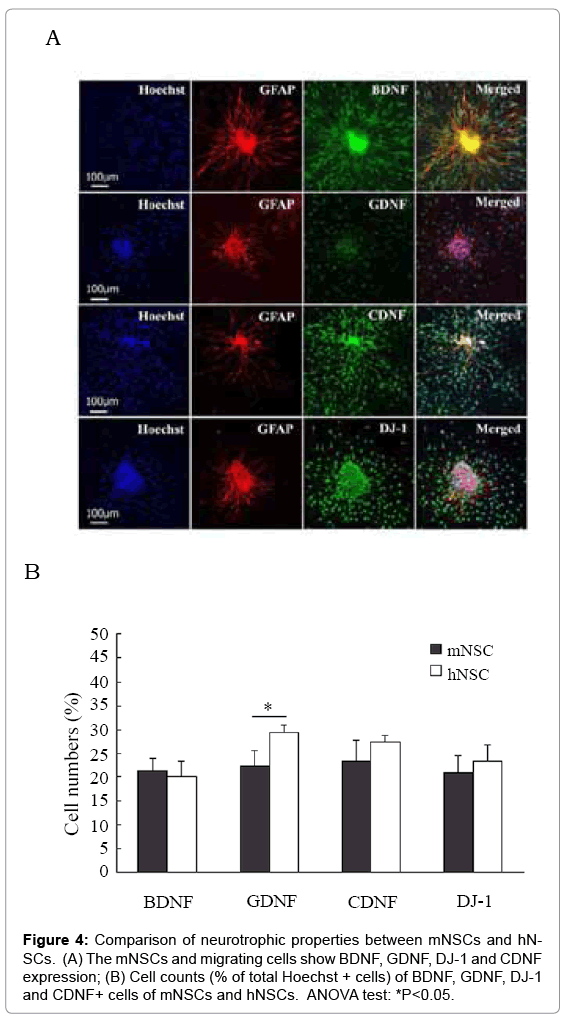
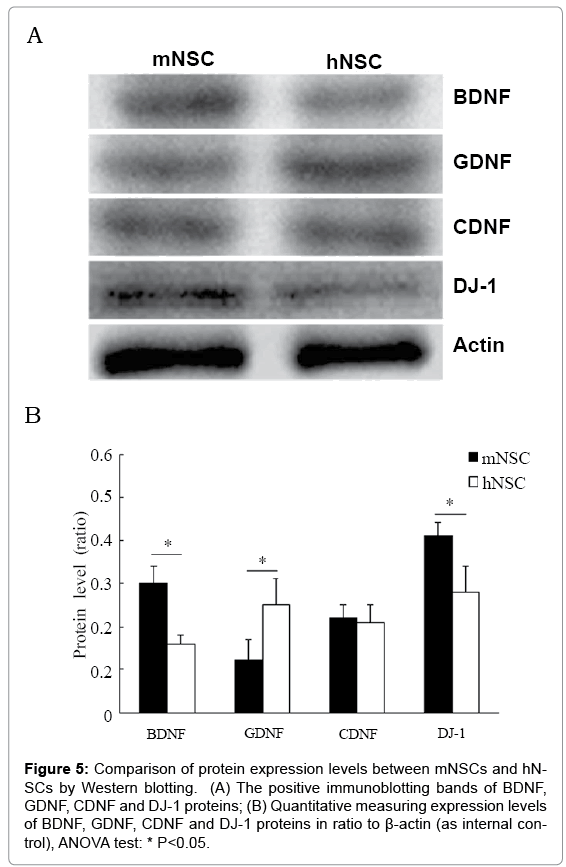
Immunocytochemistry and Western blotting were performed to detect generation of neurotrophic factor BDNF, GDNF, CDNF and DJ-1 in these neurospheres and migrating cells. Immunoreactivity to BDNF, GDNF, CDNF and DJ-1 was clearly detected in numerous cells distributing in or migrating from neurospheres under cell culture of d3 and d7 of both mNSCs and hNSCs (Figure 4A). The mNSCs and migrating cells exhibited strong BDNF and DJ-1 staining, similar CDNF staining, and weaker GFAP staining in comparison with that of hNSCs (Figure 4B). Western blotting further confirmed that mNSCs had higher expression levels of BDNF and DJ-1 proteins, similar level of CDNF protein, and lower level of GDNF protein in comparison with that of hNSCs (Figure 5). However, cell count data did not show significant difference in BDNF, CDNF and DJ-1+ migrating cells (partially being GFAP+ differentiating astrocytes) cells between mNSCs and hNSCs (Figure 4B).
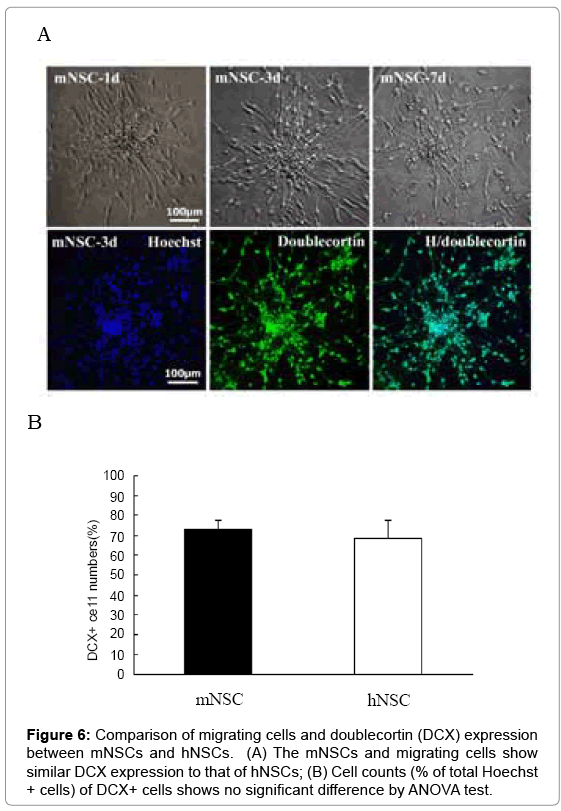
The mNSCs had moderate migrating ability and doublecortin expression
Under differentiation cell culture of serum-containing neurobasal medium, many cells grew and gradually migrated from these neurospheres at d1, d3 and d7 (Figure 6A). For detecting cell migrating ability, immunostaining to DCX (a cell migrating marker) was performed, and a number of DCX-positive cells were seen in these migrating cells from neurospheres of both mNSC and hNSCs (Figure 5A). However, quantitative analysis did not find significant difference of DCX expression levels in these migrating cells between mNSC and hNSCs (Figure 6B)
Figure 5: Comparison of protein expression levels between mNSCs and hNSCs by Western blotting. (A) The positive immunoblotting bands of BDNF, GDNF, CDNF and DJ-1 proteins; (B) Quantitative measuring expression levels of BDNF, GDNF, CDNF and DJ-1 proteins in ratio to β-actin (as internal control), ANOVA test: * P<0.05.
Discussion
This study presents unique cell proliferative, survival, dopaminergic neuronal commitment and neurotrophic properties of the mNSCs under in vitro culture. First, the mNSCs exhibit lower BrdU incorporation rate but higher cell survival capacity than hNSCs. Secondly, the mNSCs have similar Tuj-1+ neuronal differentiation, but higher Nurr1+ and TH+ dopaminergic neuronal commitment in comparison to hNSCs under differentiation culture. Thirdly, the mNSCs and hNSCs show similar migrating and strong expression levels of neurotrophic factors, i.e. BDNF, GDNF, CDNF and DJ-1, that actively function in dopaminergic neuronal maintenance or neuronal protection. Taken together, this study has provided interesting evidence that mNSCs might characterize with low cell proliferation, high survival, dopaminergic cell commitment, neurotrophic and migrating capacities, suggesting that mNSCs may appeal therapeutic potential for cell replacement transplantation or neuroprotective therapies against PD in humans [2,3,5,27,28].
It is well known that BDNF, GDNF, CDNF and DJ-1 are important types of neurotrophic factors in mammalian brains that extensively support neuronal survival, dopaminergic phenotype developing and functional recovery after injury [4-9]. For instance, BDNF acted as a critical neurotrophic factor that promoted dopaminergic neuron development and survival [5,29]. BDNF gene was recognized as a downstream target of Nurr1, an orphan receptor required for development of dopaminergic phenotype [30]. BDNF showed protective effect on dopaminergic neurons against excitotoxicity, inflammatory, neurotoxin MPTP and 6-OHDA insults [31,32], while inhibition of BDNF expression by antisense oligonucleotide resulted in loss of the dopaminergic neurons in midbrain [33]. Furthermore, growing data indicated that neurotrophic factor GDNF, CDNF and DJ-1 functioned in preventing the dopaminergic neurons from degeneration and improving behavioral recovery of PD animals. Mesencephalic astrocyte-secreted GDNF protected neurons in rat 6-OHDA model [34-36]. GDNF release from human neural progenitor cells was found in lesion event [37]. GDNF signaling could activate Nurr1 and Pitx3 and then promote survival of midbrain-derived NSCs in PD model [38], and transgenic GDNF expression in astrocytes improved cognitive deficits in aged animals [39]. CDNF is a novel neurotrophic factor showing protective role on midbrain dopamine neurons [8,40]. Anti-parkinsonian drugs might regulate both CDNF and BDNF in vivo and chronic infusion of CDNF prevented 6-OHDAinduced deficits in a rat model of PD [41,42]. Besides, DJ-1 was involved in prevention of oxidative stress, inflammation, cell injury of PD state and other age-related disorders [9,43-47]. DJ-1 deficiency in astrocytes enhanced neurotoxicity induced by mitochondrial Complex I inhibitor and impaired astrocyte-mediating neuroprotection [48,49]. Among above neurotrophic factors, BDNF showed more potent protective effect than GDNF in a model of PD [50]. In this study, interestingly, abundant generation of neurotrophic factor BDNF, GDNF, CDNF and DJ-1 was found in the mNSCs, suggesting that mNSCs may have therapeutic effect against PD progression.
Furthermore, the protective effects of neurotrophic factor-secreting cells were reported in animal models of degenerative diseases and were suggested for long-term protection in PD and Alzheimer’s disease (AD) [16,17,51]. Intranigral grafting of BDNF-secreting mesenchymal stem cells showed therapeutic effect for cell-based therapies in PD [16], and neural stem cells might improve cognition in AD model through BDNF signaling [52]. The production of neurotrophic factors was also identified in primary astrocytes and in reactive astrocytes in PD animal [12,13,53,54]. Dopamine agonists, monoaminergic neuronal activity and prostanoid receptor activation influenced or enhanced BDNF synthesis and release in astrocytes [55-58]. Retinoic acid receptor stimulation protected dopaminergic neurons via BDNF signaling [59]. The neurorescue action of rasagiline was associated induction of GDNF and BDNF [60]. The astrocytes expressing neurotrophic factor showed therapeutic effects on rat model of PD [61]. BDNF and GDNF secreted from Pitx3-transfected astrocytes also protected dopamine neurons in cell culture [62], and astrocytes derived from mesenchymal stromal cells exhibited restorative potential for PD animal [63]. Although endogenous NSCs or progenitor cells with astroglial property were found in these nigrostratal regions [22,23], their unique cell proliferation, survival and neurotrophic properties and neuronal commitment were not well delineated. Interesting data of this study further revealed that a large population of mNSCs and migrating cells (partially early-differentiating astrocytes) showed generation of neurotrophic factor BDNF, GDNF, CDNF and DJ-1, indicating that mNSCs might have cell therapeutic potential for PD patients.
In summary, inspiringly, unique properties of mNSCs with low proliferation, high cell survival and dopaminergic neuron commitment and neurotropic factor generation were identified in this study. It is obviously that mNSCs may present ideal cell source for long-term neuroprotection therapy against progression of PD in vivo possibly through their high cell survival and neurotrophic factor release. The generation of dopaminergic neurons derived from mNSCs may also be another beneficial outcome for functional improvement or recovery of PD state [27,28]. Finally, mNSCs with low proliferation should have less tumorigenicity in vivo or lower risk of uncontrolled overgrowing when they were applied for cell transplantation therapy [64,65]. It is expected that, therefore, new cell therapies may be hopefully developed by clinical translational manipulation of midbrain-derived NSCs, which exhibit the unique lower cell proliferation, higher cell survival, dopaminergic neuronal cell commitment, neurotrophic and migrating abilities for the cell replacement or neuroprotective treatment of PD in human beings.
Acknowledgements
This work was supported by grants from the National Science Basic Research Project of China (2011CB504103, 2012CB525002), National Natural Science Foundation of China (30970862, 81071609) and Science Research Project of Shaanxi Province (2011K14-07-03).
References
- Fernandez-Espejo E (2004) Pathogenesis of Parkinson’s disease: prospects of neuroprotective and restorative therapies. Mol Neurobiol 29: 15-30.
- Isacson O, Kordower JH (2008) Future of cell and gene therapies for Parkinson’s disease. Ann Neurol 64 Suppl 2: S122-138.
- Yacoubian TA, Standaert DG (2009) Targets for neuroprotection in Parkinson’s disease. Biochim Biophys Acta 1792: 676-687.
- Hagg T (1998) Neurotrophins prevent death and differentially affect tyrosine hydroxylase of adult rat nigrostriatal neurons in vivo. Exp Neurol 149: 83-192.
- Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH (2010) Neurotrophic factor therapy for Parkinson’s disease. Prog Brain Res 184: 237-64.
- Hyman C, Hofer M, Barde YA, Juhasz M, Yancopoulos GD, et al (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350: 230-232.
- Pascual A, Hidalgo-Figueroa M, Gómez-DÃaz R, López-Barneo J (2011) GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol 46: R83-92.
- Lindholm P, Voutilainen MH, Laurén J, Peränen J, Leppänen VM, et al. (2007) Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo. Nature 448: 73-77.
- Kahle PJ, Waak J, Gasser T (2009) DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med 47: 1354-1361.
- Nagatsu T, Mogi M, Ichinose H, Togari A (2000) Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural. Transm. Suppl 60: 277-290.
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH (2004) Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol 44: 399-421.
- Chen LW, Hu HJ, Liu HL, Yung KK, Chan YS (2004) Identification of brain-derived neurotrophic factor in nestin-expressing astroglial cells in the neostriatum of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-treated mice. Neuroscience 126: 941-953.
- Chen LW, Zhang JP, Kwok-Yan Shum D, Chan YS (2006) Localization of nerve growth factor, neurotrophin-3, and glial cell line-derived neurotrophic factor in nestin-expressing reactive astrocytes in the caudate-putamen of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/Bl mice. J Comp Neurol 497: 898-909.
- Ding YX, Xia Y, Jiao XY, Duan L, Yu J, et al. (2011) The TrkB-Positive Dopaminergic Neurons are Less Sensitive to MPTP Insult in the Substantia Nigra of Adult C57/BL Mice. Neurochem Res 36: 1759-1766.
- Wang YQ, Bian GL, Wei LC, Cao R, Peng YF, et al. (2008) Nigrostriatal neurons in rat express the glial cell line-derived neurotrophic factor (GDNF) receptor subunit c-RET. Anat Rec 291:449-54.
- Somoza R, Juri C, Baes M, Wyneken U, Rubio FJ (2010) Intranigral transplantation of epigenetically-induced BDNF-secreting human mesenchymal stem cells: implications for cell-based therapies in Parkinson s disease. Biol Blood Marrow Transplant 16:1530-1540
- Sadan O, Bahat-Stromza M, Barhum Y, Levy YS, Pisnevsky A, et al. (2009) Protective effects of neurotrophic factor-secreting cells in a 6-OHDA rat model of Parkinson disease. Stem Cells Dev 18: 1179-1190.
- Chen LW, Kuang F, Wei LC, Ding YX, Yung KK, et al (2011) Potential Application of Induced Pluripotent Stem Cells in Cell Replacement Therapy for Parkinson's Disease. CNS Neurol Disord Drug Targets 10: 449-458.
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, et al. (2008) Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci USA 105: 5856-5861.
- Hargus G, Cooper O, Deleidi M, Levy A, Lee K, et al. (2010) Differentiated Parkinson patient-derived induced pluripotent stem cells grow in the adult rodent brain and reduce motor asymmetry in Parkinsonian rats. Proc Natl Acad Sci USA 107: 15921-15926.
- Jönsson ME, Ono Y, Björklund A, Thompson LH (2009) Identification of transplantable dopamine neuron precursors at different stages of midbrain neurogenesis. Exp Neurol 219: 341-354.
- Hebsgaard JB, Nelander J, Sabelström H, Jönsson ME, Stott S, et al. (2009) Dopamine neuron precursors within the developing human mesencephalon show radial glial characteristics. Glia 57: 1648-1658.
- Bonilla S, Hall AC, Pinto L, Attardo A, Götz M, et al (2008) Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia 56: 809-820.
- Papanikolaou T, Lennington JB, Betz A, Figueiredo C, Salamone JD, et al. (2008) In vitro generation of dopaminergic neurons from adult subventricular zone neural progenitor cells. Stem Cells Dev 17: 157-172.
- Cameron HA, McKay RD (2001) Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol 435:406-417.
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, et al. (2008) Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci 11: 901-907.
- Ding YX, Wei LC, Wang YZ, Cao R, Wang X, et al. (2011) Molecular Manipulation Targeting Regulation of Dopaminergic Differentiation and Proliferation of Neural Stem Cells or Pluripotent Stem Cells. CNS Neurol Disord Drug Targets. 10: 517-528.
- Chen LW (2011) Advances and Perspectives on Stem Cell Therapy for Human Neurodegenerative Diseases. CNS Neurol Disord Drug Targets 10:417-418.
- Zhou J, Bradford HF, Stern GM (1997) Influence of BDNF on the expression of the dopaminergic phenotype of tissue used for brain transplants. Brain Res Dev Brain Res 100: 43-51.
- Volpicelli F, Caiazzo M, Greco D, Consales C, Leone L, et al. (2007) Bdnf gene is a downstream target of Nurr1 transcription factor in rat midbrain neurons in vitro. J Neurochem 102: 441-453.
- Canudas AM, Pezzi S, Canals JM, Pallàs M, Alberch J (2005) Endogenous brain-derived neurotrophic factor protects dopaminergic nigral neurons against transneuronal degeneration induced by striatal excitotoxic injury. Brain Res Mol Brain Res 134: 147-154.
- Gibrat C, Bousquet M, Saint-Pierre M, Lévesque D, Calon F, et al. (2010) Cystamine prevents MPTP-induced toxicity in young adult mice via the up-regulation of the brain-derived neurotrophic factor. Prog Neuropsychopharmacol Biol Psychiatry 34: 193-203.
- Porritt MJ, Batchelor PE, Howells DW (2005) Inhibiting BDNF expression by antisense oligonucleotide infusion causes loss of nigral dopaminergic neurons. Exp Neurol 192: 226-234.
- Voutilainen MH, Bäck S, Pörsti E, Toppinen L, Lindgren L, et al. (2009) Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson’s disease. J Neurosci 29: 9651-9659.
- Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, et al. (2009) Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6-OHDA cytotoxicity. Neurobiol Dis 33: 405-414.
- Laganiere J, Kells AP, Lai JT, Guschin D, Paschon DE, et al. (2010) An engineered zinc finger protein activator of the endogenous glial cell line-derived neurotrophic factor gene provides functional neuroprotection in a rat model of Parkinson’s disease. J Neurosci 30: 16469-16474.
- Behrstock S, Ebert AD, Klein S, Schmitt M, Moore JM, et al. (2008) Lesion-induced increase in survival and migration of human neural progenitor cells releasing GDNF. Cell Transplant 17: 753-762.
- Lei Z, Jiang Y, Li T, Zhu J, Zeng S (2011) Signaling of glial cell line-derived neurotrophic factor and its receptor GFRa1 induce Nurr1 and Pitx3 to promote survival of grafted midbrain-derived neural stem cells in a rat model of Parkinson disease. J Neuropathol Exp Neurol 70: 736-747.
- Pertusa M, GarcÃa-Matas S, Mammeri H, Adell A, Rodrigo T, et al. (2008) Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats. Neurobiol Aging 29: 1366-1379.
- Lindholm P, Saarma M (2010) Novel CDNF/MANF family of neurotrophic factors. Dev Neurobiol 70: 360-371.
- Gyárfás T, Knuuttila J, Lindholm P, Rantamäki T, Castrén E (2010) Regulation of brain-derived neurotrophic factor (BDNF) and cerebral dopamine neurotrophic factor (CDNF) by anti-parkinsonian drug therapy in vivo. Cell Mol Neurobiol 30: 361-368.
- Voutilainen MH, Bäck S, Peränen J, Lindholm P, Raasmaja A, et al. (2011) Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson’s disease. Exp Neurol 228: 99-108.
- Hauser DN, Cookson MR (2011) Astrocytes in Parkinson’s disease and DJ-1. J Neurochem 117: 357-358.
- Yanagida T, Tsushima J, Kitamura Y, Yanagisawa D, Takata K, et al. (2009) Oxidative stress induction of DJ-1 protein in reactive astrocytes scavenges free radicals and reduces cell injury. Oxid Med Cell Longev 2: 36-42.
- Waak J, Weber SS, Waldenmaier A, Görner K, Alunni-Fabbroni M, et al. (2009) Regulation of astrocyte inflammatory responses by the Parkinson’s disease-associated gene DJ-1. FASEB J 23: 2478-2489.
- Baulac S, Lu H, Strahle J, Yang T, Goldberg MS, et al. (2009) Increased DJ-1 expression under oxidative stress and in Alzheimer’s disease brains. Mol Neurodegener 4: 12.
- Miyazaki S, Yanagida T, Nunome K, Ishikawa S, Inden M, et al. (2008) DJ-1-binding compounds prevent oxidative stress-induced cell death and movement defect in Parkinson’s disease model rats. J Neurochem 105:2418-2434.
- Mullett SJ, Hinkle DA (2011) DJ-1 deficiency in astrocytes selectively enhances mitochondrial Complex I inhibitor-induced neurotoxicity. J Neurochem 117: 375-387.
- Mullett SJ, Hinkle DA (2009) DJ-1 knock-down in astrocytes impairs astrocyte-mediated neuroprotection against rotenone. Neurobiol Dis 33: 28-36.
- Stahl K, Mylonakou MN, Skare Ø, Amiry-Moghaddam M, Torp R (2011) Cytoprotective effects of growth factors: BDNF more potent than GDNF in an organotypic culture model of Parkinson’s disease. Brain Res 1378: 105-118.
- Giralt A, Friedman HC, Caneda-Ferrón B, Urbán N, Moreno E, et al. (2010) BDNF regulation under GFAP promoter provides engineered astrocytes as a new approach for long-term protection in Huntington’s disease. Gene Ther.
- Blurton-Jones M, Kitazawa M, Martinez-Coria H, Castello NA, Müller FJ, et al. (2009) Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc Natl Acad Sci USA 106: 13594-13599.
- Wei R, Lin CM, Tu YY (2010) Strain-specific BDNF expression of rat primary astrocytes. J Neuroimmunol 220: 90-98.
- Sato Y, Chin Y, Kato T, Tanaka Y, Tozuka Y, et al. (2009) White matter activated glial cells produce BDNF in a stroke model of monkeys. Neurosci Res 65: 71-78.
- Ohta K, Kuno S, Inoue S, Ikeda E, Fujinami A, et al. (2010) The effect of dopamine agonists: the expression of GDNF, NGF, and BDNF in cultured mouse astrocytes. J Neurol Sci 291: 12-16.
- Juric DM, Miklic S, Carman-Krzan M (2006) Monoaminergic neuronal activity up-regulates BDNF synthesis in cultured neonatal rat astrocytes. Brain Res 1108: 54-62.
- Toyomoto M, Ohta M, Okumura K, Yano H, Matsumoto K, et al. (2004) Prostaglandins are powerful inducers of NGF and BDNF production in mouse astrocyte cultures. FEBS Lett 562: 211-215.
- Hutchinson AJ, Chou CL, Israel DD, Xu W, Regan JW (2009) Activation of EP2 prostanoid receptors in human glial cell lines stimulates the secretion of BDNF. Neurochem Int 54: 439-446.
- Katsuki H, Kurimoto E, Takemori S, Kurauchi Y, Hisatsune A, et al. (2009) Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling. J Neurochem 110: 707-718.
- Weinreb O, Amit T, Bar-Am O, Youdim MB (2007) Induction of neurotrophic factors GDNF and BDNF associated with the mechanism of neurorescue action of rasagiline and ladostigil: new insights and implications for therapy. Ann N Y Acad Sci 1122: 155-168.
- Wang ZH, Ji Y, Shan W, Zeng B, Raksadawan N, et al. (2002) Therapeutic effects of astrocytes expressing both tyrosine hydroxylase and brain-derived neurotrophic factor on a rat model of Parkinson’s disease. Neuroscience 113: 629-640.
- Yang D, Peng C, Li X, Fan X, Li L, et al. (2008) Pitx3-transfected astrocytes secrete brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor and protect dopamine neurons in mesencephalon cultures. J Neurosci Res 86: 3393-3400.
- Bahat-Stroomza M, Barhum Y, Levy YS, Karpov O, Bulvik S, et al. (2009) Induction of adult human bone marrow mesenchymal stromal cells into functional astrocyte-like cells: potential for restorative treatment in Parkinson’s disease. J Mol Neurosci 39: 199-210.
- Hwang DY, Kim DS, Kim DW (2010) Human ES and iPS cells as cell sources for the treatment of Parkinson's disease: current state and problems. J Cell Biochem 109: 292-301.
- Meyer AK, Maisel M, Hermann A, Stirl K, Storch A (2010) Restorative approaches in Parkinson's Disease: which cell type wins the race? J Neurol Sci 289: 93-103.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14718
- [From(publication date):
specialissue-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10037
- PDF downloads : 4681