Review Article Open Access
Manufacturing Pharmaceutical-Grade Interferons Using Silkworm- Baculovirus System
Takashi Tanaka*Chemical Engineering Department, Toray Industries, Inc., 9-1, Oe-cho, Minato-ku, Nagoya 455-8502, Japan
- Corresponding Author:
- Takashi Tanaka
Chemical Engineering Department, Toray Industries, Inc.
9-1, Oe-cho, Minato-ku, Nagoya 455-8502, Japan
E-mail: Takashi_Tanaka2@nts.toray.co.jp
Received date: January 24, 2012; Accepted date: February 21, 2012; Published date: February 23, 2012
Citation: Tanaka T (2012) Manufacturing Pharmaceutical-Grade Interferons Using Silkworm-Baculovirus System. J Biotechnol Biomaterial S9:002. doi:10.4172/2155-952X.S9-002
Copyright: © 2012 Tanaka T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Keywords
Baculovirus; BmNPV: Silkworm; Interferon; Manufacturing
Introduction
Silkworm is scientifically named as “Bombyx mori”, belonging to Lepidoptera family. Unlike the other wild insect in the same family, the ability to move and fly long distance in search of food is weakened or lost. They have been known one of the earliest domesticated insects kept by human beings. These characteristics are very suitable for manufacturing units or using experimental insect.
The Bombyx mori nucleopolyhedrovirus (BmNPV) is a kind of baculovirus, and expression and purification of proteins using the BmNPV were established by Dr. Maeda et al. [1-2]. In this technology, recombinant virus is prepared by inserting an objective protein gene to virus genome, and is injected into silkworm for the expression of recombinant protein. Large amount of recombinant protein is produced in hemolymph or fat bodies based upon the signal sequence. The expressed protein has similar posttranslational modifications and physiological activity in many cases compared with those in mammalian cells except for glycosylations [3-5]. Cytokines such as murine IL-3k [3], human GM-CSF [6], human Interferon-β [7], and human M-CSF [8], human growth hormone [9], canine parvovirus antigen [10], and malaria antigen [11] have been reported to be expressed in silkworm.
Taking particular note of these excellent characteristics of recombinant protein production in insect, we have been developed companion animal medicines with this technology. In 1994, feline interferon was commercialized as the first medicine produced using silkworm expression system. In 2005, canine interferon-γ was commercialized subsequently.
Feline Interferon
Expression
Feline interferon cDNA was cloned from feline thymus cell line LSA-1 that has an ability to produce feline interferon. At first, COS-1 cell was transiently expressed with cDNA library prepared from LSA-1 cell, and the culture supernatant was screened with interferon activity and feline interferon cDNA was cloned [12]. Using this cDNA, interferon expression was investigated using various common recombinant expression systems: 2×105 unit/mL in E. coli, 5×103 unit/mL in yeast, 9×105 unit/mL in COS-1, and 9×105 unit/mL in CHO. However, these expression levels were insufficient for commercial applications and further improvement of the productivity was an important issue in the drug development.
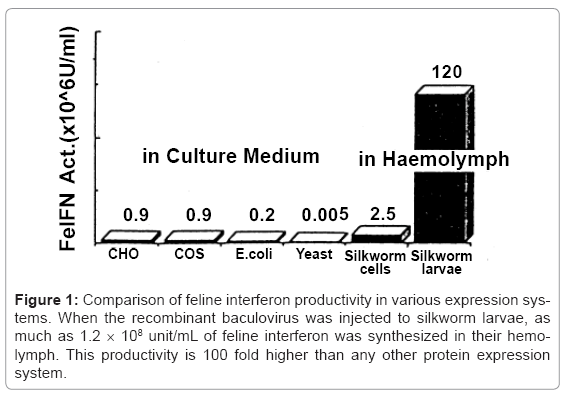
At that time, baculovirus expression system in silkworm cell lines and the silkworm using recombinant baculovirus vectors had been reported [1-2]. Recombinant BmNPV harboring feline interferon cDNA was constructed using a transfer vector pBM030 [4], in which high expression potency was demonstrated in B. mori cells and silkworm larvae. Feline interferon cDNA was inserted into downstream of polyhedron promoter in pBM030 containing untranslated regions around polyhedron gene, and co-transfected to silkworm cell together with BmNPV DNA. Recombinant BmNPVs were picked up and cloned by repeating limiting dilutions. Two and half million unit/mL of feline interferon was produced in culture supernatant of the recombinant BmNPV–infected silkworm cells. However, this production system was not satisfactory for industrial manufacture, because the cells grew slowly. Therefore, silkworm larvae were used for feline interferon production, and finally the productivity improved 1.2 × 108 unit/mL, which is 100 fold higher than any other production system [13]. The productivity from various expression systems was compared in (Figure 1).
Figure 1: Comparison of feline interferon productivity in various expression systems. When the recombinant baculovirus was injected to silkworm larvae, as much as 1.2 × 108 unit/mL of feline interferon was synthesized in their hemolymph. This productivity is 100 fold higher than any other protein expression system.
Purification
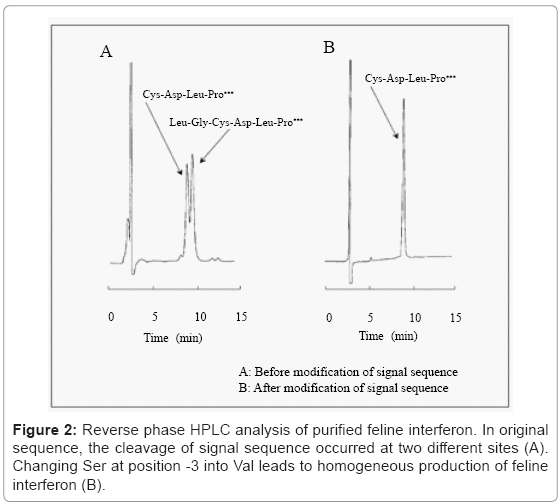
After inactivation of the recombinant virus and proteases with hydrochloric acid, the hemolymph was neutralized and centrifuged. The supernatant was purified by blue Sepharose and copper chelate Sepharose column chromatography. Reverse phase HPLC analysis shows that purified feline interferon has two peaks (Figure 2A). In N-terminal amino acid sequence analysis of the collected peak samples, one peak sample showed a consensus N-terminal amino acid sequence Cys-Asp-Leu-Pro, and the other Leu-Gly-Cys-Asp-Leu-Pro, which had additional two amino acids. As it suggested that the cleavage of signal sequence occurred at two different sites, alternate signal sequence was examined. According to report by Heijne G. von [14], Ser at -3 positions to the signal peptide sequence end was exchanged to Val, and new recombinant BmNPV was constructed. As a result, purified feline interferon from the new recombinant BmNPV–infected silkworm showed a single peak in reverse phase HPLC analysis (Figure 2B) [15]. The productivity of original and new recombinant BmNPVs was 7.7 × 107 and 1.2 × 108 unit/mL hemolymph, respectively. N-terminal sequence of the purified feline interferon from the new recombinant BmNPV was identified to be Cys-Asp-Leu-Pro as expected. But C-terminal sequence was Glu 170 in contradiction to Lys 171, which was expected as per the cDNA sequence. It was possible due to digestion by protease from silkworm or baculovirus, but the cause is not known clearly.
Canine Interferon-γ
Expression of canine interferon-γ in silkworm larvae
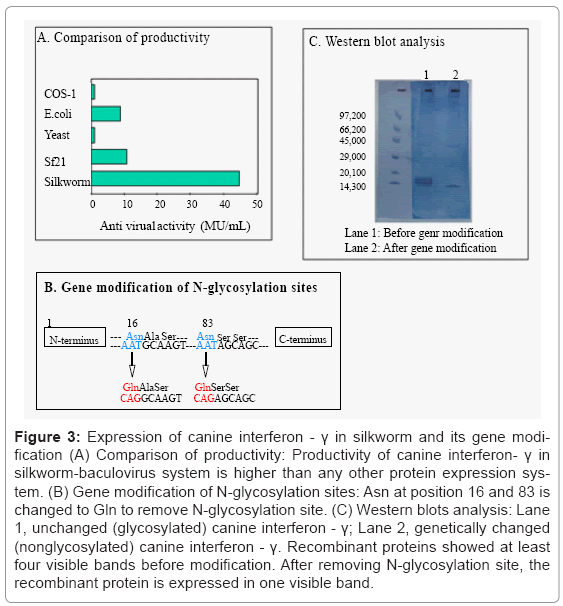
cDNA sequence of canine interferon-γ had already been reported by Devos et al. [16], cDNA of interferon-γ was obtained by reverse transcription-polymerase chain reaction. Recombinant BmNPV was prepared and expressed canine interferon-γ and the productivity was compared with various expression systems (Figure 3A). Silkworm expression system showed the highest productivity as well as feline interferon. The expressed canine interferon-γ in silkworm hemolymph showed four main bands in western blotting with anti-interferon-γ antiserum (Figure 3C), lane 1), because it formed complex protein [17]. There are two N-glycosylation sites in the canine interferon-γ which means that the presence of different bands might be due to the difference of N-glycosylation. In order to remove glycosylation, the sequence of N-glycosylation site was altered (Figure 3B) and the new BmNPV was constructed. The western blotting analysis of the newly BmNPV– infected silkworm hemolymph showed the single band of canine interferon-γ (Figure 3C, lane 2). There was no difference in antiviral activity between glycosylated and non–glycosylated canine interferon-γ produced in silkworm (1.0 × 106 unit/silkworm). In the case of the expression in insect cell line Sf21, canine interferon-γ showed a single band in western blotting analysis. This shows that glycosylation pattern and degradation pattern by protease are different between insect cell line and silkworm in the case of canine interferon-γ. In silkworm larvae hemolymph, modification and degradation pattern of recombinant protein is supposed to become varied due to tissue destruction.
Figure 3: Expression of canine interferon - γ in silkworm and its gene modification (A) Comparison of productivity: Productivity of canine interferon- γ in silkworm-baculovirus system is higher than any other protein expression system. (B) Gene modification of N-glycosylation sites: Asn at position 16 and 83 is changed to Gln to remove N-glycosylation site. (C) Western blots analysis: Lane 1, unchanged (glycosylated) canine interferon - γ; Lane 2, genetically changed (nonglycosylated) canine interferon - γ. Recombinant proteins showed at least four visible bands before modification. After removing N-glycosylation site, the recombinant protein is expressed in one visible band.
Degradation of C-terminal end
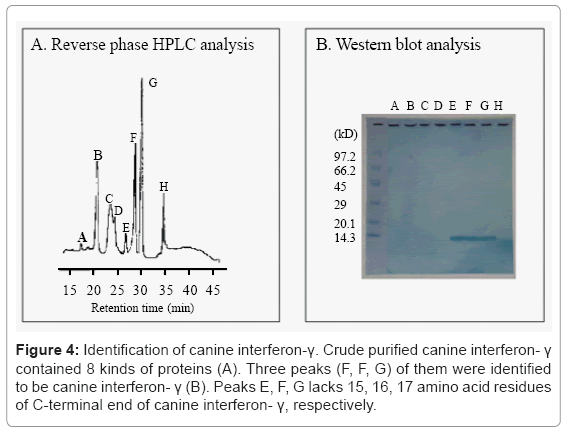
After canine interferon-γ lacking the glycosylation site was purified, further variants were found to exist. The hemolymph of recombinant BmNPV-infected silkworm larvae was treated with quaternary ammonium salts to inactivate the viruses, and the crude canine interferon-γ was purified by passing through the copper chelating Sepharose. Reverse phase HPLC analysis revealed that the crude canine interferon-γ contained 8 peaks (Figure 4A). Western blotting analysis of the collected 8 peaks showed that peaks of E, F, G were identified to be canine interferon-γ (Figure 4B).
Figure 4: Identification of canine interferon-γ. Crude purified canine interferon- γ contained 8 kinds of proteins (A). Three peaks (F, F, G) of them were identified to be canine interferon- γ (B). Peaks E, F, G lacks 15, 16, 17 amino acid residues of C-terminal end of canine interferon- γ, respectively.
From C-terminal amino acid sequence, peaks E, F, G lacks 15, 16, and 17 amino acid residues of C-terminal end of canine interferon-γ, respectively (abbreviated as CaIFN-γ/15(-), CaIFN-γ/16(-), and CaIFN-γ/17(-)). C-terminal amino acid of CaIFN-γ/15(-), /16(-), and /17(-) is Lys, Arg, and Leu, respectively. It suggests that fulllength canine interferon-γ may be degraded by trypsin-like protease or chimotrypsin-like protease in a limited way. Trypsin-like protease activity was detected in the silkworm hemolymph expressing canine interferon-γ, but chymotrypsin-like not. Addition of EDTA to crude purified canine interferon-γ reduced the generation of CaIFN-γ/17(-) significantly, suggesting that trypsin-like protease is involved in the generation of CaIFN-γ/15(-) and CaIFN-γ/16(-), and a kind of metalloprotease is involved in the generation of CaIFN-γ/17(-) (Figure 5), but the details are yet not clear.
BmNPV codes cysteine protease and the activity is detected in the BmNPV–infected silkworm larval hemolymph [18,19]. However, the cysteine protease may not be involved in the case of canine interferon-γ, because the generation of CaIFN-γ/15(-), CaIFN-γ/16(-), and CaIFN-γ/17(-) is inhibited at pH 4 and stimulated under alkaline condition.
By optimizing the operation of anion-exchange column chromatography, CaIFN-γ/16(-) was purified significantly. From the N-terminal amino acid sequence, signal peptide was cleaved correctly at single site and the purified CaIFN-γ/16(-) had the characteristic bioactivity of canine interferon-γ represented by anti-virus activity, anti-tumor activity against canine neoplastic cells, and facilitation activity of the expression of MHC class II antigen [17]. The specific activity was 9.0 × 107 unit/mg, which is comparable to that of non truncated canine interferon-γ [17].
Conclusion
Feline and canine interferon’s were developed successfully using silkworm expression system. Two kinds of interferons were already approved by European Medicines Agency, Ministry of Agriculture, Forestry and Fisheries of Japan, and others, and commercialized as medicines for companion animals. Feline interferon and canine interferon- γ were approved as a treatment of feline and canine viral diseases in 20 countries, and canine atopic dermatitis in Japan, respectively (Figure 6). In the manufacturing of medicines, a stable supply of products with constant quality is of utmost importance. For this purpose, quality control should be preceded in every step of production, purification, and formulation. A certain type of modified form of objective product is noted as impure substances, but when some of them contain active substance, the content ratio of these modified products must be controlled within the defined level. In our experience, recombinant proteins produced in silkworm had various types of variants due to degradation. In the case of feline interferon, cleavage of signal peptide occurred at two different sites. In the case of canine interferon-γ, the difference of glycosylation and limiting degradation of C-terminal end were identified. Wild-type human interferon-ω1 is cleaved at two different sites in signal peptide sequence [20]. The recombinant human interferon-γ expressed in E. coli or animal cells is degraded at its C-terminal region. These facts show that these differences in modification are partly due to the nature of expressed protein rather than due to the use of silkworm.
As the productivity of recombinant protein in silkworm larvae is higher more than any other expression system, the silkworm is a superior host for the production of pharmaceutical recombinant protein. To achieve it, the technology to unify and purify the recombinant protein is quite important and is still improved.
References
- Maeda S, Kawai T, Obinata M, Chika T, Horiuchi T, et al. (1984) Characteristics of human interferon- produced by a gene transferred by a baculovirus vector in the silkworm, Bombyx mori Proc Japan Acad 60: 423-426.
- Maeda S, Kawai T, Obinata M, Fujiwara H, Horiuchi T, et al. (1985) Production of human-interferon in silkworm using a baculovirus vector. Nature 315: 592-594.
- Miyajima A, Schreurs J, Otsu K, Kondo A, Arai K, et al. (1987) Use of the silkworm, Bombyx mori, and an insect baculovirus vector for high-level expression and secretion of biologically active mouse interleukin-3. Gene 58: 273-281.
- Horiuchi T, Marumoto Y, Saeki Y, Sato Y, Furusawa M, et al. (1987) High-level expression of the human-interferon gene through the use of an improved baculovirus vector in the silkworm, Bombyx mori. Agric Biol Chem 51: 1573-1580.
- Choudary PV, Kamita SG, Maeda S (1995) Expression of foreign genes in Bombyx mori larvae using baculovirus vectors. Methods Mol Biol 39: 243-264.
- Shi X, Qin J, Zhu J, Zhu D (1996) Expression of biologically active human granulocyte-macrophage colony-stimulating f 204 actor in the silkworm (Bombyx mori). Biotechnol Appl Biochem 24: 245-249.
- Deng J, Wang S, Yang Q, Cheng X, Li L (1995) High-level expression of human-beta-interferon gene in the silkworm with new constructed BmNPV vector. Chin J Biotechnol 11: 109–117.
- Qiu P, Qin J, Ding Y, Zhu D (1995) Yeast-prepro-alpha-factor-leader-region-directed synthesis and secretion of truncated human macrophage colony-stimulating factor in the silkworm Bombyx mori. Biotechnol Appl Biochem 21: 67-75.
- Kadono-Okuda K, Yamamoto M, Higashino Y, Taniai K, Kato Y, et al. (1995) Baculovirus-mediated production of the human growth hormone in larvae of the silkworm, Bombyx mori. Biochem Biophys 213: 389-396.
- Choi JY, Woo SD, Lee HK, Hong HK, Je YH, et al. (2000) High-level expression of canine parvovirus VP2 using Bombyx mori nucleopolyhedrovirus vector. Arch Virol 145: 171-177.
- Matsuoka H, Kobayashi J, Barker GC, Miura K, Chinzei Y, et al. (1996) Induction of anti-malarial transmission blocking immunity with a recombinant ookinete surface antigen of Plasmodium berghei produced in silkworm larvae using the baculovirus expression vector system. Vaccine 14: 120-126.
- Nakamura N, Sudo T, Matsuda S, Yanai A (1992) Molecular cloning of feline interferon cDNA by direct expression. Biosci Biotech Biochem 56: 211-214
- Yanai A, Ueda Y, Sakurai T, Satoh M (1993) Feline interferon and process for production thereof. United States Patent.
- von Heijne G (1986) A new method for predicting signal sequence cleavage sites. Nucleic Acids Res 14: 4683-4690.
- Ueda Y, Sakurai T, Yanai A (1993) Homogeneous production of feline interferon in silkworm by replacing single amino acid code in signal peptide region in recombinant baculovirus and characterization of the product. J Vet Med Sci 55: 251-258.
- Devos K, Duerinck F, Van Audenhove K, Fiers W (1992) Cloning and expression of the canine interferon-gamma gene. J Interferon Res 12: 95-102.
- Okano F, Satoh M, Ido T, Okamoto N, Yamada K (2000) Production of canine IFN-? ?in silkworm by recombinant baculovirius and characterization of the product. J Interferon Cytokine Res 20: 1015-1022.
- Takahashi S, Ushiyama S, Suzuki T, Ogawa K, Oda K (1997) Purification and characterization of cysteine proteinase from a baculovirus gene. Biosci Biotech Biochem 61: 1507-1511.
- Suzuki T, Kanaya T, Okazaki H, Ogawa K, Usami A, et al. (1997) Efficient protein production using a Bombyx mori nuclear polyhedrosis virus lacking the cysteine proteinase gene. J Gen Virol 78: 3073-3080.
- Adolf GR (1987) Antigenic structure of human interferon ?1 (Interferon aII1): Comparison with other human interferons. J Gen Virol 68: 1669-1676.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 14859
- [From(publication date):
specialissue-2012 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10232
- PDF downloads : 4627