Review Article Open Access
Microbial Synthesis of Silver Nanoparticles: A Review
Vibha Saklani*, Suman and V.K. JainAmity Institute of Advanced Research and Studies (Materials and Devices), Amity University, Noida, India
- Corresponding Author:
- Vibha Saklani
Amity Institute of Advanced Research and Studies (Materials and Devices)
Amity University, E-3 Block, IV Floor
Amity University Campus
Sector -125, Noida-201303
Gautam Buddha Nagar, Uttar Pradesh, India
Tel: 09810061072
E-mail: saklanivibha@gmail.com
Received date: October 27, 2012; Accepted date: December 06, 2012; Published date: December 09, 2012
Citation: Saklani V, Suman, Jain VK (2012) Microbial Synthesis of Silver Nanoparticles: A Review. J Biotechnol Biomaterial S13:007. doi:10.4172/2155-952X.S13-007
Copyright: © 2012 Saklani V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
This review paper proposes the synthesis of nanoparticles from microbes and its applications. Several microorganisms like E. coli, Lactobacillus, Pseudomonas, etc. are involved in the nanoparticles synthesis such as gold, silver, titanium, etc. This is a part of the microbes’ internal metabolism. The microorganism used for this research is E. coli. The paper has discussed the use of silver nanoparticles generated from this microbe. Their characterization and analysis has been briefly discussed.
Keywords
E. coli; Silver nanoparticles; MIC (Minimum Inhibitory Concentration)
Introduction
Nanotechnology is one-billionth of a meter, i.e. 10-9. This technology involves a unique combination of scientists from different fields including physicists, chemists, engineers and biologists. Many interesting nanodevices are useful in biomedical field especially for the improved cancer detection, diagnosis and treatment. Nanodevices work on two methods: The Top-down and the Bottom-up approach. One involves molding and etching materials into smaller components, and the other involves the assembling structures into useful devices (Figure 1).
In natural environment also, microbes produce nanomaterials as part of their metabolism and hence, can be utilized for various applications discussed in this paper. The microbes reproduce fast; therefore this characteristic can be well exploited for their use in various aspects. Their use in various applications is well known to everyone in the field of biological sciences. Biotechnology has joined hands and has emerged as an initiative for the study of microbes, and its various characteristics in the form of “microbiology”. Microorganisms are of size 10-6 nm, and they are referred to as nanofactories, meaning generators of nanoparticles. Since they are present in nature, they are also called as biofactories [1]. Nanoparticles are nowadays becoming very popular in various field of research, and are useful in combating various diseases in the form of their early and fast detection. These in their pure or in the form of mixtures help in the formation of sensors, in batteries, in diagnostic kits, in water treatment, also helpful in curing deadly diseases such as cancer. In the present investigation the synthesis of silver nanoparticles is through E. coli. This microbe in nature is also present in the human gut helps in digestion. E. coli generates silver nanoparticles as part of their metabolism, when encountered with silver ions from nature. The silver ions are then converted into silver atoms. It is also revealed that temperature also play an important role in nanoparticles size. Larger nanoparticles are produced at lower temperature, and size decreases when at higher temperatures.
Mechanism of Synthesis
Synthesis of Silver nanoparticles by microbes is due to their defense mechanism (resistance mechanism), and this is how the nanoparticles produced are useful to us. The resistance caused by the bacterial cell for silver ions in the environment is responsible for its nanoparticles synthesis. The silver ions in nature are highly toxic for the bacterial cells. So their cellular machinery helps in the conversion of reactive silver ions into stable silver atoms. Also, temperature and pH plays an important role in their production. At room temperature, the size of nanoparticles is 50 nm; at higher temperature, i.e. at 60°C, the size of nanoparticles reduces to 15 nm. This indicates that with the increase in temperature size decreases. Under alkaline conditions, nanoparticles synthesis by the microbe is more as compared to the acidic conditions. But, after pH 10 cell deaths occur. The first evidence of the synthesis comes from Pseudomonas stutzeri AG259, a bacterial strain that was originally isolated from silver mine [2]. The nanoparticles synthesis from E. coli varies with the change in AgNO3 concentration. 1mM concentration is the patented one. Silver when in lower concentration helps in inducing the organism to synthesize nanoparticles, whereas at higher concentration induces cell death.
Our Work Description of Silver Nanoparticle Synthesis and Its Uses
The nanoparticles can be artificially synthesized in vitro using chemical method via ethanol. But, here the synthesis was done through E. coli under room temperature. The supernatant was taken from the nutrient broth, incubated overnight inoculated with E. coli. Then 1 mM of AgNO3 (1% v/v) was added to the supernatant [3]. The formation of silver nanoparticles was observed within 10 minutes. The color change was noticed from fine yellow color to reddish brown with time, as shown in figure 2.
Antibacterial Effect
After keeping for a long time, there was a transition of color change (Figure 3), from fine yellow to deep reddish brown. We took four different series of color change namely, “fine yellow” as delta (δ); “light brown” as gamma (γ); “light reddish brown” as beta (β); and “deep reddish brown” as alpha (α). Alpha consisted of maximum nanoparticles concentration. Beta consisted of lesser no. of nanoparticles as compared to alpha. Gamma consisted of less nanoparticles concentration than beta. Delta consisted of least nanoparticle concentration of the all.
Therefore, one can conclude that with time nanoparticles production from E. coli increases and hence, their antibacterial property varies accordingly. The intensity of color is directly proportional to nanoparticles production.
To prove their efficacy, their zone of inhibition (MIC) was tested against E. coli. And for these, wells were created in a petri plate spread with micro diluted culture (25 μl). The created wells were filled with α, β, γ and δ samples, 50 μl each (Figure 4). Alpha (α), reddish brown color-showed the maximum inhibition, with no growth in its zone. Delta (δ), fine yellow color–showed the minimum inhibition of growth. Alpha and beta showing the maximum inhibition [4,5].
Water treatment
The colloidal solution of silver nanoparticles was taken to check its efficacy in water treatment. For this, 12 gm of stones were dipped in the colloidal solution for 10 min. They were then heat dried in microwave oven at 60°C for 10 min. Then the stones were again dipped in 10-5 solution of E. coli-25 μl of the solution was then spread onto the agar petriplate. It was kept for overnight incubation [6-9].
Results
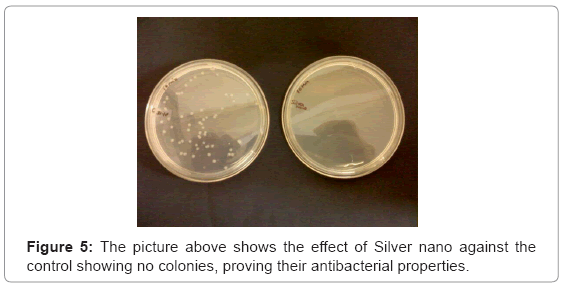
The plate contained no colonies (Figure 5), proving that the microbe generated silver nanoparticles can be effectively used in water treatment, as well where stones can be used in the form of a filter, through which water when passed, can be purified. And also the reusability of the stones showed no growth under lab conditions.
Conclusion
Interesting facts about silver nanoparticles synthesis can be very well understood when the real mechanism involved in the antimicrobial activity of silver ions is known. Silver ions are very reactive, and are known to bind to the vital cell components, inducing cell death.
However, generation of silver nanoparticles through chemical method is very tedious; whereas, through microbes such as E. coli, it is a fast and an eco-friendly approach. These can further be useful in various industrial, as well as many biomedical applications.
References
- Deepak V, Kalishwaralal K, Pandian SRK, Gurunathan S (2011) An insight into the bacterial biogenesis of silver nanoparticles, industrial production and scale-up. Metal Nanoparticles in Microbiology 17-35.
- Anil Kumar S, Abyaneh MK, Gosavi SW, Kulkarni SK, Pasricha R, et al. (2007) Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett 29: 439-445.
- Bar H, Bhui DK, Sahoo GP, Sarkar P, Priyanka S, et al. (2009) Green synthesis of silver nanoparticles using seed extract of Jatropha curcas. Colloids Surf A Physicochem Eng Asp 348: 212-216.
- Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, et al. (2009) Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces 74: 328-335.
- Kalishwaralal K, Deepak V, Ramkumarpandian S, Nellaiah H, Sangiliyandi G (2008) Extracellular biosysnthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett 62: 4411-4413.
- Nanda A, Saravanan M (2009) Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine 5: 452-456.
- Pugazhenthiran N, Anandan S, Kathiravan G, Prakash NKU, Crawford S, et al. (2009) Microbial synthesis of silver nanoparticles by Bacillus sp. J Nanopart Res 11: 1811-1815.
- Guo KW (2011) Green nanotechnology of trends in future energy. Recent Pat Nanotechnol 5: 76-88.
- El-Shanshoury AER, ElSilk SE, Ebeid ME (2011) Extracellular biosynthesis of silver nanoparticles using Escherichia coli ATCC 8739, Bacillus subtilis ATCC 6633, and Streptococcus thermophilus ESh1 and their antimicrobial activities. ISRN Nanotechnology 11:1-7.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 22939
- [From(publication date):
specialissue-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 17085
- PDF downloads : 5854