Macrophage Inflammatory Protein-1α (MIP-1 α) in Hepatitis C Virus- Related Hepatocellular Carcinoma: Relation to Clinical Staging and Tumour Angiogenesis
Received: 17-Nov-2012 / Accepted Date: 29-Dec-2012 / Published Date: 31-Dec-2012 DOI: 10.4172/2161-0681.1000133
Abstract
Abstract
Study background: Hepatitis C virus (HCV) is a major risk factor for the development of hepatocellular
carcinoma (HCC), however, the mechanism of hepatocarcinogenesis in HCV infection is still undefined. Chemokines,
which induce leukocyte migration and activate inflammatory/immune responses, have recently been implicated in the
regulation of tumour growth. This work was designed to study the role of macrophage inflammatory protein-1α (MIP-
1α), a potent macrophage chemo-attractant, in the development and progression of HCV-related HCC.
Methods: Thirty patients with HCV-related cirrhosis (15 patients with HCC who underwent surgical hepatic
resection and 15 patients without HCC) and 15 healthy subjects were enrolled in the study. Immunohistochemical
staining of HCC and adjacent non-neoplastic liver tissue was performed using antibodies against MIP-1α, CD68 [for
assessment of tumour-associated macrophage (TAM) count] and CD105 [for determination of microvessel density
(MVD)]. Pre-operative serum MIP-1α levels were measured using enzyme linked immunosorbant assay kit.
Results: HCV-related HCCs showed significantly higher MIP-1α expression, CD68+ TAM count and CD105-MVD
compared with adjacent non-neoplastic liver tissue. Serum MIP-1α levels were significantly higher in patients with and
without HCC than in healthy subjects and in HCC patients than in patients without HCC. The sensitivity and specificity
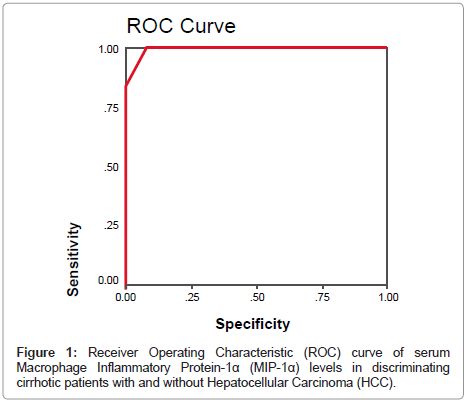
of serum MIP-1α in discriminating cirrhotic patients with and without HCC were 100% and 93.3% respectively at a
cut-off value of 17.5 pg/ml. The MIP-1α expression in HCCs showed positive correlations with serum MIP-1α levels;
tumour size, stage and histopathological grade; and intratumoural CD68+ TAM count and CD105-MVD. Moreover,
CD68+ TAM count had direct correlation with CD105-MVD in HCC.
Conclusion: MIP-lα plays an important role in the development and progression of HCC in chronic HCV infection,
possibly through recruitment of macrophages into tumour microenvironment and fostering tumour angiogenesis. MIPlα
may also serve as a potential serum biological marker and a useful therapeutic target in HCV-related HCC.
Keywords: Hepatocellular carcinoma; Macrophage inflammatory protein-1 alpha; Tumour-associated macrophages
308636Abbreviations
HCV: Hepatitis C Virus; HCC: Hepatocellular Carcinoma; MIP-1α: Macrophage Inflammatory Protein-1 alpha; TAM: Tumour-Associated Macrophages; MVD: Microvessel Density; MELD: Model for End Stage Liver Disease; CLIP: Cancer of the Liver Italian Program; HAI: Histological Activity Index; AST: Aspartate Aminotransferase; ALT: Alanine Aminotransferase; GGT: Gamma Glutamyl Transpeptidase; AFP: Alpha-Fetoprotein; INR: International Normalized Ratio; ELISA: Enzyme Linked Immunosorbant Assay; PBS: Phosphate Buffered Saline; DAB: 3,3’-diaminobenzidine
Introduction
Chronic hepatitis C virus (HCV), infection is a major risk factor for development of hepatocellular carcinoma (HCC) [1]. The incidence of HCV-associated HCC is predicted to increase over the next several years as a result of the epidemic of chronic HCV [2]. However, the precise molecular mechanism underlying the progression of chronic HCV infection to HCC is not clearly established. Recently, a critical role for chronic inflammation in tumourigenesis has generally been accepted and it has become evident that an inflammatory microenvironment is an essential component of all tumours [3]. In patients with chronic HCV infection, HCC usually arises in the setting of chronic hepatic inflammation and cirrhosis or bridging fibrosis, which favours the initiation and progression of HCC. Moreover, a persistent, nonspecific, and ineffective activation of the immune system within the chronically-inflamed liver in chronic HCV infection is thought to promote hepatocarcinogenesis [4]. Furthermore, inflammation increases vascular permeability and promotes formation of new blood vessels (angiogenesis), which is closely related to the development of HCC [5]. There is increasing evidence that the inflammatory milieu, by virtue of both its soluble signals like chemokines and cellular infiltrates may alter the behaviour of HCC [6].
The importance of chemokines in cancer-associated inflammation has been highlighted. Chemokines are responsible for the migration of various types of chemokine receptor-positive leukocytes to the tumour site [7]. Both tumour cells and stromal cells such as fibroblasts and macrophages elaborate chemokines in the tumour microenvironment that can promote tumour growth via cell proliferation, angiogenesis, invasion and metastasis [8]. Chemokines can also suppress immune responses resulting in tumour escape from immune surveillance [9].
Macrophage inflammatory protein-1α (MIP-1α), also known as CC chemokine ligand 3 (CCL3), is a member of the pro-inflammatory CC chemokine subfamily [10]. It binds to and activates cells via two chemokine receptors, namely CCR1 and CCR5, which are expressed by a variety of cells [11]. Several lines of evidence indicate that MIP-1α and its receptors may be involved in tumour growth and associated inflammation and may determine the destination of metastasis of different types of tumours [12-14]. Moreover, MIP-1α secreted by endothelial cells may promote vessel remodeling and tumour neovascularization through its potent angiogenic activities [15]. Locally-produced MIP-1α is a potent chemoattractant of different immune cells, particularly macrophages from the circulation to inflammatory sites and tumour microenvironment [16]. Tumour-associated macrophages (TAMs) recruited by MIP-1α form a major component of the inflammatory infiltrate seen in the stroma of many tumours and can affect different aspects of the neoplastic process [17].
Therefore, the present work was designed to study the role of MIP-1α in the pathogenesis of HCV-related HCC in relation to tumour progression and angiogenesis.
Subjects and Methods
Thirty treatment-naïve patients with HCV-related cirrhosis (15 patients with HCC who underwent surgical hepatic resection and 15 patients without HCC) referred to the Hepatobiliary Unit, Department of Medicine, Main Alexandria University Hospital; and 15 age- and sex-matched healthy subjects were enrolled in this study. The diagnosis of HCC was based on serum levels of alpha-fetoprotein (AFP), ultrasonography/triphasic computed tomography (CT) and/or dynamic MRI and was confirmed by histopathological examination of surgically-resected tumours. The presence of cirrhosis was determined by clinical, biochemical and ultrasonographical evidences and by histopathological examination of adjacent non-neoplastic liver tissue in patients with HCC. Exclusion criteria included patients with hepatitis B virus infection; history of alcohol consumption; concomitant schistosomiasis; evidence of hepatocellular decompensation; inflammatory disorders; other malignancies; cardiac, respiratory or renal diseases and known chronic diseases such as diabetes mellitus or connective tissue disorders. None of the patients has previously received anti-viral therapy or treatment for HCC such as systemic anti-cancer therapy, locoregional therapy or transcatheter arterial embolization before the study. The study protocol was approved by the Research Review Committee of the Alexandria Faculty of Medicine and was conformed to the 1975 Declaration of Helsinki. An informed consent was obtained from each subject included in the study.
Upon entry into the study, the following clinical and biochemical parameters were assessed: age, gender, the presence of palpable focal hepatic lesions, symptoms and signs of chronic liver disease, liver and spleen size, serum albumin, serum bilirubin, serum aspartate and alanine aminotransferases (AST and ALT respectively), serum gamma glutamyl transpeptidase (GGT), prothrombin time, international normalized ratio and serum creatinine. Serum AFP levels were measured using standardized enzyme linked immunosorbant assay kit (ELISA). The severity of liver disease in patients with cirrhosis was graded according to the Child-Pugh classification [18] and the Model for End Staging Liver Disease (MELD) score [19]. Abdominal ultrasonographic and triphasic CT examination were used for initial diagnosis of HCC and for assessment of tumour characteristics, and the presence of cirrhosis, ascites and splenomegaly. The HCC stage was determined by the scoring system proposed by the Cancer of the Liver Italian Program (CLIP) investigators [20]. This program includes Child-Pugh class of hepatic function, where classes A , B and C are scored as 0, 1 and 2 respectively; tumour morphology, where uninodular tumours involving ≤50% of liver are scored as 0, multinodular tumours involving ≤ 50% of liver are scored as 1 and diffuse tumours involving >50% of liver are scored as 2; alpha fetoprotein level, where <400 ng/ml and ≥400 ng/ml are scored as 0 and 1 respectively; and portal vein thrombosis, which is scored as 0 if absent and as 1 if present. Tumours are divided into: CLIP stages 0-6 as follows: CLIP 0, 0 points; CLIP 1, 1 point; CLIP 2, 2 points; etc
Measurement of serum MIP-1α levels
Pre-operative serum levels of MIP-1α were measured using commercially-available solid phase sandwich ELISA kit (Invitrogen Corporation, USA) according to the manufacturer’s instructions [21]. Fifty μl of serum samples (obtained from all patients and healthy subjects), standards and controls were added to the appropriate microtiter wells coated with human MIP-1α and 50 μl of the standard diluent buffer were added to the zero standard wells. Wells reserved for chromogen blank were left empty. Fifty μl of biotinylated anti-MIP-1α (Biotin conjugate) solution were then pipetted into each well except the chromogen blank(s) and the plate was incubated for 2 hours at room temperature. Then, 100 μl of streptavidin-horseradish peroxidase working solution were added to each well except the chromogen blank(s) and the plate was incubated for 30 minutes at room temperature. Solution from wells was thoroughly aspirated and discarded and the wells were washed 4 times after each step. Then, 100 μl of stabilized chromogen were added and the plate was incubated for 30 minutes at room temperature in the dark. The liquid in the wells began to turn blue. Finally, 100 μl of stop solution were added. The solution in the wells changed from blue to yellow. The absorbance of each well at 450 nm was read having blanked the plate reader against a chromogen blank composed of 100 μl each of stabilized chromogen and stop solution. The plate was read within 2 hours after adding the stop solution. A curve-fitting software was used to generate the standard curve, from which the concentrations of MIP-1α for serum samples and controls were read. Serum MIP-1α was measured in pg/ml.
Pathological examination of HCC and adjacent non-neoplastic liver tissue
Surgically-resected specimens from patients with HCV-related HCC were examined grossly for determination of tumour size and multiplicity. Representative samples of tumours and adjacent non-neoplastic liver tissue were fixed in 10% formalin, embedded in paraffin, sectioned (5μm) and stained with hematoxylin and eosin for histopathological diagnosis and determination of tumour grade and presence of capsule. Tumours were graded according to Edmondson and Steiner’s criteria [22], as follows: grade 1 HCC (well differentiated): the nuclear cytoplasmic ratio is nearly normal and the tumour is recognized by the overall growth pattern; grade 2 HCC (moderately differentiated) has larger more hyperchromatic nuclei, esinophilic cytoplasm and acini, trabecular or papillary patterns; grade 3 HCC (poorly differentiated) has more variable larger hyperchromatic nuclei with multiple nucleoli, with a loss of trabecular pattern, more numerous giant cells and less evident bile plugs; grade 4 HCC has less mature cells with large nuclei and little cytoplasm, and is hard to recognize as being of HCC origin. The adjacent non-neoplastic liver tissue was assessed as regards presence of cirrhosis and steatosis as well as the grade and stage of hepatitis using modified histological activity index (HAI) [23]. Steatosis was evaluated by estimating the approximate amount of parenchyma involved as follows: ‘-’=absent; ‘+’=mild, less than one third; ‘++’=moderate, one third to two thirds and ‘+++’=marked, more than two thirds [24].
Immunohistochemistry for MIP-1α, CD68 and CD105
Immunohistochemical staining for MIP-1α, CD68 and CD105 was performed as previously described [25-27] on 5 μm-thick paraffin sections cut from HCCs and adjacent non-neoplastic hepatic tissue. The tissue sections were deparaffinized in xylene, rehydrated in descending grades of alcohol, then immersed in 0.3% hydrogen peroxide in methanol for 20 minutes to inhibit endogenous peroxidase activity. The following primary mouse monoclonal antibodies were used: anti-human MIP-1α/CCL3 antibody, clone 93321 (R&D Systems, USA) diluted 1:20; anti-CD68 Ab-3, clone KP1 (Neomarkers, Labvision, USA) (ready to use) for determination of macrophage count; and anti-CD105/endoglin Ab-3 clone SN6h (Neomarkers, Labvision, USA) (ready to use) for assessment of microvessel density (MVD).
Before immunostaining, antigen retrieval was performed as follows: for anti-human MIP-1α antibody, slides were placed in citrate buffer (0.01 M, pH 6.0) in a 700W microwave oven, and then allowed to cool to room temperature; for anti-CD68 and anti-CD105 antibodies, enzymatic digestion was performed using protease XXV at 1mg/ml phosphate-buffered saline (PBS) for 5 minutes at 37°C. Then, Ultra V block was applied for 3-5 minutes to block nonspecific background staining. Thereafter, tissue sections were incubated with the primary antibodies at 4°C overnight in a humid chamber. Slides were then incubated with biotinylated goat anti-polyvalent (linking reagent), followed by peroxidase-conjugated streptavidin, each for 20 minutes at room temperature. Tissue sections were washed with PBS for 5 minutes after each step. The reaction product was developed using 3,3’-diaminobenzidine (DAB) mixture for 10 minutes. The slides were finally dehydrated, counterstained with hematoxylin and mounted. Negative control sections (where the primary antibody has been omitted), were included in each run.
Assessment of MIP-1α expression: MIP-1α immunostaining was evaluated semiquantitatively according to the percentage of positively-stained cells in non-overlapping microscopic fields, and scored as follows: ‘-’ = <10% of cells were stained; ‘+’ = 10-20% of cells showed positive reaction; ‘++’ = 20-50% of cells exhibited positive reaction; and ‘+++’ = >50% of cells were positive [25].
Determination of macrophage count: The three areas of densest macrophage infiltration (hot spots) were first identified by scanning the entire CD68-stained section at low power (x100), then all CD68-positive cells were counted in three high power microscopic fields (x400), and the mean count was calculated [26].
Determination of microvessel density: CD105-stained sections were first screened at low magnification (x100) to identify the three areas with the highest vascularity (hot spots). Then, the absolute number of microvessels in three x200-power fields, one in each hot spot, was counted. The mean of these 3 counts was considered as the MVD, expressed as the absolute number of microvessels per 0.74mm2 (x200 field) [27]. Any brown-stained endothelial cell or endothelial cell cluster that was clearly separate from adjacent microvessels was counted as one microvessel irrespective of the presence of a vessel lumen [26,27].
Statistical analysis
All data were statistically analyzed using the SPSS program (version 13.0) (SPSS Inc., Chicago, IL, USA) for Windows. The Student’s t test was used for comparison between two arithmetic means and the Mann-Whitney U-test was used for comparison between two means of non-normally distributed variables. The one-way ANOVA test was used for comparing the three groups with post hoc comparisons. Comparison between proportions was determined by the Chi square (χ2) test or Fisher’s Exact test (FET). Correlations between variables were analyzed by using Pearson’s correlation coefficient or Spearman’s rank test where appropriate. Analysis was statistically significant at P<0.05. The sensitivity and specificity of serum MIP-1α in discriminating cirrhotic patients with and without HCC were assessed by plotting a receiver-operating characteristic (ROC) curve and determining its cut-off valu
Results
The clinical and biochemical characteristics of cirrhotic patients with and without HCC and healthy subjects are summarized in Table 1, and the radiological and histopathological features of patients with HCC are shown in Table 2.
| Parameters | HCV patients with HCC (n=15) |
HCV patients without HCC (n=15) |
Healthy Subjects (n=15) |
P value |
|---|---|---|---|---|
| Age (years) | 51.27 ± 5.56 | 52.73 ± 7.23 | 50.93 ± 6.77 | 0.844 |
| Gender: - Male (%) - Female (%) |
15 (100.0) 0 (0.0) |
15 (100.0) 0 (0.0) |
15 (100.0) 0 (0.0) |
|
| AST (U/L; mean ± SD) | 68.40 ± 30.38a b | 50.87 ± 25.11a | 20.13 ± 3.78 | <0.0001 |
| ALT (U/L; mean ± SD) | 71.47 ± 29.44a b | 40.53 ± 19.18a | 20.40 ± 4.55 | <0.0001 |
| GGT (U/L; mean ± SD) | 102.27 ± 80.4 a b | 43.47 ± 21.62 | 20.93 ± 3.20 | 0.0001 |
| Serum albumin (g/dl; mean ± SD) | 3.53 ± 0.20a | 3.49 ± 0.18a | 4.60 ± 0.46 | <0.0001 |
| Serum bilirubin (mg/dl; mean ± SD) | 1.08 ± 0.13 a | 1.05 ± 0.23 a | 0.64 ± 0.16 | <0.0001 |
| INR (mean ± SD) | 1.19 ± 0.08 a | 1.25 ± 0.13 a | 1.07 ± 0.04 | <0.0001 |
| AFP (ng/ml; mean ± SD) | 1302.36 ± 1890.19a b | 6.00 ± 2.35 | 4.60 ±1.60 | 0.002 |
| Child-Pugh class: - Class A (%) - Class B (%) |
11 (73.3) 4 (26.7) |
7 (46.7) 8 (53.3) |
NA | 0.132 |
| MELD score | 7.13 ± 1.19 | 8.40 ± 1.96 | NA | 0.119 |
| Serum MIP-1a (pg/ml) | 27.33 ± 4.58a b | 8.67 ± 6.11a | 1.67 ± 2.44 | <0.0001 |
NA=not applicable
a=Statistically-significant difference from healthy subject;
b=Statistically-significant difference from cirrhotic patients without HCC.
Table 1: Clinical and biochemical characteristics of HCV-related cirrhotic patients with and without Hepatocellular Carcinoma (HCC) and healthy subjects.
| Parameters | HCV patients with HCC (n=15) |
|---|---|
| Tumour size (cm): - Range - Mean ± SD |
4.0–8.0 5.60 ± 1.21 |
| Number of nodules: - Uninodular (%) - Multinodular (%) |
10 (66.7) 5 (33.3) |
| Echopattern: - Hypoechoic (%) - Heterogenous (%) |
10 (66.7) 5 (33.3) |
| Tumour Location: - Left lobe (%) - Right lobe (%) |
15 (100.0) 0 (0.0) |
| Tumour Extension: - < 50% (%) - > 50% (%) |
15 (100.0) 0 (0.0) |
| Tumour encapsulation (%) | 11 (73.3) |
| CLIP stage: - CLIP 0 (%) - CLIP 1 (%) - CLIP 2 (%) |
3 (20.0) 7 (46.7) 5 (33.3) |
| HCC Grade: - Grade 1 (%) - Grade 2 (%) - Grade 3 (%) |
2 (13.3) 7 (46.7) 6 (40.0) |
| Adjacent non-neoplastic liver tissue: - HAI grade (Range) - Steatosis (%): - (%) + (%) ++ (%) +++ (%) |
6-10 4 (26.7) 5 (33.3) 6(40.0) 0 (0.0) |
Table 2: Radiological and histopathological characteristics of patients with Hepatocellular Carcinoma (HCC).
Serum levels of MIP-1α
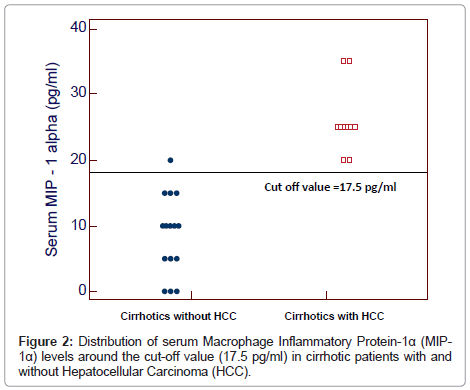
The serum levels of MIP-1α ranged between 20-35 pg/ml and 0-20 pg/ml in cirrhotic patients with and without HCC respectively and between 0-5 pg/ml in healthy subjects. Serum MIP-1α levels were significantly higher in patients with and without HCC than in healthy subjects (27.33 ± 4.58 pg/ml and 8.67 ± 6.11 pg/ml vs 1.67 ± 2.44 pg/ml) and in patients with HCC than in those without HCC (P<0.0001) (Table 1).
By plotting a ROC curve, the sensitivity and specificity of serum MIP-1α in discriminating cirrhotic patients with and without HCC were found to be 100% and 93.3% respectively at a cut-off value of 17.5 pg/ml (Figures 1 and 2).
Expression of MIP-1α in HCC and adjacent non-neoplastic liver tissue
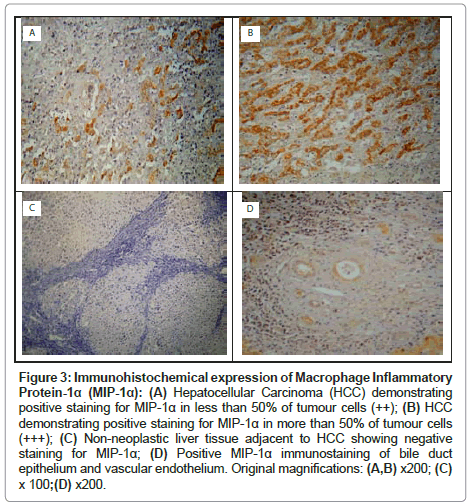
In HCCs, positive cytoplasmic immunostaining for MIP-1α was demonstrated in the tumour cells in 14 (93.3%) patients. The distribution of MIP-1α expression in HCCs was as follows: ‘-’ in 1 (6.7%) patient; ‘+’ in 3 (20.0%) patients; ‘++’ in 4 (26.7%) patients; and ‘+++’ in 7 (46.7%) patients (Table 3, Figures 3A and 3B). In the adjacent non-neoplastic liver tissue, the distribution of MIP-1α expression in hepatocytes was as follows: ‘-’ in 9 (60.0%) patients and ‘+’ in 6 (40.0%) patients (Table 3 and Figure 3C). In addition, strong MIP-1α staining was observed in vascular endothelial cells and bile duct epithelial cells (Figure 3D). The MIP-1α expression was significantly higher in HCCs than in the adjacent non-neoplastic liver tissue, (χ2=18.40, P=0.0004) (Table 3).
| Parameters | HCC (n=15) |
Adjacent non-neoplastic liver tissue |
P value |
|---|---|---|---|
| MIP-1a expression: - (%) + (%) ++ (%) +++ (%) |
1 (6.7) 3 (20.0) 4 (26.7) 7 (46.7) |
9 (60.0) 6 (40.0) 0 (0.0) 0 (0.0) |
0.0004 |
| CD68+ TAM count (/x400 field): - Range - Mean ± SD |
28.7–108.7 73.47± 23.01 |
15.7–55.0 31.79 ± 11.55 |
<0.001 |
| CD105-MVD (/x200 field): - Range - Mean ± SD |
13.3-58.7 39.55 ± 12.55 |
5.7-21.0 13.05 ± 4.62 |
<0.001 |
Table 3: Macrophage Inflammatory Protein-1α (MIP-1α) expression, CD68-positive Tumour-Associated Macrophage (TAM) count and CD105-Micro Vessel Density (MVD) in Hepatocellular Carcinoma (HCC) and the adjacent non-neoplastic liver tissue.
Figure 3: Immunohistochemical expression of Macrophage Inflammatory Protein-1α (MIP-1α): (A) Hepatocellular Carcinoma (HCC) demonstrating positive staining for MIP-1α in less than 50% of tumour cells (++); (B) HCC demonstrating positive staining for MIP-1α in more than 50% of tumour cells (+++); (C) Non-neoplastic liver tissue adjacent to HCC showing negative staining for MIP-1α; (D) Positive MIP-1α immunostaining of bile duct epithelium and vascular endothelium. Original magnifications: (A,B) x200; (C) x 100;(D) x200.
Macrophage count in HCC and adjacent non-neoplastic liver tissue
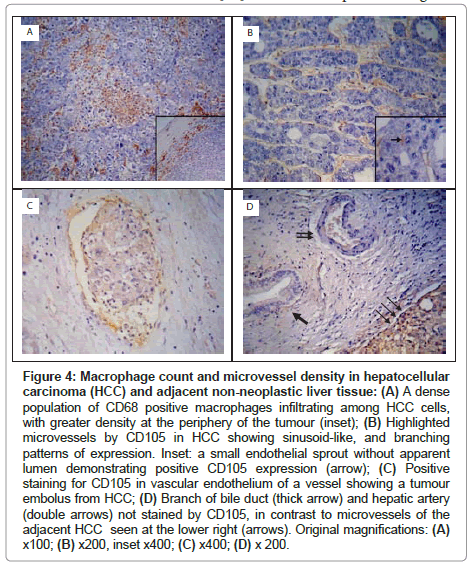
In HCCs, CD68+ TAMs were numerous, and were primarily distributed at the periphery of the tumour (Figure 4A). The CD68+ TAM count (at x 400 magnification) ranged between 28.7-108.7 and 15.7-55.0 in HCC and adjacent non-neoplastic liver tissue respectively. The CD68+ TAM count was significantly higher in HCCs than in the adjacent non-neoplastic liver tissue (73.47 ± 23.01 vs 31.79 ± 11.55, P<0.001) (Table 3).
Figure 4: Macrophage count and microvessel density in hepatocellular carcinoma (HCC) and adjacent non-neoplastic liver tissue: (A) A dense population of CD68 positive macrophages infiltrating among HCC cells, with greater density at the periphery of the tumour (inset); (B) Highlighted microvessels by CD105 in HCC showing sinusoid-like, and branching patterns of expression. Inset: a small endothelial sprout without apparent lumen demonstrating positive CD105 expression (arrow); (C) Positive staining for CD105 in vascular endothelium of a vessel showing a tumour embolus from HCC; (D) Branch of bile duct (thick arrow) and hepatic artery (double arrows) not stained by CD105, in contrast to microvessels of the adjacent HCC seen at the lower right (arrows). Original magnifications: (A) x100; (B) x200, inset x400; (C) x400; (D) x 200.
Microvessel density in HCC and adjacent non-neoplastic liver tissue
CD105 immunostaining highlighted the cell membrane of endothelial cells. All of the HCC specimens were immunoreactive with anti-CD105 antibody. The density of microvessels was higher in the peripheral part of the tumour than in the central areas. Three patterns of expression were encountered: sinusoid-like, branching and small without apparent lumina (endothelial sprouts) (Figure 4B). Occasionally, CD105 highlighted vessels containing tumour emboli (Figure 4C). In the adjacent non-neoplastic liver tissue, CD105 stained scattered hepatic sinusoidal endothelial cells predominantly around the tumour, whereas portal veins, hepatic arteries and bile ducts showed no CD105-positivity (Figure 4D).
The CD105-MVD (at x 200 magnification) ranged between 13.3-58.7 and 5.7-21.0 in HCC specimens and adjacent non-neoplastic liver tissue respectively. The CD105-MVD was significantly higher in HCC tissues than in the adjacent non-neoplastic liver tissue (39.55 ± 12.55 vs 13.05 ± 4.62, P<0.001) (Table 3).
Statistical correlations between MIP-1α expression, CD68+ TAM count and CD105-MVD in HCC and other studied parameters: (Table 4)
| Parameters | MIP-1a expression* | CD68+TAM count (/x400 field) | CD105- MVD(/x200 field) | |||
|---|---|---|---|---|---|---|
| "r" value | Pvalue | "r" value | P value | "r" value | Pvalue | |
| Serum AST (U/L) | 0.221 | 0.428 | 0.301 | 0.275 | 0.289 | 0.296 |
| Serum ALT (U/L) | 0.333 | 0.225 | 0.348 | 0.204 | 0.303 | 0.273 |
| Serum GGT (U/L) | 0.126 | 0.655 | 0.149 | 0.596 | 0.121 | 0.666 |
| Serum AFP (ng/ml) | 0.489 | 0.065 | 0.279 | 0.314 | 0.276 | 0.320 |
| Child-Pugh score* | 0.326 | 0.236 | 0.133 | 0.637 | 0.175 | 0.533 |
| MELD score* | 0.260 | 0.349 | 0.196 | 0.484 | 0.317 | 0.249 |
| Serum MIP-1a (pg/ml) | 0.671 | 0.006 | 0.410 | 0.129 | 0.401 | 0.138 |
| HCC diameter (cm) | 0.730 | 0.002 | 0.642 | 0.010 | 0.641 | 0.010 |
| CLIP stage* | 0.637 | 0.011 | 0.602 | 0.018 | 0.625 | 0.013 |
| HCC grade* | 0.728 | 0.002 | 0.838 | <0.001 | 0.762 | 0.001 |
| HAI grade | -0.314 | 0.255 | -0.206 | 0.461 | -0.165 | 0.556 |
| Steatosis* | -0.203 | 0.468 | -0.224 | 0.422 | -0.175 | 0.533 |
| CD68+ TAM count | 0.844 | <0.001 | - | - | - | - |
| CD105-MVD | 0.615 | 0.015 | 0.917 | <0.001 | - | - |
*Spearman’s rank test
Table 4: Statistical correlations (“r” value) between Macrophage Inflammatory Protein-1α (MIP-1α) expression, CD68-positive Tumour-Associated Macrophage (TAM) count and CD105-MicroVessel Density (MVD) in Hepatocellular Carcinoma (HCC) and other studied parameters.
The MIP-1α expression in HCCs showed positive correlations with serum MIP-1α levels (r=0.671, P=0.006), intratumoural CD68+ TAM count (r=0.844, P<0.001) and CD105-MVD (r=0.615, P=0.015). Also, CD68+ TAM count and CD105-MVD in HCCs were directly correlated (r=0.917, P<0.001). Moreover, positive correlations were found between MIP-1α expression, CD68+ TAM count and CD105-MVD in HCCs on one hand and HCC maximum diameter (r=0.730, P=0.002; r=0.642, P=0.010 and r=0.641, P=0.010 respectively), CLIP stage (r=0.637, P=0.011; r=0.602, P=0.018 and r=0.625, P=0.013 respectively) and histopathological grade (r=0.728, P=0.002; r=0.838, P<0.001 and r=0.762, P=0.001 respectively) on the other hand. In the mean time, there were no statistically significant correlations between intratumoural MIP-1α expression, CD68+ TAM count and CD105-MVD with serum levels of AST, ALT, GGT and AFP, Child-Pugh score, MELD score and HAI grade and steatosis in the adjacent non-neoplastic liver tissue (P>0.05).
Discussion
Using immunohistochemical analysis, the present study demonstrated that MIP-1α expression in HCC cells was significantly higher than in the adjacent non-neoplastic liver tissues in patients with HCV-related HCC unrelated to the severity of liver disease. Vascular endothelial cells and bile duct epithelial cells were stained as well. Previous investigators also found that MIP-1α was aberrantly expressed in hepatoma cells as well as in vascular endothelium, and small bile duct epithelium in surgically-obtained HCC tissues from HCV-infected patients whereas their immunoreactivities were weakly detected in normal liver tissues [25]. In murine hepatocarcinogenesis models, aberrant expression of MIP-1α and its receptor CCR1 has been demonstrated in HCC cells arising in N-nitrosodiethylamine (DEN)-treated mice and HBsAg transgenic mice [28]. In addition, Takai et al. [29] revealed that MIP-1α gene was significantly up-regulated in tumour cells in mouse HCC using microarray and quantitative real-time polymerase chain reaction analyses. The finding that MIP-1α has been detected within HCC cells may indicate that the tumour itself is responsible for secretion of this chemokine.
In the mean time, the present study also showed a significant increase in serum levels of MIP-1α in cirrhotic patients with HCC compared with those without HCC and uninfected subjects. These findings suggest that MIP-1α may play a role in the development of HCC. Rajkumar et al. [30] detected a higher level of MIP-1α in the plasma from gastric cancer patients than in patients with normal/non-malignant gastric conditions, which showed a significant drop after surgical resection. Also, increased serum levels of MIP-1α have been reported in patients with chronic lymphocytic leukemia [31]. Moreover, the present study demonstrated that serum MIP-1α levels were directly correlated with the intratumoural chemokine expression in patients with HCC and showed high sensitivity and specificity in discriminating cirrhotic patients with and without HCC at a cut-off value of 17.5 pg/ml, suggesting that quantitative estimation of MIP-1α in serum may reflect the chemokine production in HCC and could be a potential diagnostic biomarker for HCC.
The possible role of HCV infection in enhancing MIP-1α production in the liver may be linked directly or indirectly to HCV. Even before the development of HCC, MIP-1α production has been found to increase either in the liver or in serum of patients with different stages of HCV-associated liver disease [32]. In the present study, serum levels of MIP-1α were significantly higher in HCV-positive cirrhotic patients without HCC than in uninfected subjects, with a further increase observed in patients with HCC suggesting that MIP-1α production is increasing with the progression of liver disease in chronic HCV infection.
Furthermore, the current work showed that MIP-1α expression in HCC cells was directly correlated with tumour size, stage and histopathological grade suggesting that MIP-1α may play a role in HCC progression. Several lines of evidence suggest a potential contribution of MIP-1α to tumour growth [13,28,33]. Yang et al. [28] also demonstrated that tumour foci number and sizes were dramatically reduced in CCL3- and CCR1-deficient mice compared with those in wild-type mice after treatment with DEN, a known inducer of HCC. Given that MIP-1α was expressed by HCC cells, it is likely that this chemokine may interact with CCR1 expressed on hepatoma cells in an autocrine and/or paracrine manner resulting in tumour progression [28].
An additional role for MIP-1α during tumourigenesis might be the regulation of tumour angiogenesis [34]. The current work showed that CD105-MVD was significantly higher in HCC than in the adjacent non-neoplastic liver tissue and was positively correlated with tumour size, stage and histopathological grade suggesting that MIP-1α plays a role in tumour neovascularization, an indispensable process for HCC growth [5]. A previous study showed that tumour angiogenesis was markedly reduced in CCL3- and CCR1-knockout mice compared with wild-type mice in murine hepatocarcinogenesis [28]. Also, a deficiency of the MIP-1α gene reduced neovascularization in a lung metastasis model [34]. MIP-1α may affect neovessel formation by directly activating endothelial cells expressing CCR1 and CCR5 in a paracrine manner [25,28].
Being a potent chemoattractant factor, MIP-1α may indirectly promote tumourigenesis by recruitment of various types of leukocytes particularly macrophages into tumour tissues [16,17]. The current study demonstrated the presence of a large number of infiltrating CD68+ macrophages in HCCs compared with the adjacent non-neoplastic liver tissue, which was positively correlated with intratumoural MIP-1α expression. Enrichment of macrophages in tumour tissues compared with pericancerous tissues has been previously reported in HCC [35-37]. Hussein et al. [36] found that the transitions from normal liver to the subsequent lesional steps (chronic hepatitis, cirrhotic nodules, dysplastic nodules and HCCs) in chronic HCV infection were associated with significantly increased density of tumour infiltrating CD68+ macrophages. It has been reported that HCV can replicate in monocytes/macrophages causing their prolonged activation and accumulation of inflammatory cytokines that contribute to DNA damage and carcinogenesis [38]. The role of MIP-1α as a key macrophage chemoattractant in the tumour microenvironment, has been evidenced in previous studies. Yang et al. [28] found that the numbers of macrophages in tumour foci in chemically-induced HCC were remarkably depressed in both CCL3- and CCR1-deficient mice compared with that of wild-type mice. Also, MIP-1α was detected in tumour cells during murine lung metastasis process together with an infiltration of macrophages expressing CCR5 [34].
In addition, the present study demonstrated that the number of infiltrating CD68+ macrophages in HCC tissues was correlated with tumour size, stage and histopathological grade suggesting that TAMs are linked to HCC progression. There is substantial evidence that macrophages play an important role in promoting tumour growth. Ding et al. [35] showed that high intratumoural macrophage infiltration predicts poor prognosis in patients with HCC. In experimental murine hepatocarcinoma, stimulation of mouse tissue macrophages one day before intravenous injection of tumour cells, increased the number and weight of implants (experimental metastases) in the liver [39]. Also, Zhang et al. [40] reported that treating HCC by ecteinascidin-743, an anti-neoplastic alkaloid agent, can suppress tumour growth through attacking tumour cells and TAMs. In the mean time, soluble mediators like VEGF and TNF-α secreted from primary tumours may stimulate macrophages to produce MIP-1α, which in turn stimulates primary tumour cells in a paracrine manner to enhance tumour progression [41]. Thus, both MIP-1α and TAMs may have a mutual relationship, which favours their fundamental role in hepatocarcinogenesis [42].
Moreover, TAMs can promote tumour growth by stimulating angiogenesis [8]. The present study showed that the number of CD68+ macrophages and CD105-MVD were positively correlated in HCC tissues as previously demonstrated [35-37]. Moreover, the infiltrating macrophages and enhanced angiogenesis were primarily distributed at the periphery of HCC, which is the predominant place for tumour cells to invade into surrounding normal tissue and/or blood vessels, thereby fostering tumour progression and metastatic spread. Zhang et al. [43] found that depletion of macrophages in combination with the administration of the anti-angiogenic agent, sorafenib significantly inhibited tumour progression, tumour angiogenesis, and lung metastasis compared with sorafenib monotherapy. Moreover, when TAM infiltration into tumours was prevented by depleting circulating monocytes, tumour angiogenesis and growth were suppressed [44]. Macrophages in the tumour microenvironment play a pivotal role in the angiogenic switch. They are an important source of various angiogenic factors including VEGF and MMP-9 [45,46]. Thus, tumour-derived MIP-1α may indirectly induce neovascularization through recruitment of macrophages into HCC microenvironment.
All together, the present study shed the light on the role of cancer-associated inflammation in HCC progression. The findings that mark MIP-1α and infiltrating TAMs as key players in tumour growth and angiogenesis in HCV-related HCC might be translated into clinical implications. Pharmacological inhibition of MIP-1α by neutralizing antibodies or blocking its binding to CCR1 may result in disruption of macrophage recruitment into tumour site and reduction in tumour growth and angiogenesis. Moreover, selectively depleting or modulating tumour-promoting characteristics and/or activity of TAMs, might be another potential therapeutic target.
References
- But DY, Lai CL, Yuen MF (2008) Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol 14: 1652-1656.
- Levrero M (2006) Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene 25: 3834-3847.
- Porta C, Larghib P, Rimoldic M, Totarob MG, Allavenac P, et al. (2009) Cellular and molecular pathways linking inflammation and cancer. Immunobiology 214: 761-777.
- Castello G, Scala S, Palmieri G, Curley SA, Izzo F (2010) HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol 134: 237-250.
- Sanz-Cameno P, Trapero-Marugán M, Chaparro M, Jones EA, Moreno-Otero R (2010) Angiogenesis: from chronic liver inflammation to hepatocellular carcinoma. J Oncol 2010: 272170.
- Schrader J, Iredale JP (2011) The inflammatory microenvironment of HCC - the plot becomes complex. J Hepatol 54: 853-855.
- Rollins BJ (2006) Inflammatory chemokines in cancer growth and progression. Eur J Cancer 42: 760-767.
- Kimura YN, Watari K, Fotovati A, Hosoi F, Yasumoto K, et al. (2007) Inflammatory stimuli from macrophages and cancer cells synergistically promote tumor growth and angiogenesis. Cancer Sci 98: 2009-2018.
- Ben-Baruch A (2006) Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol 16: 38-52.
- Maurer M, von Stebut E (2004) Macrophage inflammatory protein-1. Int J Biochem Cell Biol 36: 1882-1886.
- Kaufmann A, Salentin R, Gemsa D, Sprenger H (2001) Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J Leukoc Biol 69: 248-252.
- Konishi T, Okabe H, Katoh H, Fujiyama Y, Mori A (1996) Macrophage inflammatory protein-1 alpha expression in non-neoplastic and neoplastic lung tissue. Virchows Arch 428: 107-111.
- Silva TA, Ribeiro FL, Oliveira-Neto HH, Watanabe S, Alencar Rde C, et al. (2007) Dual role of CCL3/CCR1 in oral squamous cell carcinoma: implications in tumor metastasis and local host defense. Oncol Rep 18: 1107-1113.
- Milliken D, Scotton C, Raju S, Balkwill F, Wilson J (2002) Analysis of chemokines and chemokine receptor expression in ovarian cancer ascites. Clin Cancer Res 8: 1108-1114.
- Lu P, Li L, Wu Y, Mukaida N, Zhang X (2008) Essential contribution of CCL3 to alkali-induced corneal neovascularization by regulating vascular endothelial growth factor production by macrophages. Mol Vis 14: 1614-1622.
- DiPietro LA, Burdick M, Low QE, Kunkel SL, Strieter RM (1998) MIP-1alpha as a critical macrophage chemoattractant in murine wound repair. J Clin Invest 101: 1693-1698.
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2008) The inflammatory microenvironment in tumour progression: the role of tumour-associated macrophages. Crit Rev Oncol Hematol 66: 1-9.
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60: 646-649.
- Wiesner RH, McDiarmid SV, Kamath PS, Edwards EB, Malinchoc M, et al. (2001) MELD and PELD: application of survival models to liver allocation. Liver Transpl 7: 567-580.
- [No authors listed] (1998) A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology 28: 751-755.
- Fisher NC, Neil DA, Williams A, Adams DH (1999) Serum concentrations and peripheral secretion of the beta chemokines monocyte chemoattractant protein 1 and macrophage inflammatory protein 1alpha in alcoholic liver disease. Gut 45: 416-420.
- Edmondson HA, Steiner PE (1954) Primary carcinoma of the liver. A study of 100 cases among 48,900 necropsies. Cancer 7: 374-376.
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, et al. (1995) Histological grading and staging of chronic hepatitis. J Hepatol 22: 696-699.
- Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR (1999) Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol 94: 2467-2474.
- Lu P, Nakamoto Y, Nemoto-Sasaki Y, Fujii C, Wang H, et al. (2003) Potential interaction between CCR1 and its ligand, CCL3, induced by endogenously produced interleukin-1 in human hepatomas. Am J Pathol 162: 1249-1258.
- Kwon GY, Lee SD, Park ES (2005) Mast cell and macrophage counts and microvessel density in invasive breast carcinoma-comparison analysis with clinicopathological parameters. Cancer Res Treat 37: 103-108.
- Yang LY, Lu WQ, Huang GW, Wang W (2006) Correlation between CD105 expression and postoperative recurrence and metastasis of hepatocellular carcinoma. BMC Cancer 6: 110.
- Yang X, Lu P, Fujii C, Nakamoto Y, Gao JL, et al. (2006) Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer 118: 1869-1876.
- Takai H, Ashihara M, Ishiguro T, Terashima H, Watanabe T, et al. (2009) Involvement of glypican-3 in the recruitment of M2-polarized tumor-associated macrophages in hepatocellular carcinoma. Cancer Biol Ther 8: 2329-2338.
- Rajkumar T, Vijayalakshmi N, Gopal G, Sabitha K, Shirley S, et al. (2010) Identification and validation of genes involved in gastric tumorigenesis. Cancer Cell Int 10: 45.
- Zucchetto A, Benedetti D, Tripodo C, Bomben R, Dal Bo M, et al. (2009) CD38/CD31, the CCL3 and CCL4 chemokines, and CD49d/vascular cell adhesion molecule-1 are interchained by sequential events sustaining chronic lymphocytic leukemia cell survival. Cancer Res 69: 4001-4009.
- Apolinario A, Diago M, Lo Iacono O, Lorente R, Pérez C, et al. (2004) Increased circulating and intrahepatic T-cell-specific chemokines in chronic hepatitis C: relationship with the type of virological response to peginterferon plus ribavirin combination therapy. Aliment Pharmacol Ther 19: 551-562.
- Masih-Khan E, Trudel S, Heise C, Li Z, Paterson J, et al. (2006) MIP-1alpha (CCL3) is a downstream target of FGFR3 and RAS-MAPK signaling in multiple myeloma. Blood 108: 3465-3471.
- Wu Y, Li YY, Matsushima K, Baba T, Mukaida N (2008) CCL3-CCR5 axis regulates intratumoral accumulation of leukocytes and fibroblasts and promotes angiogenesis in murine lung metastasis process. J Immunol 181: 6384-6393.
- Ding T, Xu J, Wang F, Shi M, Zhang Y, et al. (2009) High tumour-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Human Pathol 40: 381-389.
- Hussein MR, Ahmed RA (2005) Analysis of the mononuclear inflammatory cell infiltrate in the cirrhotic, dysplastic nodules and hepatocellular carcinomas in patients with chronic hepatitis C infection. Cancer Biol Ther 4: 1075-1078.
- Peng SH, Deng H, Yang JF, Xie PP, Li C, et al. (2005) Significance and relationship between infiltrating inflammatory cell and tumor angiogenesis in hepatocellular carcinoma tissues. World J Gastroenterol 11: 6521-6524.
- Radkowski M, Bednarska A, Horban A, Stanczak J, Wilkinson J, et al. (2004) Infection of primary human macrophages with hepatitis C virus in vitro: induction of tumour necrosis factor-alpha and interleukin 8. J Gen Virol 85: 47-59.
- Lin CY, Lin CJ, Chen KH, Wu JC, Huang SH, et al. (2006) Macrophage activation increases the invasive properties of hepatoma cells by destabilization of the adherens junction. FEBS Lett 580: 3042-3050.
- Zhang CH, Xu GL, Jia WD, Ge YS, Wang W (2010) Can hepatocellular carcinoma be treated by Yondelis through targeting both tumor cells and tumor-associated macrophages? Hepatogastroenterology 57: 114-116.
- Lin YJ, Lai MD, Lei HY, Wing LY (2006) Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology 147: 1278-1286.
- Maru Y (2007) Which came first, tumor cells or macrophages? Cell Adh Migr 1: 107-109.
- Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, et al. (2010) Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res 16: 3420-3430.
- Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, et al. (2007) Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol 127: 2031-2041.
- Coffelt SB, Hughes R, Lewis CE (2009) Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta 1796: 11-18.
- Egeblad M, Werb Z (2002) New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2: 161-174.
Citation: El Aggan HA, Helmy MA, El Deeb NMF, Zeid AE, Yehia MFA (2012) Macrophage Inflammatory Protein-1α (MIP-1 α) in Hepatitis C Virus-Related Hepatocellular Carcinoma: Relation to Clinical Staging and Tumour Angiogenesis. J Clin Exp Pathol 3:133. DOI: 10.4172/2161-0681.1000133
Copyright: © 2012 El Aggan HA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14966
- [From(publication date): 11-2012 - Jul 12, 2025]
- Breakdown by view type
- HTML page views: 10212
- PDF downloads: 4754