Case Report Open Access
Markers of Inflammation and Lineage on Exfoliated Colonic Cells In Pediatric Inflammatory Bowel Disease
| Padmanabhan P. Nair1,2, Alka Kamra1, George Kessie1, Shilpa Kalavapudi1, June-Home Chen1, Robert Shores1, Lisa Madairos3, Alessio Fasano3 and Prasanna Nair4* | |
| 1NonInvasive Technologies, Elkridge, Maryland, USA | |
| 2Department of International Health, Johns Hopkins University Bloomberg School of Public Health, Baltimore, Maryland, USA | |
| 3Mucosal Biology Research Center and Division of Pediatric Gastroenterology, Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA | |
| 4Division of General Pediatric Medicine, Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA | |
| Corresponding Author : | Prasanna Nair, MBBS, MPH, FAAP Professor Emeritus, Department of Pediatrics University of Maryland School of Medicine Baltimore, Maryland 21201, USA Tel: 410 328 2533 E-mail: pnair@peds.umaryland.edu |
| Received November 15, 2011; Accepted December 14, 2011; Published December 16, 2011 | |
| Citation: Nair PP, Kamra A, Kessie G, Kalavapudi S, Chen J, et al. (2011) Markers of Inflammation and Lineage on Exfoliated Colonic Cells In Pediatric Inflammatory Bowel Disease. J Gastrointest Dig Syst S8:001. doi:10.4172/2161-069X.S8-001 | |
| Copyright: © 2011 Nair PP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Objectives: The diagnosis (endoscopy, and biopsy) and continued clinical management of Inflammatory Bowel Disease (IBD), remain highly invasive, expensive, and inconvenient for the pediatric patient. The objective of this study was to see if colonocytes obtained from stools of subjects with IBD and normal controls would demonstrate higher levels of inflammatory markers (Cox 2 in CD45+ and CD45- cells) and if the inflammatory process and treatment effects would be reflected in an altered cytokine expression in the subjects compared to controls. Setting: Outpatient hospital based pediatric gastroenterology clinic. Methods and Main outcome measures: Stool samples (~ 1 gm), were obtained from 18 children between the ages of 4 and 18 diagnosed with IBD, and from a normal first degree relative. Colonocytes were isolated using the Somatic Cell Sampling Recovery (SCSR) system and assessed for the expression of COX-2, CD-45, IgA, IgG, IL6, IL18, TGF ß, TNF, and IL16ß using flow cytometry. In addition, levels of COX-2 and cytokeratin 19 transcripts were measured by microwell plate hybridization assay. Results: Expression of COX-2 and co-expression of IgA and IgG were significantly higher in the IBD cases compared to the controls. In ulcerative colitis, the expression of COX-2 and co-expression of COX-2 and CD45 were greater than that in patients with Crohn’s disease. In contrast, cells expressing IgA and IgG were higher in Crohn’s. Subjects on immunosuppressants and/or anti-inflammatory medications, expressed significantly lower levels of COX-2 and IL-18 compared to those who were not on treatment. Conclusions: This study indicates that the use of disease markers on exfoliated colonic cells can be used for noninvasive assessment of disease status, for follow-up of response to treatment and for forecasting flare-up of disease before its symptomatic manifestations.
| Keywords |
| Inflammatory Bowel Disease; Pediatric patient; Cox 2; CD45+ and CD45- cells; TNF; Cytokine expression |
| Abbreviations |
| IBD: Inflammatory Bowel Disease; SCSR: Somatic Cell Sampling and Recovery; IgA: Immunoglobulin A; IgG: Immunoglobulin G; COX-2: Cyclooxygenase 2; TNF: Tumor Necrosis Factor; IL: Interleukin |
| Introduction |
| Inflammatory Bowel Disease (IBD) is a major chronic illness among children, adolescents and young adults (recent reviews) [1-4]. In ulcerative colitis and Crohn’s disease, the two major forms of IBD, a persistent inflammatory reaction is an integral component of the clinical manifestations of this disease. Ulcerative colitis typically presents with blood in the stools and diarrhea. The onset may be insidious and only if symptoms last for over 2 weeks is a diagnosis of IBD rather than an infectious etiology considered. Crohn’s disease may present with GI symptoms including abdominal pain and bloody diarrhea, however systemic manifestations are more common than with ulcerative colitis. These include fever, malaise, easy fatigability, growth failure, anemia, skin rashes, and arthritis [3,4]. Diagnosis of ulcerative colitis is made by endoscopy and colonic biopsy; barium enema has no place in the diagnosis of UC. A diagnosis of Crohn’s disease is made from the history, physical, laboratory findings (Hb, Hct, WBC, ESR, CRP), endoscopy and radiologic examination [5]. These diagnostic studies, although essential for establishing the diagnosis, are highly invasive, expensive, and inconvenient for the patient. This is particularly true because the disease is chronic and these tests may need to be repeated. Nevertheless, the differential diagnosis between the two IBD forms is complicated by the overlapping clinical presentations, similar outcomes in non-invasive tests, and by the histological evaluation of intestinal biopsies that sometimes does not show typical features of either condition. Once a diagnosis is made the clinical course of the disease during treatment is assessed largely by following the subjective reports of the patient and frequent repeat endoscopies. The diagnosis and continued clinical management of IBD is expensive and time consuming (in terms of clinician time per patient). Previous studies have shown that colonic cells can be recovered in a viable state from stool samples and examined for the presence of cells carrying specific biomarkers of neoplastic transformation [6-16]. Recent studies have indicated that alterations in cytokine synthesis may play a role in inflammatory bowel disease (IBD) pathogenesis. Cytokines studied in IBD patients included TNF-alpha, TGF-beta, IL-1, IL-10, IL-6, IL-12, IL-18, IL- 23, IL-27, and IFN-gamma [17-20]. Following confirmatory endoscopy, this relatively inexpensive laboratory procedure could assist in those cases in which the differential diagnosis between Crohn’s disease and ulcerative colitis is difficult and could be used to monitor the patient’s clinical progress during treatment, repeatedly, without undergoing the usual invasive procedures. The purpose of this study was to see if colonocytes obtained from stool of subjects with IBD would demonstrate higher levels of inflammatory markers compared to normal controls and if inflammatory process and treatment effects would be reflected in an altered cytokine expression in the subjects compared to controls. This study was designed as an initial proof of concept for this new approach to the pathophysiology of IBD at the cellular level, noninvasively. |
| Materials and Methods |
| Patients and Procedures |
| Children between 4 and 18 years, with a diagnosis of IBD, followed in the Pediatric Gastroenterology Clinic of the University of Maryland Hospital in Baltimore, were eligible for enrollment. The Institutional Review Board approved the study. A nurse patient coordinator reviewed the study protocol with the patient and/or parents to ascertain eligibility and obtained informed consent for participation. Non-symptomatic first degree relatives (siblings or parents) of subjects who were willing to cooperate were enrolled as control subjects. Twenty four families consented to participate in the study. Study participants, were asked to provide two 0.5 gm stool samples collected on consecutive days within five days prior to clinically indicated colonoscopy. A clinical research nurse administered a questionnaire to obtain patient’s subjective perception of health status. |
| Sample collection |
| The patients were provided with a stool collection kit consisting of two screw-capped collection vials containing 15 ml of a nontoxic transport medium. Approximately, 0.5 gm of stool was collected using the plastic ladle attached to the screw cap and placed in the liquid medium. The tube was capped and the sample sent to the research laboratory via courier without refrigeration. When the samples arrived in the laboratory, they were logged in and processed for the isolation of exfoliated colonic cells [13]. |
| Immunological Assays on Exfoliated Cells |
| COX-2, CD45, IgA, and IgG expression on exfoliated colonocytes: Cell surface markers were assessed using flow cytometry. For each of the patients and controls 2 stool samples were collected on two separate days, a week before the patient’s scheduled colonoscopy. Colonic cells from the interface and pellet were isolated for analysis. |
| Quantification of mRNA by Microtiter Plate Hybridization Assay |
| Levels of COX-2 and cytokeratin 19 transcripts in colonocyte samples were measured using microwell plate hybridization assay. COX-2 and cytokeratin 19 synthetic oligonucleotides (sense) aminated at the 5’ end via a C12 linker were used as capture probes, covalently attached to a 96 microwell plate (Costar) coated with reactive N-hydroxysuccinimide ester groups (NOS) (DNA-BIND, Corning, New York). Following coupling and blocking of residual NOS groups, denatured biotinylated target amplicons were hybridized to the probes in the wells. Hybridized PCR products were detected by the colorimetric signal generated by the standard streptavidin-horseradish peroxidase enzyme system. The amount of hybridized PCR product is an indirect measure of the amount of target transcripts in colonocyte samples and is quantitated from a standard curve generated using varying amounts of synthetic biotinylated antisense oligonucleotide hybridized to immobilized capture probes. |
| Measurement of Intracellular Cytokines |
| Flow Cytometry assays for surface antigens of COX- 2, IgA, and IgG were performed using a direct staining technique as described by Nair et al. [13] LS180 (colon carcinoma cell line, ATCC # CL-187) was used as a positive control in all the gene expression assays. |
| Oligonucleotide probes and primers |
| All synthetic oligonucleotides used for the study were designed using Primer3 software (MIT, Boston, MA) from published sequences (GenBank Accession# M90100 for Cox-2 & GenBank Accession# BC007628 for Cytokeratin 19). The oligonucleotides were synthesized by Sigma Genosys (The Woodlands, TX). Sequences are as follows: |
| Capture probes: Cox-2: 5’- H2N-TGG CTA CAA AAG CTG GGA AGC CTT-3’ (nt 457-480). |
| Cytokeratin19: 5’H2N-AGC TGC CTT GGA AGA CAC ACT G-3’) (nt 997-1018). |
| Biotinylated control oligonucleotides: Cox-2: 5’-biotin-GGT TAG AGA AGG CTT CCC AGC TTT TGT AGC CAT AGT CAG C-3’ (nt 488-449). |
| Cytokeratin 19: 5’-biotin-CGT TTC TGC CAG TGT GTC TTC CAA GGC AGC TTT CAT GCT C-3’ (40-mer; 1027-988). |
| PCR primers: Cox-2 (F): 5’-GGC GCT CAG CCA TAC AGC AAA-3’ |
| Cox-2 (R): 5’-biotin-ATC ATC AGG CAC AGG AGG AAG GGC3’ |
| Cytokeratin 19 (F): 5’-ATC CTG AGT GAC ATG CGA AGC-3’ |
| Cytokeratin 19 (R): 5’-biotin-CAT GAG CCG CTG GTA CTC CTG-3’ |
| RNA Extraction |
| Total RNA was isolated from colonocytes and LS180 cells with the Pico PureTM RNA isolation kit (Arcturus, Mountain View, CA) according to the manufacturer’s instructions. Genomic DNA was degraded using on-column digestion with 25 U of RNase-free DNAse I (Ambion, Austin, TX) for 30 min and the RNA eluted with 30 μl of nuclease-free water. |
| RT-PCR |
| First strand cDNA was prepared from 200 ng of total RNA extracts using a commercial kit (Thermoscript RT-PCR system for First- Strand cDNA synthesis; Invitrogen, Carlsbad, CA). Negative controls consisted of cDNA templates with no reverse transcriptase to test for the absence of genomic DNA. |
| Agarose gel electrophoresis |
| RT-PCR products were analyzed by electrophoresis on 2% agarose gels, stained with SYBR green I (Invitrogen, Carlsbad, CA) and visualized under UV illumination. |
| Quantification of mRNA by microtiter plate hybridization assay |
| Levels of Cox-2 and cytokeratin 19 transcripts in colonocyte samples were measured using microwell plate hybridization assay. |
| Immobilization of capture probes |
| Immobilization of capture probes was performed according to the manufacturer’s instructions. Cox-2 and cytokeratin 19 capture probes (25 pmoles) in 100 μl of coating buffer (50 mM sodium phosphate, pH 8.5, 1 mM EDTA) was added to each well of DNA-Bind plate. |
| Statistical analyses |
| Statistical analyses were performed using the Statistical Package for Social sciences 11.5 (SPSS Inc., Chicago, Il, 2002). Inflammatory surface markers and intracellular cytokine expression in patients and controls were compared. Statistical significance was determined using t tests and Chi-Square Tests. We considered a P value of ≤ 0.05 significant. As recommended by Lipsey for some of the variables we set an alpha at 0.10, rather than the standard of 0.05 because of the small sample size [21]. Although this approach increases the possibility of a Type Ð�? error (erroneously reporting a difference), it decreases the likelihood of a Type Ð�?Ð�? error (reporting no difference when a difference exists) [21]. A multivariate analysis of variance was conducted to estimate the effect of severity of clinical symptoms, colonoscopy findings, pathological findings/diagnosis, treatment and demographic characteristics on inflammatory markers/cytokines. |
| Results |
| Clinical Characteristics of the Patients |
| Twenty four families agreed to participate, of which 22 families completed the study as designed. Adequate stools were received from 18 subjects whose clinical symptoms are listed on (Table 1). There was an equal distribution of males to females (m=9, f=9), 9.1% reported a family history of IBD, 77.7% were Caucasian and 22.3 % African American. Seven subjects reported mild abdominal pain (38.9%), 5 moderate pain, (27.8%) and 6 none (33.3%). Total signs on the Harvey Bradshaw index ranged from 0 to 7 [22]. |
| Assessment of severity by colonoscopy and histology are listed on (Table 2) Seven (38.9%) had evidence of ulcerative colitis, 11 (61.1%) had diagnosis of Crohn’s by colonoscopy or pathology. Histopathology showed evidence of severe inflammation in 4 (22.2%), moderate inflammation in 10 (55.5%), mild or none in 4 (22.2%). Eight (44.4%) of the subjects were on immunosuppresants and/or antimetabolite treatment. |
| Expression of COX-2 in exfoliated cells from patients compared with controls |
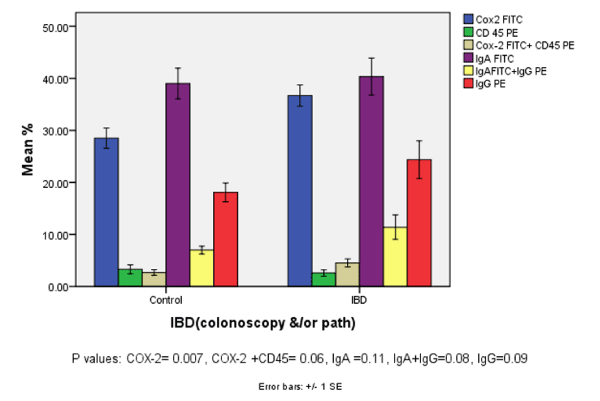
| Figure 1 shows that the expression of COX-2 was significantly higher in the IBD cases compared to the controls (p value 0.007). Coexpression of COX-2 +CD45 and IgA + IgG as determined by twocolor flow cytometry was elevated in IBD but did not reach statistical significance (p value 0.06 and 0.08 respectively). Individually, CD 45, IgA, and IgG were similar in patients compared to controls. In this analyses all patients, regardless of their disease status were compared to their first degree relatives (control). |
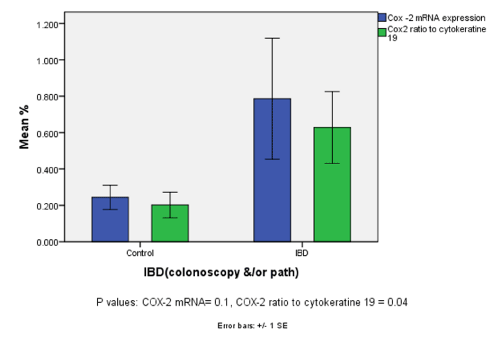
| Figure 2 presents results of COX-2 mRNA expression (pellet and interface). In general COX-2 expression in IBD patients was consistently higher than in the corresponding normal subjects but did not reach statistical significance (p value 0.07). However the values reached statistical significance when adjusted for the expression of housekeeping protein cytokeratin 19 (p value 0.04) |
| Expression of intracellular cytokines in patients compared with controls |
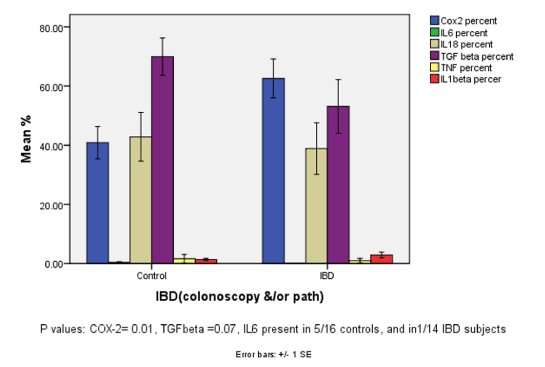
| Intracellular cytokines IL6, IL18, TGF β, TNF and COX-2 are presented in Figure 3. COX-2 was significantly higher in subjects compared to controls (p value 0.04) Cytokine levels were lower in IBD compared to normal subjects, TGF β was also lower in IBD patients (p value 0.01). IL6 was detected in only one of the IBD patients, but was present in 5 of the normal subjects. |
| Response of inflammatory markers to treatment with immunosupressants |
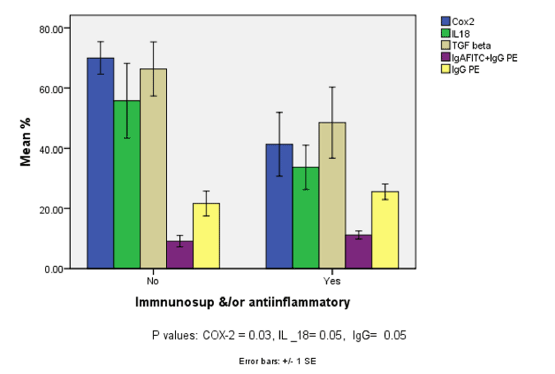
| Within the group of IBD patients, those who were on immunosuppressants and/or anti-inflammatory medications, expressed significantly lower levels of COX-2 and IL-18 compared to those who were not on treatment (Figure 4). In contrast, in this treated group the number of cells expressing IgG by itself was enhanced (Figure 4) compared to the untreated group. |
| Characteristics distinguishing Crohn’s from ulcerative colitis |
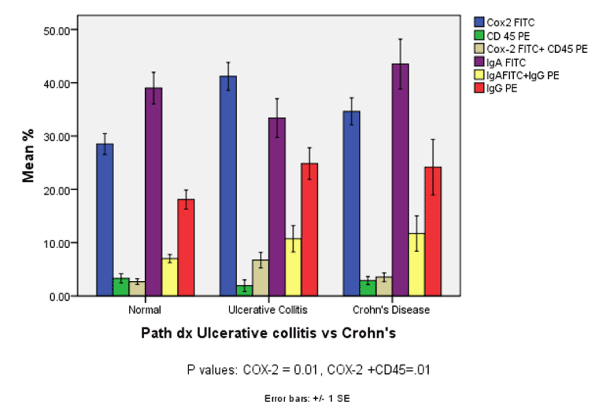
| Analysis of cells from patients with Crohn’s or ulcerative colitis and their normal first degree relatives indicated that children with ulcerative colitis had significantly higher levels of COX-2 and coexpression of COX-2 and CD45 compared to the other two groups (Figure 5). Patients with Crohn’s had significantly higher numbers of IgA and IgA + IgG positive cells when compared to patients with ulcerative colitis and normal first degree relatives. |
| Discussion |
| We applied this noninvasive approach to screen patients suspected of suffering from IBD by demonstrating the presence of inflammatory cells in stools. Following confirmatory endoscopy, this relatively inexpensive laboratory procedure could assist in those cases in which the differential diagnosis between Crohn’s disease and ulcerative colitis is difficult and could be used to monitor the patient’s clinical progress during treatment, repeatedly, without undergoing the usual invasive procedures. Enumeration of inflammatory cells by two-color immunofluorescent flow cytometry provides a means of monitoring inflammatory activity to follow epithelial restitution of the colonic mucosa during periods of remission. The feasibility of this approach has been demonstrated in an earlier report [23]. |
| Using a noninvasive approach for isolating colonic epithelial cells from stool samples, we were able to study the expression of COX2, IgA, IgG, cytokines and CD45 as a measure of disease severity in children diagnosed with IBD. The expression of COX-2 and coexpression of IgA & IgG as determined by two-color flow cytometry were significantly higher in the IBD patients compared to controls. CD45 alone as a measure of inflammatory cells showed no difference between patients and controls, suggesting that inflammatory response may not be primarily a result of recruitment of inflammatory cells of lymphoid lineage (CD45+ cells) to the diseased sites. Multivariate analysis, controlling for sex, race, and age did not alter the significance in the differences between patients and control first degree relatives. |
| In addition to being noninvasive, this technique suggests features that might distinguish the inflammatory response between ulcerative colitis and Crohn’s disease. Since the higher COX-2 response in ulcerative colitis compared to Crohn’s disease is associated with CD45+ inflammatory cells, it appears that the inflammatory response associated with cells of lymphoid lineage (CD45+) is a distinguishing feature of ulcerative colitis. |
| This is consistent with previous observations that ulcerative colitis patients had significantly more positive COX-2 expression in inflammatory cells [24]. Studies conducted on intestinal mucosal biopsies of patients with IBD have shown a significantly higher expression of CD45RA in CD4(+) T lymphocytes from patients with ulcerative colitis compared with those from patients with Crohn’s disease [25]. This signifies that in ulcerative colitis, the inflammatory cells are predominantly from the lamina propria and lymphoid follicles in contrast to epithelial cells from the mucosa. |
| In our study, cells expressing IgA and IgG were more numerous in Crohn’s disease compared to those from ulcerative colitis. Our hypothesis is that this is indicative of a hyperimmune response at the epithelial level of the mucosa, characteristic of this condition. |
| Analyses of biomarkers on exfoliated colonic cells can be used for the assessment of disease status, for follow-up of response to treatment and for forecasting flare-up of disease before symptomatic manifestations. Furthermore, in earlier studies we have established the fact that cells isolated by the SCSR technique is anatomically representative of the entire colonic mucosa starting at the ileo-caecal junction [8,15,26]. This is based on the fact that cells expressing the blood group antigen, detectable in our cell isolates, originate only from the proximal segments (caecum and proximate colon) of the colon. The distinct advantage of this procedure is that it can be performed repeatedly without any discomfort to the patient. This study has shown the feasibility of monitoring patients with IBD in a noninvasive manner. |
| Funding |
| This project has been funded by an SBIR grant R44-DK56567 from the US National Institutes of Health, National Institutes of Diabetes, Digestive and Kidney Diseases. |
| Competing Interests |
| PPN and PN are Principals in NonInvasive Technologies LLC |
| Ethics approval |
| This study was conducted with the approval of the Institutional Review Board of the University of Maryland School of Medicine and Hospital. |
| Significance of this study |
| What is already known about this subject? |
| • Inflammatory Bowel Disease is associated with an inflammatory process of the bowel mucosa. |
| • Endoscopy followed by colonic biopsy to detect the presence of an inflammatory process by means of microanatomic pathology is the established standard of diagnosis and follow-up during treatment. |
| • These procedures, though essential for establishing a diagnosis, are invasive, and particularly traumatic for children when the disease is chronic and may need to be repeated. |
| What are the New Findings? |
| • Significant numbers (in the millions) of viable colonic cells can be recovered from a small sample of stool (1.0 gm) from these patients and quantitatively assessed for the presence of inflammatory cells while delineating them by lineage (epithelial versus lymphoid). |
| • This procedure (Somatic Cell Sampling and Recovery, SCSR™) allows for a true sampling of all exfoliated cells, anatomically representative of the entire colonic mucosa, from the ileo-caecal junction to the rectum. |
| • Patients with inflammatory bowel disease are distinguishable from their unaffected first degree relatives by the presence of cells of both lymphoid (CD 45+) and non-lymphoid lineage (CD 45-) expressing markers of inflammation (COX-2 and cytokines). |
| • The quantitative burden of inflammatory cells in a stool sample is consistent with disease severity as assessed by endoscopy and microanatomical pathology. |
| • Response to therapy with immunomodulators was reflected in a concomitant decrease in the percentage of inflammatory cells. |
| How might it impact on clinical practice in the foreseeable future? |
| • The technology allows for a non-invasive approach to the repeated monitoring of disease severity as well as response to treatment of pediatric IBD patients. |
| • The feasibility of obtaining viable exfoliated colonic cells, non-invasively should encourage further investigations into the characterization of these cells for their disease propensities. |
References
- Mizoguchi A, Mizoguchi E (2008) Inflammatory bowel disease, past, present and future: lessons from animal models. J Gastroenterol 43: 1-17.
- Sands BE (2007) Inflammatory bowel disease: past, present and future. J Gastroenterol 42: 16-25.
- yams J (2004) Inflammatory Bowel Disease. In: Behrman RE, Kliegman RM, Jenson HB, editors. Nelson Text Book of Pediatrics, 17th ed. Philadelphia: Saunders Elsevier 1248-1255.
- Fish D, Kugathasan S (2004) Inflammatory bowel disease. Adolesc Med Clin 15: 67-90.
- IBD Working Group of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (2005) Inflammatory bowel disease in children and adolescents: recommendations for diagnosis--the Porto criteria. J Pediatr Gastroenterol Nutr 41: 1-7.
- Iyengar V, Albaugh GP, Lohani A, Gold D, Nair PP (1990) Cytologic and immunochemical characterization of colonic cells isolated from human stools by density gradient. FASEB J 4: 1165.
- Iyengar V, Albaugh GP, Lohani A, Nair PP (1991) Human stool as a source of viable colonic epithelial cells. FASEB J 5: 2856-2859.
- Albaugh GP, Iyengar V, Lohani A, Malayeri M, Bala S, et al. (1992) Isolation of exfoliated colonic epithelial cells: a novel non-invasive approach to the study of cellular markers. Int JCancer 52: 347-350.
- Nair PP, Bala S, Albaugh GP (1991) Lymphoid cell differentiation markers are expressed on exfoliated human colonocytes. FASEB J 5: 945.
- Cremins J, Salata K, Kikendall JW, Hershey J, Nair PP, et al. (1994) Colonocyte expression of CD and HLA markers in patients with normal, and neoplastic colons. Gastroenterology 106: A379.
- Dutta SK, Ashktorab H, Vinayek SM, Shami S, Nair PP (1996) Evaluation of CD44 expression in isolated fecal colonocytes as a marker for non-invasive detection of colonic polyp and cancer. Gastroenterology 110: A570.
- Dutta SK, Nair PP (1998) Noninvasive detection of colorectal cancer by molecular tools: Coming of age. Gastroenterology 114: 1333-1335.
- Yamao J, Matsumura Y, Shimada Y, Moriya Y, Sugihara K, et al. (1998) Abnormal expression of CD44 variants in the exfoliated cells in the feces of patients with colorectal adenocarcinoma. Gastroenterology 114: 1196-1205.
- Desilets DJ, Davis KE, Nair PP, Salata KF, Maydonovitch CL, et al. (1999) Lectin binding to human colonocytes is predictive of colonic Neoplasia. Am J Gastroenterol 94: 744-750.
- Nair PP, Lagerholm S, Dutta S, Shami S, Davis K, et al. (2003) Coprocytobiology: On the nature of cellular elements from stools in the pathophysiology of colonic disease. J Clin Gastroenterol 36: S84-S93.
- Lagerholm S, Lagerholm S, Dutta S, Nair PP (2005) Noninvasive detection of c-myc p64, c-myc p67, and c-erbb-2 in colorectal cancer. Scand J Gastroenterol 40: 1343-1350.
- Wang J, Fu YX (2005) Tumor necrosis factor family members and inflammatory bowel disease. Immunol Rev 204: 144-155.
- Schmidt C, Giese T, Ludwig B, Mueller-Molaian I, Marth T, et al. (2005) Expression of interleukin-12-related cytokine transcripts in inflammatory bowel disease: elevated interleukin-23p19 and interleukin-27p28 in Crohn's disease but not in ulcerative colitis. Inflamm Bowel Dis 11: 16-23.
- Kader HA, Tchernev VT, Satyaraj E, Lejnine S, Kotler G, et al. (2005) Protein microarray analysis of disease activity in pediatric inflammatory bowel disease demonstrates elevated serum PLGF, IL-7, TGF-beta1, and IL-12p40 levels in Crohn's disease and ulcerative colitis patients in remission versus active disease. Am J Gastroenterol 100: 414-423.
- Aizawa Y, Sutoh S, Matsuoka M, Negishi M, Torii A, et al. (2005) Association of interleukin-18 gene single-nucleotide polymorphisms with susceptibility to inflammatory bowel disease. Tissue Antigens 65: 88-92.
- Lipsey MW (1990) Design sensitivity: Statistical power for experimental research. Newbury Park, California, SAGE Publication Ltd.
- Harvey RF, Bradshaw JM (1980) A simple index of Crohn’s disease activity. Lancet 1: 514.
- Lagerholm SA, Dutta SK, Merchant NB, Nair PP (2001) COX-2 expression in fecal colonocytes from patients with inflammatory bowel disease (IBD). Gastroenterology 120: A4.
- Paiotti AP, Artigiani Neto R, Forones NM, Oshima CT, Miszputen SJ,et al. (2007) Immunoexpression of cyclooxygenase-1 and -2 in ulcerative colitis. Braz J Med Biol Res 40: 911-918.
- Ten Hove T, The Olle F, Berkhout M, Bruggeman JP, Vyth-Dreese FA, et al. (2004)Expression of CD45RB functionally distinguishes intestinal T lymphocytes in inflammatory bowel disease. J Leukoc Biol 75: 1010-1015, Epub 2004 Mar 12.
- Kamra A, Kessie G, Chen JH, Kalavapudi S, Shores R, et al. (2005) Exfoliated colonic epithelial cells: Surrogate targets for evaluation of bioactive food components in cancer prevention. J Nutr 135: 2719-2722.
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 13415
- [From(publication date):
specialissue-2012 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 8890
- PDF downloads : 4525
