Research Article Open Access
Long-term Electromagnetic Field Treatment Increases Brain Neuronal Activity: Linkage to Cognitive Benefit and Therapeutic Implications for Alzheimer's Disease
Takashi Mori1 and Gary W. Arendash2,3*
1Departments of Biomedical Sciences and Pathology, Saitama Medical Center and Saitama Medical University, Kawagoe, Saitama 350-8550, Japan
2Department of Cell Biology, Microbiology, and Molecular Biology, University of South Florida, Tampa, FL 33620, USA
3The Florida Alzheimer's Disease Research Center, Tampa, FL 33620, USA
- Corresponding Author:
- Gary W. Arendash, Ph.D,
Department of Cell Biology, Microbiology, and Molecular Biology
University of South Florida, Tampa, FL 33620
Tel: (813) 974-8434
Fax: (813) 974-1614
E-mail: arendash@cas.usf.edu
Received date: October 01, 2011; Accepted date: November 09, 2011; Published date: November 11, 2011
Citation: Mori T, Arendash GW (2011) Long-Term Electromagnetic Field Treatment Increases Brain Neuronal Activity: Linkage to Cognitive Benefit and Therapeutic Implications for Alzheimer's Disease. J Alzheimers Dis 1:102. doi: 10.4172/2161-0460.1000102
Copyright: © 2011 Mori T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
Although a single exposure to high frequency electromagnetic fields (EMF) appears to increase neuronal activity based on PET/EEG monitoring, the "long-term" effects of daily EMF treatment on neuronal activity have not been evaluated in either humans or animals. In the present study, we report daily EMF treatment over a two-month period to enhance neuronal activity in entorhinal cortex of aged (23 - 28 month old) Alzheimer\'s transgenic mice and littermate normal mice, as indexed by the expression of c-Fos in neurons. Moreover, this enhanced neuronal activity was temporally linked to cognitive benefit in the same animals. In view of the impaired neuronal activity that occurs very early and progressively in Alzheimer\'s Disease, we suggest EMF treatment as a viable approach to counter this neuronal hypo-activity and possibly enhance/stabilize cognitive function.
Keywords
Neuronal c-Fos expression; Entorhinal cortex; Transgenic mice; High frequency; Cell phone level; β-amyloid
Abbreviations
Aβ: β-amyloid; AD: Alzheimer’s Disease; ANOVA: Analysis of Variance; APPsw: Swedish Mutation of Amyloid Precursor Protein; EEG: Electroencephalograph; EMF: Electromagnetic Field; FDG-PET: Flurodeoxyglucose-Positron Emission Tomograph; GSM: Global System for Mobile Communications; MCI: Mild Cognitive Impairment; NT: Non-Transgenic; SAR: Specific Absorption Rate; Tg: Transgenic
Introduction
A progressive reduction in neuronal activity, as indexed by fluorodeoxyglucose-positron emission tomography (FDG-PET) scan analysis, is an early characteristic of Alzheimer’s Disease (AD) that is correlated with cognitive decline and highly predictive of conversion from mild cognitive impairment (MCI) to AD [1,2]. As such, therapeutics that enhance neuronal activity in AD brains could have substantial cognitive benefit. Although high frequency electromagnetic field (EMF) treatment at cell phone levels has been reported to increase neuronal activity (indexed by FDG-PET scan analysis) and EEG alpha-wave activity in normal adults during a “single” EMF exposure [3,4], no “long-term” daily EMF treatment studies have been done in normal or AD subjects to determine effects on neuronal activity. We have, however, recently performed the first “long-term” EMF treatment studies in AD transgenic (Tg) mice and adult normal mice [5,6]. These studies indicate a surprising ability of such high frequency EMF treatment (over months) to result in: 1) protection against, or reversal of, cognitive impairment in AD mice, 2) improved cognitive performance of normal mice, and 3) no deleterious effects on brain oxidative/inflammatory markers or brain hyperthermia. In suggesting that long-term EMF treatment could be a viable therapeutic against AD with multiple mechanisms of action (i.e., Aβ anti-aggregation, mitochondrial enhancement), we have hypothesize that increases in neuronal activity could be an important additional mechanism of longterm EMF action [5,6]. In the present study, we wanted to determine what the long-term effects of cell phone level EMF treatment are on neuronal activity, as indexed by neuronal expression of c-Fos – and we wished to do so in both Alzheimer’s Tg and normal mice that were very old. Neuronal expression of c-Fos immunostaining is an established indirect marker for neuronal activity [7], with c-Fos being expressed within hours in neurons firing action potentials.
Methods
At 21 - 26 months of age, APPsw transgenic (Tg) mice carrying the mutant APPK670N, M671L gene and non-transgenic (NT) littermates were divided into the following sub-groups for EMF or sham control treatment: Tg controls (n=6), Tg+EMF (n=10), NT controls (n=4), and NT+EMF (n=5). Tg and NT mice to be exposed to EMFs had their cages placed within a large Faraday cage, which contained an EMF generator antenna that provided two 2-hour periods of EMF treatment (early morning and late afternoon) per day at typical cell phone levels (GSM 918 MHz, SAR at 0.25 - 1.05 W/kg, pulsed and modulated) according to our established protocol [5,6]. Because brain c-Fos expression is sensitive to animal handling and stress-inducing factors, control/sham-treated mice were handled identically to EMF-treated mice and housed identically to EMF-treated mice in a separate room with the same environment (i.e., temperature, background noise level, etc.) as the room housing EMF-treated mice. Thus, any non-specific effects of handling/environmental stress on c-Fos were controlled for.
At one month into EMF treatment and during the OFF period (mid-way between ON periods), mice were evaluated in the Y-maze task of spontaneous alternation for general mneumonic memory [5] and percent spontaneous alternation determined during a single 5-minute trial. At 2 months in EMF treatment (23 - 28 months of age), all animals were euthanized during the OFF period (mid-way between ON periods). Brains were initially perfused with isotonic phosphate buffered saline (PBS; 0.1M, pH 7.4). The left caudal brain was then paraffin-embedded and processed for c-Fos immunostaining/image analysis. At the level of the posterior hippocampus (bregma - 2.92 mm to - 3.64 mm), five 5 μm sections (150 μm apart) were taken from each mouse brain using a sliding microtome. Immunohistochemical staining was performed following the manufacturer’s protocol using a Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) coupled with the diaminobenzidine reaction. Used as the primary antibody was a rabbit N-terminus of human c-Fos polyclonal antibody (sc-52 1:500, Santa Cruz Biotechnology, Santa Cruz, CA). Brain sections were retrieved for c-Fos epitope using citric acid buffer (0.1 M, pH 6.0) and an autoclave (100°C, 10 minutes) prior to the pre-blocking step. PBS or normal rabbit serum (isotype control) was used instead of primary antibody or ABC reagent as a negative control.
For quantitative image analysis, images were acquired using an Olympus BX60 microscope with an attached digital camera system (DP-70, Olympus, Tokyo, Japan), and the digital image was routed into a Windows PC for quantitative analysis using SimplePCI software (Hamamatsu Photonics, Hamamatsu, Shizuoka, Japan). Images of five 5-μm sections (150 μm apart) through anatomic regions of interest (entorhinal cortex, hippocampus, and posterior neocortex) were captured from each animal. For quantification of c-Fos positive neurons, sections were manually edited to eliminate artifacts, with the data reported as number of immune-labeled cells in entire region of entorhinal cortex for each section. Each analysis was done by a single examiner blinded to sample identities. All statistical comparisons involved one-way ANOVA, followed by post hoc Tukey HSD test where appropriate.
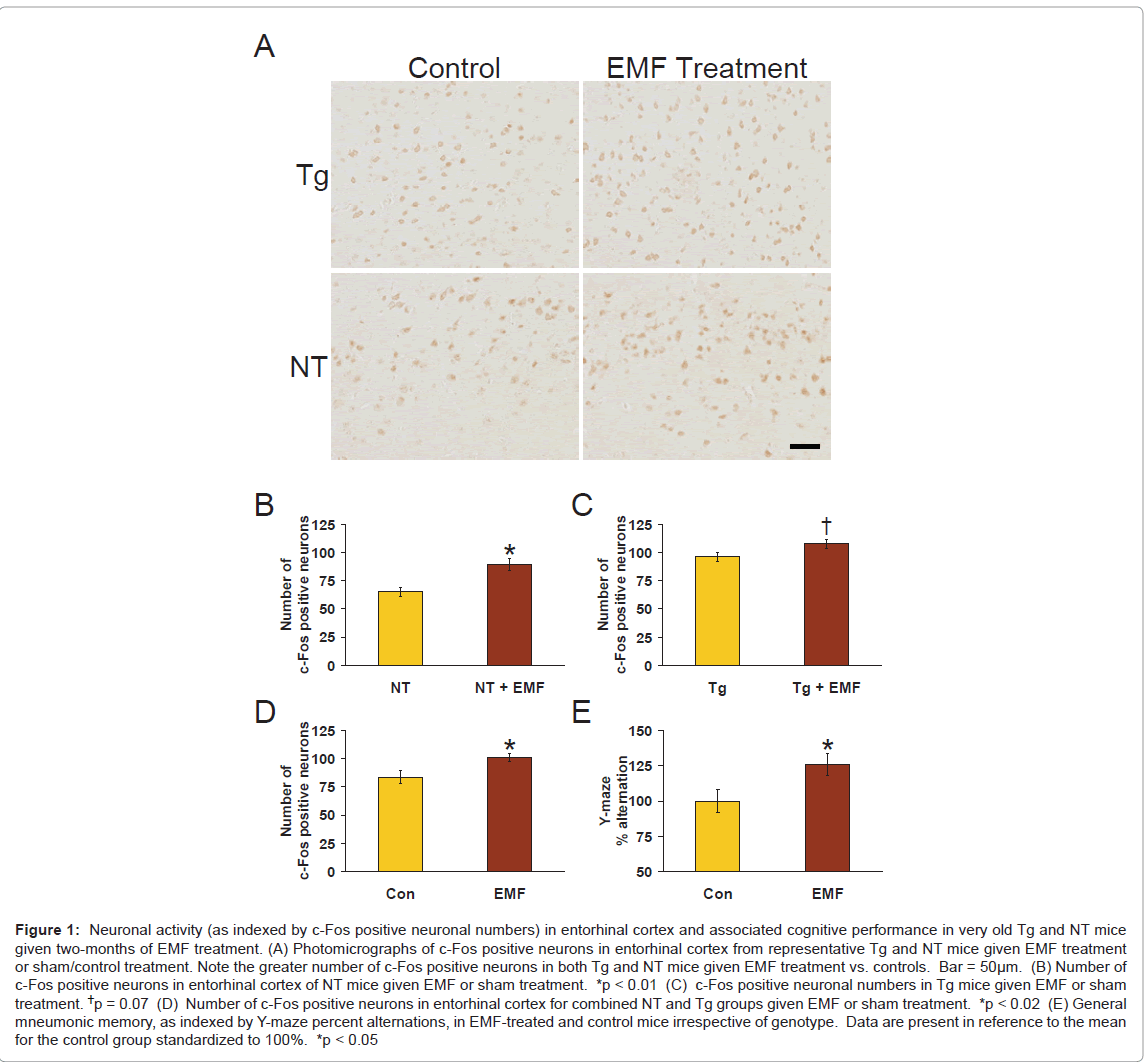
Figure 1: Neuronal activity (as indexed by c-Fos positive neuronal numbers) in entorhinal cortex and associated cognitive performance in very old Tg and NT mice given two-months of EMF treatment. (A) Photomicrographs of c-Fos positive neurons in entorhinal cortex from representative Tg and NT mice given EMF treatment or sham/control treatment. Note the greater number of c-Fos positive neurons in both Tg and NT mice given EMF treatment vs. controls. Bar = 50μm. (B) Number of c-Fos positive neurons in entorhinal cortex of NT mice given EMF or sham treatment. *p < 0.01 (C) c-Fos positive neuronal numbers in Tg mice given EMF or sham treatment. †p = 0.07 (D) Number of c-Fos positive neurons in entorhinal cortex for combined NT and Tg groups given EMF or sham treatment. *p < 0.02 (E) General mneumonic memory, as indexed by Y-maze percent alternations, in EMF-treated and control mice irrespective of genotype. Data are present in reference to the mean for the control group standardized to 100%. *p < 0.05
Results and Discussion
As indexed by number of c-Fos positive neurons, neuronal activity in entorhinal cortex was significantly increased (↑ 37%) in aged NT mice given 2 months of EMF treatment compared to sham-treated normal mice (Figure 1B; p<0.01); a nearly significant increase in number of c-Fos positive neurons (↑12%; p=0.07) was also present in Tg mice given EMF treatment (Figure 1C). When results from both genotypes were combined, a significant (p<0.02) overall enhancing effect of EMF treatment on neuronal activity (↑21%) was present (Figure 1D). Thus, long-term EMF treatment provided a “generalized” enhancement in entorhinal cortex neuronal activity, as indexed by number of c-Fos positive neurons. Representative photomicrographs of c-Fos immunostaining in entorhinal cortex of NT and Tg mice are provided in Figure 1A.
As is standard methodology for evaluation of c-Fos staining, only neurons in entorhinal cortex showing a clearly-labeled nuclear signal for c-Fos were counted as c-Fos positive. Since very few strongly positive c-Fos neurons were observed in two other regions of interest (i.e., hippocampus and posterior neocortex), no quantification of c-Fos positive neuronal numbers could be done in those regions. The minimal number of c-Fos positive neurons in these other brain areas of interest may reflect the fact that the c-Fos gene is an immediate early gene whose neuronal expression is dependent on time after stimulation (by EMF treatment). In this regard, animals were euthanized hours following their final EMF treatment (when they would have otherwise been behaviorally evaluated). The number of c-Fos positive neurons in hippocampus and/or posterior neocortex may have been appreciably higher during, or more immediately following, EMF treatment.
Prior to euthanasia, testing of mice mid-way between daily EMF ON periods in the Y-maze task revealed a significant overall effect (p<0.05) of EMF treatment to enhance Y-maze percent alternation (↑ 26%; Figure 1E) irrespective of genotype. Therefore, just as for number of c-Fos positive neurons in entorhinal cortex, EMF treatment induced a “generalized” increase in Y-maze performance. Importantly, both c-Fos analysis (at euthanasia) and Y-maze testing were done during the same window of time following morning EMF treatment (i.e., at 2 - 5 hours into the OFF period). These two measurements are therefore temporally linked and underscore that cognitive and physiologic benefits of long-term EMF exposure occur during the 8 hours between EMF treatments, as we previously reported [5,6]. However, because c-Fos is an immediate early gene, it is likely that EMF-induced increases in c-Fos staining are even greater during ON periods, with a gradual attenuation of this effect during OFF periods.
The present study is the first to investigate “long-term” effects of EMF exposure on brain neuronal activity. In that context, the observed enhancement of neuronal activity, as indexed by c-Fos staining, occurred with long-term exposure at “cell phone level” EMF parameters. Consistent with our results, a recent PET-based study in humans reported a significant 7% increase in neuronal activity in cortical areas immediately beneath where a cell phone was being held for a single 50-minute exposure [3]. Regarding cognitive performance, “longterm” EMF exposure over years at cell phone level parameters has been reported to improve cognitive performance in an interference task [8] and to result in 30 - 40% less hospitalizations for AD/vascular dementia [9]. In view of the progressive decline in neuronal activity that begins in MCI well before ensuing diagnosis of AD [1,2], early intervention with therapeutics that increase neuronal activity could act to stabilize or improve cognitive function. EMF treatment is particularly attractive in that regard because we have previously shown that it directly affects AD pathogenesis in AD transgenic mice by: 1) suppressing/reversing Aβ aggregation [5], and 2) enhances brain mitochondrial function [6]. We now add enhancement of neuronal activity, as indexed by c-Fos staining, to this list of beneficial EMF mechanisms. Because toxic intraneuronal Aβ is only removed/cleared from neurons through synaptic release at nerve terminals [10], EMF-enhanced neuronal activity may be of particular value to clear disaggregated Aβ from neurons and from the AD brain. Since high levels of EMF exposure can induce body hyperthermia and hyperthermia can in turn increase brain c-Fos staining [11], it is important to note that brain temperatures during both ON and OFF periods of our chronic, low-level EMF treatment remain normal [5,6].
Several caveats should be mentioned in the context of our present results. First, animals received full body EMF treatment. Although we have established that this does not result in any remarkable changes in body physiology or any pathologic effects [5,6], our future studies will emphasize head-only EMF treatment. Second, only one brain region was quantified for neuronal activity due to low c-Fos neuronal numbers in other areas we intended to examine from the same posterior forebrain slab. Examination of additional brain areas, both during and at intervals after EMF exposure, is now warranted. Finally, although the present study (and essentially all prior human studies) involved “cell phone level” EMF parameters, more efficacious EMF parameters are likely for enhancing neuronal activity/cognitive performance and are currently being explored.
Acknowledgements
This work was supported by funds from the NIA-designated Florida Alzheimer’s Disease Research Center (AG025711) to G.A. and the USF/Byrd Alzheimer’s Institute to G.A. We thank Maggie Dorsey and Lilly Wang for their technical assistance in this work.
References
- Jaqust WJ, Landau SM, Shaw LM, Trojanowski JQ, Koeppe RA, et al. (2009) Relationships between biomarkers in aging and dementia. Neurology 73: 1193- 1199.
- Landau SM, Harvey D, Madison CM, Koeppe RA, Reiman EM, et al. (2011) Associations between cognitive, functional, and FDG-PET measures of decline in AD and MCI. Neurobiol Aging 32: 1207-1218.
- Volkow ND, Tomasi D, Wang GJ, Vaska P, Fowler JS, et al. (2011) Effects of cell phone radiofrequency signal exposure on brain glucose metabolism. JAMA 305: 808-813.
- Kwon MS, Hamalainen H (2011) Effects of mobile phone electromagnetic fields: critical evaluation of behavioral and neurophysiological studies. Bioelectromagnetics 32: 253-272.
- Arendash GW, Sanchez-Ramos J, Mori T, Mamcarz M, Lin X, et al. (2010) Electromagnetic field treatment protects against and reverses cognitive impairment in Alzheimer's mice. J Alzheimers Dis 19: 191-210.
- Dragicevic N, Bradshaw PC, Mamcarz M, Lin X, Wang L, et al. (2011) Longterm electromagnetic field treatment enhances brain mitochondrial function of both Alzheimer's transgenic mice and normal mice: A mechanism for electromagnetic field-induced cognitive benefit? Neuroscience 185: 135-149.
- Zhang J, Zhang D, McQuade J, Behbehani M, Tsien JZ, et al. (2002) c-fos regulates neuronal excitability and survival. Nat Genetics 30: 416-420.
- Arns M, Luijtelaar G, Sumich A, Hamilton R, Gordon E (2007) Electroencephalographic, personality, and executive function measures associated with frequent mobile phone use. Int J Neurosci 117: 1341-1360.
- Schuz J, Waldemar G, Olsen J, Johansen C (2009) Risks for central nervous system diseases among mobile phone subscribers: A Danish retrospective cohort study. PLoS One 4: e4389.
- Cirrito J, Yamada K, Finn M, Sloviter R, Bales K, et al. (2005) Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48: 913-922.
- du Plessis I, Mithcell D, Niesler C, Laburn HP (2006) c-Fos immunoreactivity in selected brain regions of rats after heat exposure and pyrogen administration. Brain Res 1120: 124-130.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15172
- [From(publication date):
November-2011 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10543
- PDF downloads : 4629