Research Article Open Access
Microbially Enhanced Compost Extract: Does It Increase Solubilisation of Minerals and Mineralisation of Organic Matter and Thus Improve Plant Nutrition?
| Karuna Shrestha*, Kerry B. Walsh, and David J. Midmore | |
| Engineering & Health, Faculty of Sciences, Centre for Plant and Water Science (CPWS), Central Queensland University, Australia | |
| Corresponding Author : | Karuna Shrestha Engineering & Health, Faculty of Sciences Centre for Plant and Water Science (CPWS) Central Queensland University, Rockhampton 4702 QLD, Australia Tel: +61 749232315 Fax: +61 749309255 E-mail: k.shrestha@cqu.edu.au |
| Received March 23, 2012; Accepted April 27, 2012; Published April 29, 2012 | |
| Citation: Shrestha K, Walsh KB, Midmore DJ (2012) Microbially Enhanced Compost Extract: Does It Increase Solubilisation of Minerals and Mineralisation of Organic Matter and Thus Improve Plant Nutrition? J Bioremed Biodegrad 3:149. doi: 10.4172/2155-6199.1000149 | |
| Copyright: © 2012 Shrestha K et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Compost extracts may potentially benefit plant growth through a direct nutritional benefit, through increased mineralisation or solubilisation, through disease protection or by detoxification of the soil environment. Tomato ( Lycopersicum esculentum cv. Tiny Tim) and a subsequent sorghum ( Sorghum bicolor cv. Sweet Jumbo LPA) grown on a ferrosol and a vertisol was treated with soil drenches of a cattle rumen content compost extract (aerated and non-aerated, sterilised or not). Soil type was the primary factor in determining growth rate of crops. Compost extracts produced by either extraction method had similar effects on plant growth, and there was no difference between sterilised and non-sterilised treatments. Thus the noted growth benefit of compost extracts was not directly biological in nature. The positive impact of compost extract application on plant growth was ascribed to a nutritional effect, attributed to the high doses of compost extract application used (2 Lpot -1 , equivalent to 34,000 Lha -1 ), delivering 3.4 g of Npot -1 . There was no evidence of increased mineralisation or solubilisation in this exercise.
| Keywords |
| Compost extract; Compost tea; Mineralisation; Solubilisation; Respiration; δ15N analysis |
| Abbreviations |
| Cont: Non-fertilised; ACE: Aerated Compost Extract; NCE: Non-aerated Compost Extract; SACE: Sterilised Aerated Compost Extract; SNCE: Sterilised Non-aerated Compost Extract; Chem: Chemical Fertilizer |
| Introduction |
| Compost extract, popularly known as ‘compost tea’, is an extract of compost incubated with or without a microbial food source with the aim of extracting soluble nutrients and plant beneficial microbes into the solution [1]. Although there is an extensive anecdotal evidence to support the claim of improved plant growth in response to compost extract application, studies conducted under controlled conditions are limited. However, the direct cause of this response is often not elucidated – the effect could be due a direct fertilisation effect, increased mineralisation or solubilisation, chelating of heavy metals, or a disease suppression effect. |
| The mineral nutrients extracted from compost can improve soil fertility directly [2-4]. Other claims include a role for the extracted microbiota in improved mineralisation of soil organic matter and solubilisation of soil minerals, chelation of ions [5], suppression/ biocontrol of certain plant root and foliar diseases [6,7], and microbial production of plant growth promoting hormones such as auxins [8], or cytokinin-like substances [9]. For example, Ekin [10] reported increased shoot biomass and seed yield of sunflowers associated with a soil application of phosphate solubilising bacteria (Bacillus M-13) and a phosphorus fertiliser, compared to fertiliser alone. This result was ascribed to the effect of soil microbiota on soil mineral solubilisation at a level significant to plant growth. |
| Many reports of the use of compost extract involve high rates of application, such that a direct nutritional effect will dominate. For example, Hargreaves et al. [11] reported that compost extract (produced at the ratio of 1:10 w/v) applied at weekly intervals to a raspberry crop at the rate of 150-300 mLplot-1 (2.4 m2) per application [12] promoted the growth of raspberries as effectively as applying compost at the rate of 150 kg total N per ha in 2004 and 75 kg N per ha in the following years to the soil. However, 27 applications made over 3 years will have delivered the equivalent of 17-34 kLha-1, surely a significant source of nutrients in its own right. In another study, Hargreaves et al. [13] produced compost extracts by steeping compost for 72 h at the ratio of 1:5 (w/v), with application at the rate of 1 L and 2 Lplot-1 (of 2 m2 containing six strawberry plants) per week continuously for twelve weeks in 2006 and for five weeks in 2007 (i.e., 120,000 Lha-1, or 24 tonha-1 of compost). Compost extracts may thus be used to maintain soil nutrient levels by growers who are restricted from using inorganic fertilisers by the norms of organic farming, with the advantage of easier distribution (e.g. through irrigation systems) than is possible for solid compost. |
| Compost extracts, however, can vary in composition. There are limited studies comparing methods of preparation of compost extracts [14]. For example, compost extracts can be produced following aerobic (aerated compost extract) or anaerobic methods (non-aerated compost extract), presumably resulting in distinctly different microbial populations, however there is a lack of evidence as to the superiority of one or other method for agricultural purposes [15]. Ingham [16] has advocated the use of aerobic compost extracts, whereas other studies have found a significantly positive effect of non-aerated compost extracts on plant growth due to disease suppression [17-20]. |
| The present exercise was designed to test three hypotheses: (a) that application of compost extract to the soil results in an enhancement of the microbial population of the soil, (b) that extract microorganisms may enhance solubilisation of relatively inaccessible inorganic pools and/or increase mineralisation of organic matter to release mineral nutrients, and (c) that the effect of a compost extract is primarily due to the mineral nutrients contained in the extract. Two pot trials were conducted to determine the effects of extraction methods (aerobic and non-aerobic) on the growth of tomato and sorghum supplied with extracts (either sterilised or non-sterilised) derived from a cattle rumen content compost. In one trial, the soil medium contained sand and leached compost (to test mineralisation capacity), while in the second trial two soils were used, both of low nutrient availability and organic matter content (to test solubilisation capacity), with a rotation crop planted into the root residues of the first crop (to test mineralisation capacity). |
| Tomato was chosen as a likely horticultural crop for ‘compost tea’ application, being Australia’s second largest commercial vegetable crop [21]. |
| Materials and Methods |
| Compost and compost extract |
| Cattle rumen content (paunch material) was used for compost given the relative uniformity of this material (e.g. relative to urban green waste) and its local abundance (with up to 1700 head of cattle slaughtered per day in Rockhampton abattoirs). A commercial composting operation in Rockhampton utilises a thermophilic windrow composting process, with the windrows (2 m height, 2.5 m width and 50 m length of material) turned mechanically at monthly intervals, for nine months to ensure homogeneity and aeration. Four consignments of nine month old compost were used in the course of this study. |
| Compost extracts were produced at the ratio of 1:10 (w/v) of compost submerged in dechlorinated tap water following aerated and non-aerated methods of extraction (at between 18ºC and 25ºC). Dissolved oxygen, measured with a TPS WP 82 Dissolved Oxygen Meter (EnviroEquip, Australia), of aerated and non-aerated compost extracts at 24 h of the incubation period was 71% and 1.5% of airsaturation, respectively. Addition of 0.5% v/v of molasses and 1% v/v of soluble ‘fish and kelp hydrolysate’ (Fish and Kelp Product, Australia) was made prior to application of the compost extract to soil/growing media. Compost extracts were prepared on the day of soil application. |
| Nutrient analysis of composite samples of the aerated compost extract (ACE) and the non-aerated compost extract (NCE), i.e., representatives of all extracts used, was undertaken by the Soil and Plant Laboratory, Wesfarmers CSBP Limited, Australia. The samples were shipped in cooled liquid form. |
| Experiment 1 |
| The hypothesis was tested that non-sterile compost extract would enhance plant growth over an equivalent amount of sterile compost extract through the enhancement of soil mineralisation in an ‘artificial’ soil of organic matter and sand mix. |
| The growing media was prepared by mixing the thoroughly washed (i.e., leached) sterilised rumen compost with sterilised sand at the ratio of 1:5 v/v. Materials (sand, compost, compost extract) were sterilised by autoclaving at 121ºC for 15 minutes. Three-week old tomato seedlings (Lycopersicum esculentum cv. Tiny Tim) were transplanted one each into 13 cm diameter pots and maintained in a growth chamber. Each pot contained 1 kg of the growing media. |
| A completely randomised experimental design was implemented, with five treatments: (i) control (i.e., equivalent amount of water only), (ii) aerated compost extract (ACE), (iii) non-aerated compost extract (NCE), (iv) sterilised aerated compost extract (S ACE) and (v) sterilised non-aerated compost extract (S NCE). Pots were placed on a 45 cm by 25 cm grid, with eight replicate pots per treatment. Treatments, including the water control, were applied twice at the rate of 100 mLkg-1 compost-sand mix, the first being applied one week after transplanting and the second a week later. |
| Two replicate trials (I and II) were conducted. In trial I, the growth chamber was set at 28ºC, 720 μmolm-2s-1 PAR and 75% relative humidity (RH). In trial II, the growth chamber was set with lower temperature and light intensity, but with the same relative humidity as in trial I (24ºC, 480 μmol m-2s-1 PAR and 75% RH). |
| The leaf chlorophyll concentration was measured at 10 day intervals starting from 18 and 31 days after transplanting in trials I and II, respectively. Dry weight of all plants (roots and shoot biomass) was measured following harvest at 45 and 51 days after transplanting in trials I and II, respectively. |
| Experiment 2 |
| The hypothesis was tested that microbial enhancement of the soil could serve to improve solubilisation of nutrients (and mineralisation of any soil organic matter) and so improve plant growth. |
| A vertisol was sourced (courtesy of Eric Coleman) from a Gracemere site (latitude: 23.44ºand longitude: 150.46º) that had been maintained as uncultivated grassland for over thirty years. A ferrosol was sourced (courtesy of Tony Wolfenden) from an organic sweet potato farm at Rossmoya (latitude: 23.05º and longitude: 150.48º), from a site that had been cultivated regularly over the past decade. Soil was collected to a depth of 30 cm, and homogenised using a small front end loader. Replicated samples from both soils were sent for nutrient analysis (Wesfarmers CSBP Limited, Soil and Plant Laboratory, Australia). |
| Soil was air dried and each pot (30 cm diameter, 40 cm height and 20 L volume) was filled with 20 kg of vertisol or 22 kg of ferrosol. All pots were watered to achieve field capacity (40% v/v and 38% v/v for the vertisol and ferrosol, respectively). Pots were maintained in a greenhouse (ambient temperature ranging from 18 to 40ºC, with 60 to 100% relative humidity) and spaced at 75 x 50 cm. Tomato (cv. Tiny Tim) seeds were germinated and seedlings transplanted to achieve one plant per pot. |
| The experiment used a factorial design within a randomised complete block design, with four application treatments applied to the two soil types, and four replicate pots per treatment. Treatments were (i) aerated compost extract (ACE), (ii) non-aerated compost extract (NCE), (iii) chemical fertilizer and (iv) a water control. All treatments were applied as a soil drench, with 0.5 L solution applied weekly to each pot for four weeks (equivalent to approximately 8,500 Lha-1 per application), beginning from one week after transplanting. The chemical fertiliser treatment utilised the same volume of solution containing 0.9 g of “Thrive” (Yates, NSW Australia), which consisted of 15: 4: 26 NPK and other essential nutrients. Culturable heterotrophic bacterial and fungal counts (cfu Log10 mL-1) of representative samples of aerated and non-aerated compost extracts (n = 3), sampled immediately prior to the application to the pots, were determined following the pour plate method [22]. |
| Tomato plants (the same cultivar as used in Experiment 1) were grown for 76 days, being watered to field capacity at weekly intervals. Soil respiration and soil temperature were measured per pot (n = 3) 76 days after transplanting, to estimate plant root and microbial activity in the soil using an EGM3 respirometer (PP Systems International Ltd., Hertfordshire, UK). Plant leaf chlorophyll concentration (SPAD-502 meter, Minolta Corporation, Japan), fruit yield and above ground biomass were assessed at harvest on all plants (76 days after transplanting). |
| The microbial population of the soil was quantified in triplicate at 36 days after tomato transplanting, that is, one day after the last compost extract application, using a plate count method (Table 4) [22]. Soil was sampled with a 2 cm diameter core sampler from the top 10 cm depth, composited, and 1 g of representative soil sample was taken from each treatment. |
| A nitrogen budget was constructed. Shoot material, compost extract and fertiliser, dried at 65ºC, were ground using a CQ University manufactured ball mill grinder. Subsamples (10 mg) were packed in tin capsules. Sample nitrogen elemental and isotopic content (natural abundance) was determined using an automated continuous flow isotope cube utilising Dumas flash combustion (Elementar, Germany) coupled with a continuous flow mass spectrometer (Isoprime, England) at the University of Melbourne. Machine precision and standardisation were established with acetanilide (Merck, Germany) as a tertiary standard (10.36% N and 0.2‰ δ15N) with analytical precision (1σ) of < 0.1%. |
| Experiment 3 |
| The hypothesis was tested that microbial enhancement of the soil could serve to improve mineralisation of residual tomato roots, and hence improve plant growth. |
| Following harvest of the tomato crop (removal of shoot system only, with the soil left undisturbed) and a fallow period of one month, 10 sorghum seeds (Sorghum bicolor cv. Sweet Jumbo LPA) were directly sown in each pot, and thinned out after one week to maintain six plants per pot. From the previous tomato trial, there was no significant effect of blocks on yield (above-ground biomass). In that trial, blocks were arranged down the length of the greenhouse. Blocks were rearranged across the width of the greenhouse to test for an environmental gradient in this direction (i.e., a possible wind speed gradient away from the windsock located at one edge of the greenhouse). |
| Two treatments, namely, aerated compost extract (ACE) and sterilised aerated compost extract (SACE), were applied at three week intervals at the rate of 5 mL per kg of soil. The rate of compost extract application was reduced to minimise nutrient input (equivalent to approximately 1700 Lha-1 per application). These treatments were applied to two pots each of the original four replicates of the first (tomato) rotation (i.e. across the two soils (vertisol and ferrosol) and four previous treatments (control, ACE, non-aerated compost extract ‘NCE’, and chemical). The pots were randomly assigned treatments within three blocks. |
| Percent light interception at the base of the sorghum plant per pot was measured using a Ceptometer, LAI-2000 (Decagon, USA) (Minolta Corporation, Japan). Soil respiration (root + microbial respiration) per pot (n = 3) was measured with an EGM3 respirometer just before harvest. The vegetative growth (shoot biomass), as measured by aboveground plant dry weight, of the forage sorghum crop was measured on all pots at 50 days after sowing. |
| Statistical analyses |
| Data were analysed using the GLM procedure of GENSTAT [23] and statistical significance was evaluated at P < 0.05 and P < 0.001. The effect of treatments on different parameters including plant biomass was analysed using either one-way or two-way analysis of variance (ANOVA). For the glasshouse trial, tomato shoot biomass (plant dry weight in g) was used as a covariance-factor on the sorghum shoot biomass for the analysis of variance. |
| Results |
| Characterisation of compost extract |
| The soluble nutrient contents of compost extracts produced under aerated and non-aerated conditions were similar (table 1). |
| In each compost extract, 3 kgfwt of compost at 60% moisture (i.e. 1.2 kgdwt) was added to 30 L of water. Given N content of the compost of 1.9% dwt, 22.8 g N was added to the 30 L extract in the form of compost. Each compost extract also contained 1% of Searles Fish and Kelp. Given N content of 10.3% w/v in this product (as reported on label), 30.9 g N was added to the 30 L brew in the form of this product. Thus, a 30 L compost extract is expected to contain 53.7g N. In supplying the extract at the rate of 2 Lpot-1, 3.58 g N should have been delivered. |
| Experiment 1: Tomato growth in sand-compost mix |
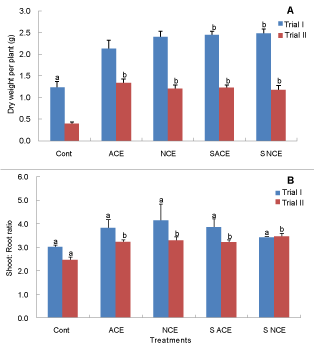
| Plant biomass: In both trials, total plant biomass (dry weight, shoot + root) was significantly different (P < 0.001) between imposed treatments and the control (Figure 1A), however there was a nonsignificant difference among the compost extract treatments (both sterilised and non-sterilised). |
| In all treatments receiving compost extracts, tomato plant shoot to root ratio was increased compared to the control, but no difference was found between compost extract treatments (Figure 1B). |
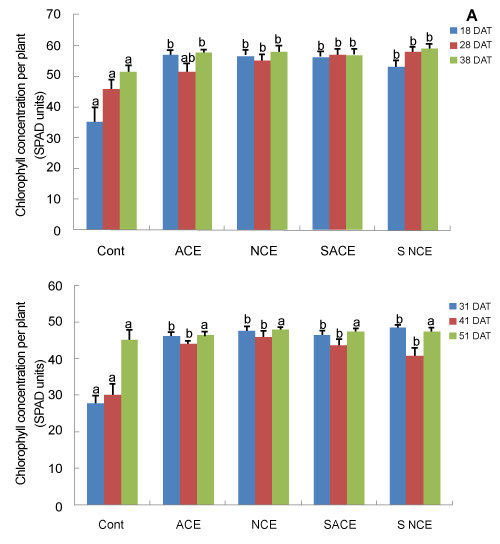
| Tomato leaf chlorophyll concentration: In trial I (Figure 2A), chlorophyll concentrations (SPAD Units) were significantly higher in compost extract treated plants than in the control at 18, 28 (except for ACE) and 38 days after transplanting (P < 0.001, P < 0.01 and P < 0.05, respectively). In trial II (Figure 2B), higher chlorophyll concentrations were found in leaves of plants treated with both sterilised and nonsterilised compost extracts (aerated and non-aerated) compared to the control at 31 (P < 0.001) and 41 (P < 0.05) days after transplanting. |
| However, in trial II, no difference in leaf chlorophyll concentrations between treatments was recorded at 51 days after transplanting. |
| δ15N of fertilisers (compost and compost extracts) and plants grown in compost-sand mix: Mean values for δ15N content of the compost (at 15‰), used in the compost-sand mix, were lower than that for the two compost extracts (at 17‰), a result consistent with denitrification and volatilisation in the compost extracts, however this difference was not significantly different due to replicate variability (typical SE of approx 0.5‰) (Figure 3). However, plant δ15N was similar in all three treatments, and significantly lower than that of the compost extract. Mean values for δ15N content of the compost (at 15‰), used in the compost-sand mix, were lower than that for the two compost extracts (at 17‰), a result consistent with denitrification and volatilisation in the compost extracts, however this difference was not significantly different due to replicate variability (typical SE of approx 0.5‰) (Figure 3). However, plant δ15N was similar in all three treatments, and significantly lower than that of the compost extract. |
| Experiment 2: Tomato growth on soil |
| Soil: The two soils were characterised with respect to their basic properties (Table 2). The ferrosol was comparatively nutrient poor, in terms of P and K, and slightly more acidic than the vertisol. |
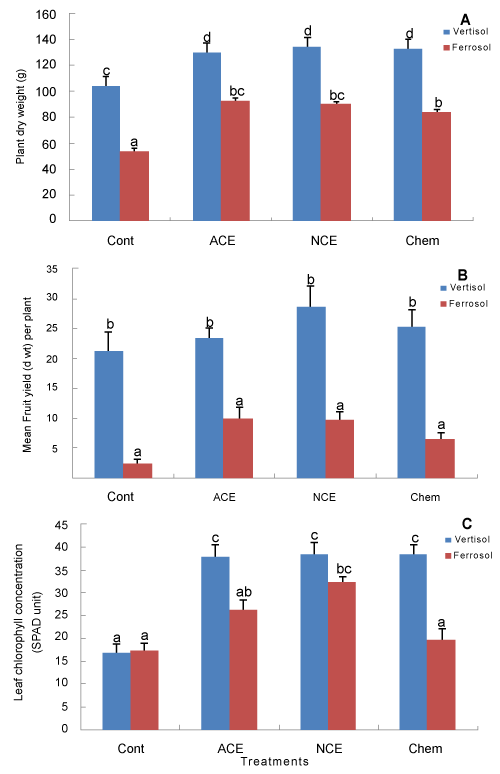
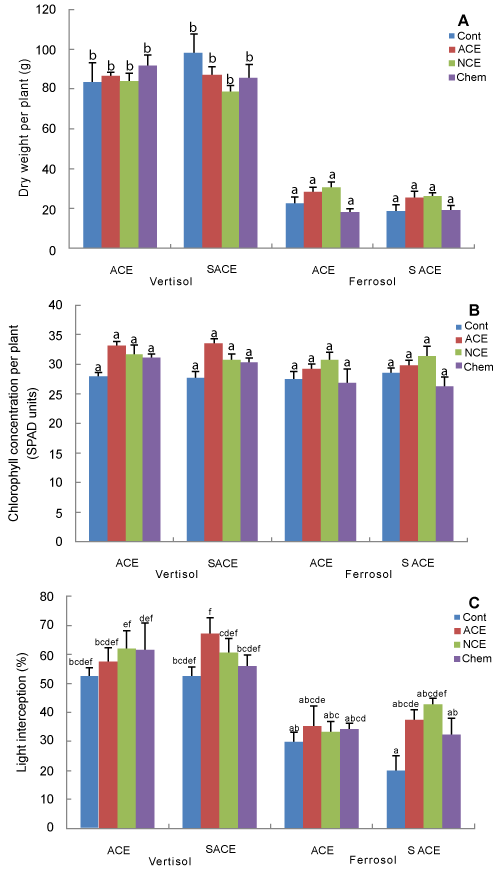
| Tomato growth, yield and chlorophyll concentration: At harvest (i.e., 76 days after transplanting), shoot biomass (gdwt) was significantly lower (P < 0.001) for plants grown in the ferrosol than those grown in the vertisol, irrespective of the amendment treatments (Figure 4A). In both soil types, shoot biomass was significantly lower in the control treatment (P < 0.001) compared to the other three treatments, however there was no significant difference among the three (two compost extract and one inorganic) treatments in both vertisol and ferrosol soils (Figure 4A). Although soil type showed a significant effect (P < 0.001) on fruit yield per plant, there was no significant difference in fruit yield among any of the treatments (Figure 4B). There was a proportionally greater benefit of NCE on leaf chlorophyll concentration on the ferrosol than the vertisol (Figure 4C). Chlorophyll concentration was increased in plants treated with either compost extract, relative to the control and the chemically treated plants (Figure 4C). |
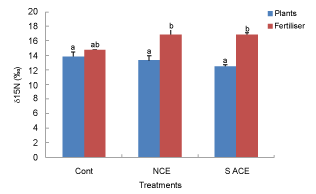
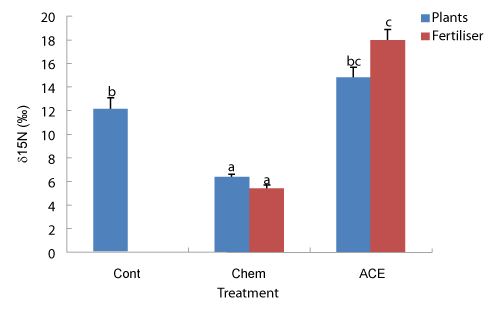
| δ15N of fertilisers and plants grown on soil: The δ15N isotopic composition of the inorganic fertiliser used was low, at 5‰, while that of the compost extract was high, at 18‰ (Figure 5). The latter result is consistent with denitrification occurring in the composting and extraction processes, with 14N preferentially lost. Tomato plants grown on a ferrosol without amendment possessed a δ15N isotopic composition of approximately 12‰, while that of plants grown on the same substrate but with addition of inorganic fertiliser was 6‰, and that of the fertiliser itself was 5‰. This result indicates that (12-6)/ (12-5)*100 = 86% of N sourced by the plant was from the chemical fertiliser, with the remainder sourced from the soil. The observed low contribution of N from the soil is consistent with a low rate of N mineralisation and thus availability. |
| In contrast, the δ15N isotopic composition of plants treated with compost extract (but no inorganic fertiliser) was 15‰, with that of the compost extract itself, 18‰, indicating that (15-12)/(18-12)*100 = 50% of N sourced by the plants in this treatment was sourced from the extract, although the δ15N values of ACE treated plants was not significantly different to that of the control plants (Figure 5). Note that these calculations assume that the compost extract was homogenous in its δ15N composition (e.g. soluble and insoluble pools). |
| Microbial enumeration of ACE and NCE: The bacterial colony forming units (cfu) load of NCE was approximately two orders of magnitude lower than that of ACE, at all assessment dates, while the fungal load was similar in the two extract preparations (Table 3). The addition of 2 L of ACE per pot will have involved the addition of approximately 2 x 1012 cfu of bacteria and approximately 7 x 107 cfu of fungi to each pot. |
| Soil microbial populations: The population (log values) of culturable bacteria was significantly higher in compost extract (both ACE and NCE) treated soils, 36 days after the transplanting of tomato, compared to control and chemical fertiliser treatments (P < 0.001) (Table 4). Consistent with the fungal count in the compost extract itself, there were no significant differences in either soil in terms of fungal populations in the two compost extract treatments. Additionally, there was no significant difference in fungal load between any of the four treatments including control and inorganic chemical fertilisation (Table 4). |
| Soil respiration and temperature: The measurement of soil respiration at harvest (76 days after transplanting) represents a sum of both soil microbial and root respiration. There was no significant difference evident in respiration rate, either between soil types or treatments (data not shown). |
| Soil temperature (monitored 30 cm below the soil surface) did not differ significantly between any of the treatments and soil types (data not shown). |
| Mass balance of nutrients: The four applications of 0.5 L of ACE (total of 2 L per pot) resulted in an application of approximately 162 mg soluble N as NH4+ and NO3−, 371 mg soluble P and 668 mg soluble K, respectively to each pot. An additional 3580 mg insoluble N was present in the compost extract. |
| A mass balance of N, as an example, for each treatment in the vertisol was calculated and is presented in Table 5. The ACE treatment, for which there was an above-ground biomass of 130 gdwt, and given an estimated root to shoot ratio of 1 and N content of 1.1%, the calculated plant N content was 2860 mg (Table 5). Thus, the N provided in the compost extract was equivalent to approximately 125% (N) of that in the plant. |
| Experiment 3: Sorghum crop rotation |
| Plant growth, shoot biomass, chlorophyll concentration and light interception: There were no visual differences between the growth of plants treated with aerated and sterilised aerated compost extracts in either soil type while a dramatic difference (P < 0.001) was observed in above-ground sorghum biomass (harvested 50 days after sowing) between soil types (Figure 6A). |
| However, above-ground biomass did not differ between treatments imposed on the sorghum plants (Figure 6A), or between the treatments imposed on the preceding tomato crop as shown by the covariance test (p = 0.98). For percent light interception (Figure 6C), significant difference was again found only between soil types (P < 0.001). |
| There was no significant difference in sorghum leaf chlorophyll concentration between any treatments (Figure 6B). |
| Discussion |
| Effects of treatments on growth and nutrition of tomato plants |
| In the tomato growth trial (Experiment 1), conducted in sandcompost mix, the lack of a plant growth difference between the sterilised and non-sterilised treatments is consistent with the interpretation of a direct nutritional benefit from the applied compost extract, rather than from a secondary benefit of the microbial activity, such as mineralisation of organic matter in the sand-compost mix. |
| The mass balance of N (Table 5) is again consistent with the interpretation that the differences in plant growth between the applied treatments is due to the nutrients supplied in the compost extract itself, rather than an enhancement of soil mineralisation by the enhanced soil microbiota (Experiment 2). |
| The soluble and insoluble nutrient concentration of compost extracts produced under aerated and non-aerated conditions was similar (table 1,5). However, the N present in insoluble components of the compost extract (e.g. microbial biomass) was approximately 20 times higher than in the soluble fraction (Table 5). Potentially the nutrients in the microbial biomass would not be immediately available for plant growth, and a greater plant response might be expected from autoclaved extracts. As there was no such difference, the majority of the applied microbial biomass presumably dies on application to the compost-sand mix, freeing nutrients for plant growth. |
| No differences between aerated and non-aerated compost extract treatments were observed in the current study in terms of growth (e.g. dwt biomass) of tomato plants (Figures 1,4). Pant et al. [14] tested the growth of pak choi under greenhouse conditions treated with aerated, non-aerated and microbially enhanced vermicompost extracts prepared at the ratio of 1:10 (w/v) and applied at the rate of 150 mLpot-1 for four continuous weeks, compared to an aerated water-control. They also observed non-significant differences in growth for plants treated with aerated and non-aerated compost extracts, with all vermicompost regimes increasing above-ground fresh weight of pak choi compared to the water-control. |
| In Experiment 1, tomato plants receiving compost extracts had a higher shoot to root ratio compared to the control plants (Figure 1B). An increased shoot : root ratio is consistent with a relief of a nutrient limitation in the extract treated plants [24]. |
| The higher shoot to root ratio of tomato plants in the first trial compared to the second trial could be attributed to the lower light level employed in the second experiment. |
| Effects of treatments on tomato leaf chlorophyll concentration |
| In both trials I and II of Experiment 1, chlorophyll concentrations were significantly higher in both sterilised and non-sterilised compost extract treated plants compared to control. Fayed [25] also observed higher leaf chlorophyll concentration in pomegranate trees treated with two foliar applications of compost extract mixed with antioxidants at the rate of five litres per tree. Such observations are consistent with N or Fe fertilization effect by the compost extract. |
| However, leaf chlorophyll concentrations between treatments did not differ 51 days after transplanting in trial II. The gradual increase in chlorophyll concentration in plants of the control treatment must reflect the mineralisation of organic matter present in the soil mix. |
| In Experiment 2, the effect of compost extract on leaf chlorophyll concentration (measured 76 days after transplanting) varied with soil type, with a significant interaction (P < 0.001) between soil type and treatments noted (Figure 4C). The significant interaction effect was attributed to the lack of response of tomato (in terms of chlorophyll concentration) to the chemical fertiliser treatment on the ferrosol. Chlorophyll concentration in tomato plants grown in the vertisol was higher compared to those grown in the ferrosol (Figure 4C), which could be related to the higher nutrient availability in the former, especially, phosphorus and potassium (Table 2). |
| Higher leaf chlorophyll concentration was found in the first trial compared to that in the second trial (Figure 2B), a result which could be due to higher light intensity and higher temperature employed in the first trial. Low temperature and low light intensity can lead to an inhibition or impairment of formation of chlorophyll in tomato [26,27]. |
| Plants supplied with compost extract had a significantly lower δ15N level than those of the compost extract itself, suggesting that plants sourced N from the mineralisation of the solid rumen compost in addition to the liquid compost extracts (Figure 3). Presumably the N of the compost extracts remained unavailable to the plant as it was within microbial biomass. |
| Influence of soil types on crop growth |
| Soil type had a stronger influence on plant growth than the influence of soil amendments used in the present study. In Experiment 2, vertisol produced higher shoot biomass of tomato plants compared to those grown in the ferrosol, which was consistent with the higher fertility (especially P) of the former soil. Chemical fertiliser supported increased growth on ferrosol, however only 36 mg P was given while the difference in soil P in the pots was 2200 g. |
| The grazing soil from which vertisol was collected has no known history of fertiliser addition and is likely to be deficient in both Zn and B. Since compost extracts have concentrations of Zn and B that are substantially higher than those of the commercial fertiliser, plants grown in this soil are highly likely to respond to the addition of the trace elements present in the extracts. |
| The vertisol also supported greater sorghum plant growth (aboveground dry weight biomass) and high light interception compared to the ferrosol (Figure 6A, 6C). This result is again attributed to the significantly greater nutritional status of the vertisol in comparison to the ferrosol (Table 2). |
| Nutritional and microbial supply from compost extracts |
| The measured dissolved N (as ammonium and nitrate) content of the extract represented only a small fraction (5% and 4% for ACE and NCE, respectively) of the total N content (table 1). The difference (e.g. 3418 mg N per 2 L of aerated compost extract) must either be present as microbial biomass, or insoluble or soluble but organic forms of N in the compost extract, and/or this N was lost from the compost extract through denitrification and volatilisation. The observed microbial load and the high 15N values of the compost extract are consistent with both processes occurring. |
| Given the rate of extract application (2 Lpot-1), the level of plant nutrients in the compost extracts was certainly significant in terms of plant nutrition. For example, the harvested (above-ground) biomass in Experiment 2 was approximately 100 g d wt per pot. The average N concentration of above-ground biomass across all treatments was 1.2% (from elemental analyzer – mass spectrometry analysis, data not shown). Given this, harvested plant biomass contained 1.2 g N (pot-1). Assuming a conservative shoot root ratio of 1:1, the total plant N content was 2.4 gpot-1. Thus, a direct fertilisation effect is expected for the extract treatments (3.4 g N delivered per pot) [28]. |
| The chemical fertiliser treatment was intended as a control exercise on the effect of nutrient addition, as opposed to other possible effects of extract addition (e.g. mineralisation). The chemical fertiliser application involved a similar order of magnitude of soil inorganic elements per pot as the compost extract treatments, except for N (which at 534 mg Npot-1 was much higher than the soluble inorganic N pool in the compost extracts (table 1), but also much lower than the total N pool of the extracts). Little difference existed in the level of assessed soluble nutrients between ACE and NCE (table 1). |
| However, the four compost extracts, prepared at weekly intervals, were not consistent in their bacterial or fungal loads, with differences of up to two orders of magnitude between batches. Of course, some variation is expected, given the non-sterile, non-controlled incubation conditions and variation in the compost inoculum, however this level of variation is large. Further, such variation in bulk bacterial and fungal numbers also hints at variation in species composition as well. More attention to the incubation process is recommended to reduce the observed variation in final microbial load. |
| Effects of treatments on soil microbial properties |
| In Experiment 2, although there was a difference in bacterial counts in compost extract treated soils compared to the control and chemical treatments, no differences existed in fungal loads between the treatments (Table 4). This indicates that while compost extract application may have changed fungal species composition; there was no immediate effect on total fungal (cfu) load. |
| Soil temperature and soil respiration did not differ between any of the treatments and soil types. The level of soil microbiota activity was unlikely to be sufficiently high to raise soil temperature. Soil type (as related to solar absorptivity) is likely to impact soil temperature, and therefore perhaps soil respiration, but sample variability was sufficiently high to disguise any such relationship. |
| Conclusions |
| It was hypothesised that compost extracts could increase soil solubilisation and mineralisation rates, and thus increase plant growth. In the experiments undertaken in this study, data suggest that the impact of compost extract on plant nutrition and growth was apparently though the direct addition of nutrients, rather than through an indirect effects of soil microbiota. This result is ascribed to the high rate of extract addition, at 2 L per pot (equivalent to 34,000 Lha-1). Compost extracts produced using the two extraction methods (with and without aeration), did not vary in their effect on plant growth as demonstrated by the responses of tomato and sorghum crops. Further experimentation is needed to assess compost extract contribution to plant nutrition through increasing soil microbiota, adding microbiota key to certain nutrient cycles. |
| Acknowledgements |
| The authors would like to acknowledge Rob Lang from Broadmeadows Pty Ltd, Rockhampton, Australia for supply of the rumen compost for this experiment, Brett Kuskopf (The University of Melbourne) for δ15N analysis and Endeavour Postgraduate Awards (EPA), Australia, for providing the PhD scholarship to Karuna Shrestha. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 | Table 5 |
Figures at a glance
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 |
 |
 |
 |
| Figure 4 | Figure 5 | Figure 6 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 17134
- [From(publication date):
May-2012 - Nov 21, 2025] - Breakdown by view type
- HTML page views : 12110
- PDF downloads : 5024
