Editorial Open Access
Leptin, Sonic Hedgehogs, and Neurogenesis- A Primary Cilium's Taleon
Ubaldo Armato1*, Balu Chakravarthy2, Anna Chiarini1, Franco Chioffi3, Ilaria Dal Prà2 and James F. Whitfield2
1Histology & Embryology Section, Department of Life & Reproduction Sciences, University of Verona Medical School,Verona, Venetia, Italy
2Molecular Signaling Group, Institute for Biological Sciences, National Research Council of Canada, Ottawa, Ontario, Canada
3Neurosurgery Operative Unit, St. Chiara Hospital, Trento, Trentino, Italy
- Corresponding Author:
- Prof. Dr. Ubaldo Armato, MD
Histology & Embryology Section
Department of Life & Reproduction Sciences
8 Strada Le Grazie, I-37134 Verona, Venetia, Italy
Tel: 0039-045-8027159
Fax: 0039-045-8027159
E-mail: uarmato@gmail.com
Received date: November 18, 2011; Accepted date: November 19, 2011; Published date: November 21, 2011
Citation: Armato U, Chakravarthy B, Chiarini A, Chioffi F, Dal Prà I, et al. (2012) Leptin, Sonic Hedgehogs, and Neurogenesis- A Primary Cilium’s Tale. J Alzheimers Dis 2:e105. doi:10.4172/2161-0460.1000e105
Copyright: © 2012 Armato U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abbreviations
DG: Dentate gyrus; GrC: Granule Cell; Gli‐A: Gli‐Activator; Gli‐R: Gli‐Repressor; Lep: Leptin; LepR: Lep Receptor; RG‐NSCs: Radial Glial Neuronal Stem Cells; PM: Post‐Mitotic; POMC: Proopiomelanocortin; Ptch: Patched; SGZ: Sub‐Granular Zone; Shh: Sonic hedgehog; Smo: Smoothened; TAN: Transit Amplifying Neuroblast.
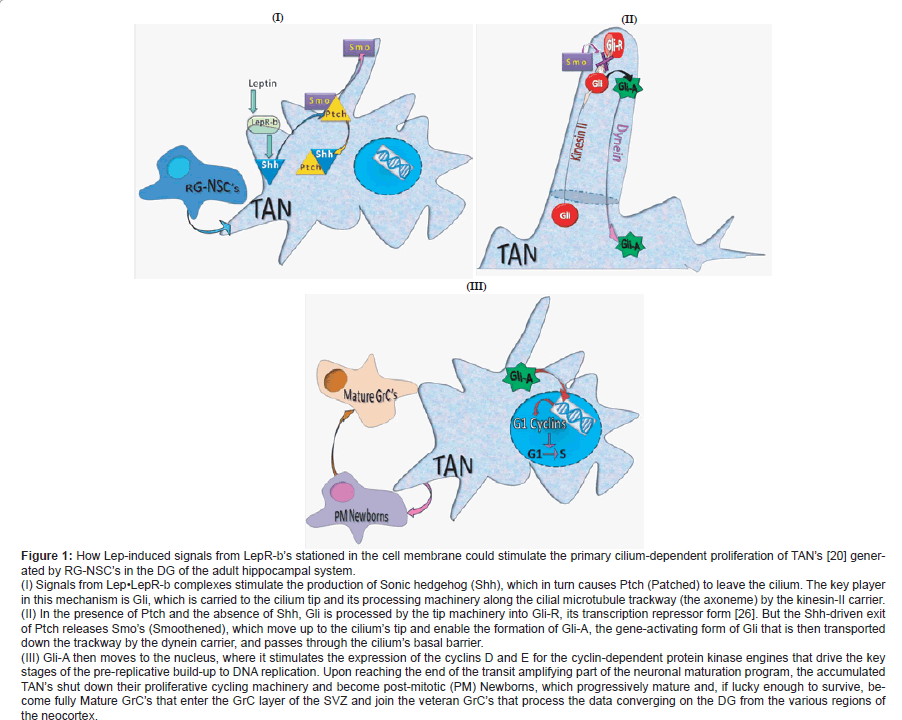
This story begins in the brain but not with neurogenesis. It starts with the 16-kDa Leptin (Lep) cytokine-hormone’s first known role as a controller of body energy reserves stored as white fat [1-5]. Lep is produced by white-fat adipocytes and then carried into the brain across the bloodbrain barrier by endothelial short isoform Lep-a receptors (LepR-a’s) or via the cerebrospinal fluid [1-5]. When it arrives in the hypothalamus, Lep induces arcuate nuclear anorexigenic proopiomelanocortin (POMC)-expressing neurons and orexigenic neuropepetide Y/Agouti-related peptide (NPY/AgRP)-expressing neurons to suppress appetite and prevent hyperphagic obesity by respectively stimulating and silencing these two types of neurons [1-5]. It does this via JAK2/STAT3 signaling from the long isoform LepR-b’s (also known as ObR-b’s) on these neurons [1-5]. But where do the arcuate neurons put their LepR-b’s? Would they not just put them into their cytoplasmic membranes? Then the Lep story took an unexpectedly exciting turn when novel experimental results strongly suggested that the primary cilia protruding from the arcuate neurons carried the hyperphagia- and obesity-preventing LepR-b’s. Reportedly, these tiny antennae, neurons throughout the brain are endowed with, probe their extracellular environments for relevant chemical agents and trigger proper responses to these and maybe to any cilia-bending mechanical stresses [6,7]. What were these game-changing results? Knocking out POMC neurons’ cilia by disabling intraflagellar transport [8] or knocking out cilial adenylyl cyclase III in the hypothalamic neurons made adult mice unresponsive to Lep, hyperphagic, obese, and consequently hyper-leptinemic due to the build-up of Lep-producing adipocytes [9-15]. Then there was the Lep-resistant hyperphagia and extreme obesity of persons with the Bardet-Biedl syndrome caused by the failure of multi-protein complexes (one of which, BBS1, binds LepR-b) that transport components for cilial maintenance and functions from the Golgi apparatus to the ciliary basal body and from there into the cilium [14,16]. There were even more indications of cilial LepR-b. In fact, Lep was reported (i) to stimulate the proliferation of transit amplifying neuroblasts (TAN’s), the granule cell (GrC) progenitors, in the hippocampal dentate gyrus (DG)-and, hence, the adult neurogenesis that is instead reduced in neurodegenerative conditions-and (ii) to improve memory in Alzheimer’s disease (AD)- model transgenic mice [17-19]. Because of the known role of cilia in driving adult neurogenesis [20,21], these findings suggested that LepRb’s are concentrated in the cilia of the TANs in the sub-granular zone (SGZ) of the adult DG, one of the principal conditions-and al regions of adult neurogenesis in rodents and humans [22]. But despite these exciting and very convincing indications of LepR-b cilial localization, no one seems to have found these receptors in the cilia of hypothalamic arcuate neurons or DG GrC’s [23]. Up to now, only Stratigopoulos and co-workers [24] have reported that exposing cultured murine arcuate neurons to Lep caused Lep•LepR-b complexes to cluster around the cilial basal bodies, the closest points anyone has seen LepR-b’s come to cilia, but without actually entering the cilia. However, strong signals from Lep•LepR-b complexes clustering around the cilial basal barrier, a selective gateway to the cilial inner sanctum [25], might drive Lep intracellular signaling mediators into the cilium. The mystery engendered by the lack of convincing indications that LepR-b’s operate from the primary cilia in hypothalamic arcuate neurons and hippocampal DG GrC’s in the absence of the otherwise expected flurry of reports from LepR-b-loaded cilia, could be solved if Lep functions first by binding to LepR-b’s located in the cell membrane. Then, the activated extra-cilial LepR-b’s would stimulate a cilium-based mechanism that can drive different processes, including adult neurogenesis. So, what could this downstream cilium-based mechanism be and how could extra-cilial Lep•LepR-b complexes stimulate it? A very likely possibility is the Sonic hedgehog (Shh) signaling mechanism, known to be housed in the primary cilium, which Goetz and co-workers have vividly labeled a “hedgehog signal-transduction machine” [26] (Figure 1I). Indeed, it has very recently been shown that Lep triggers a phosphoinositide-3 kinase (PI3K)/Akt-mediated stimulation of Shh expression in rat hepatic stellate cells [27]. But to find out how Lep and the Shh’s it generates might stimulate neurogenesis, we must first look into the SGZ of the DG before the appearance of Lep and the Shh’s. Here we see a few slowly cycling self-renewing radial glial neuronal stem cells (RG-NSC’s) generating rapidly cycling Shh-responsive TAN’s, which, unlike their ancestral RG-NSC’s, need their primary cilia and the primary cilium Shh mechanism to drive their proliferation [20,21]. In the mouse, by about four weeks after the generation of their ancestral progenitors from RG-NSC’s in their SGZ niche, TAN’s’ surviving postmitotic progeny start ripening. By about four weeks later, they have acquired fully mature dendritic spines and mossy fiber boutons and have moved up into the GrC layer. There they finally join the veteran GrC’s encoding the data converging on them from various regions of the neocortex [26,27]. When Lep appears, the Shh’s produced by the extracilial Lep•LepR-b-signaling from the cell membrane binds to Ptch (Patched) and pulls it out of the cilial membrane [26]. This releases Smo (Smoothened) from its cytoplasmic cage, from which it climbs up to the tip of the cilium (Figure 1I). There, Smo stops a processing machinery producing Gli-R (Gli-repressor) from Gli, but promotes the synthesis of the Gli-A (Gli-activator) transcription factor. Incidentally, Gli is carried by the kinesin-II transporter along the cilium’s microtubular axonemal trackway to the cilial tip (Figure 1II). Now, the Lep-triggered Gli-A is carried by the dynein transporter down to the cilial basal body gateway through which it is released and reaches the nucleus to activate many target genes, among which are those for the G1 cyclins (cyclins D1, D2, E) that drive the build-up to the initiation of DNA replication and TAN’s proliferation [19,26,30] (Figure 1III). Therefore, it seems that the neurogenesis-stimulating Lep might be a potential daily administrable arrestor of the development of Alzheimer’s disease if it be given early enough, perhaps in the mild cognitive impairment (MCI) stage of the ailment. Incidentally, it also appears, from Lep’s ability to strongly stimulate peri-lesional neurogenesis and angiogenesis in the poststroke cerebral cortices of mice, that Lep might also be used to attenuate the extent of stroke damage in humans [31]. Indeed, Lep can be safely given to human patients. Thus, a daily subcutaneous injection of recombinant methionyl human Lep has been safely given for a decade as the replacement therapy to four genetically obese humans to replace their missing Lep and with it to reduce their obesity and improve their cognitive abilities [32]. Of course, in the case of Alzheimer’s disease, Lep would have to be part of a cocktail also containing an agent that can arrest the de novo production of the toxic, synapse-disrupting amyloid-β1-42 oligomers. However, only time and much more work will be needed to test this idea.
Acknowledgements
The authors deeply thank Dr. Raffaella Pacchiana for the artwork in Figure 1.
Figure 1: How Lep-induced signals from LepR-b��?s stationed in the cell membrane could stimulate the primary cilium-dependent proliferation of TAN��?s [20] generated
by RG-NSC��?s in the DG of the adult hippocampal system.
(I) Signals from Lepâ�?¢LepR-b complexes stimulate the production of Sonic hedgehog (Shh), which in turn causes Ptch (Patched) to leave the cilium. The key player
in this mechanism is Gli, which is carried to the cilium tip and its processing machinery along the cilial microtubule trackway (the axoneme) by the kinesin-II carrier.
(II) In the presence of Ptch and the absence of Shh, Gli is processed by the tip machinery into Gli-R, its transcription repressor form [26]. But the Shh-driven exit
of Ptch releases Smo��?s (Smoothened), which move up to the cilium��?s tip and enable the formation of Gli-A, the gene-activating form of Gli that is then transported
down the trackway by the dynein carrier, and passes through the cilium��?s basal barrier.
(III) Gli-A then moves to the nucleus, where it stimulates the expression of the cyclins D and E for the cyclin-dependent protein kinase engines that drive the key
stages of the pre-replicative build-up to DNA replication. Upon reaching the end of the transit amplifying part of the neuronal maturation program, the accumulated
TAN��?s shut down their proliferative cycling machinery and become post-mitotic (PM) Newborns, which progressively mature and, if lucky enough to survive, become
fully Mature GrC��?s that enter the GrC layer of the SVZ and join the veteran GrC��?s that process the data converging on the DG from the various regions of
the neocortex.
References
- Gautron L, Elmquist JK (2011) Sixteen years and counting: an update on leptin in energy balance. J Clin Invest 121: 2087-2093.
- Levin BE, Magnan C, Dunn-Meynell Ale Foll C (2011) Metabolic sensing in the brain: who, what, where, and how? Endocrinology 152: 2552-2557.
- Li MD (2011) Leptin and beyond: an odyssey to the central control of body weight. Yale J Biol Med 84: 1-7.
- Villanueva EC, Myers MG (2008) Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes (Lond) 32: S8-12.
- Wauman J, Taverner J (2011) Leptin receptor signaling: pathways to leptin resistance. Front Biosci 17: 2771-2793.
- Lee JE, Gleeson JG (2011) Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Curr Opin Neurol 24: 98-105.
- Arellano JI, Guadiana SM, Breunig JJ, Rakic P, Sarkisian MR (2011) Development and distribution of neuronal cilia in mouse neocortex. J Comp Neurol. Oct 20. doi: 10.1002/cne.22793. [Epub ahead of print].
- Louvi A, Grove EA (2011) Cilia in the CNS: the quiet organelle claims center stage. Neuron 69: 1046-1060.
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, et al. (2007) Conditional disruption of intraflagellar transport in adult mice leads to hyperphagic-induced obesity and slow-onset cyclic kidney disease. Curr Biol 17: 1586-1594.
- Gupta PS, Prodromu NV, Chapple JP (2009) Can faulty antennae increase adiposity? The link between cilia proteins and obesity. J Endocrinol 293: 327-336.
- Mok CA, H�?�?�?©on E, Zhen M (2010) Ciliary dysfunction and obesity. Clin Genet 77: 18-27.
- Myers MG, M�?�?�?¼nzberg H, Leinninger GM, Rebecca L (2009) The geometry of leptin action in the brain. More complicated than a simple ARC. Cell Metab 9: 117-123.
- Satir P (2007) Cilia biology: stop overeating now! Curr Biol 17: R963-R965.
- Seo P, Guo D-F, Bugge K, Morgan DA, Rahmouni K, et al. (2009) Requirement of Bardet-Biedl syndrome proteins for leptin receptor. Hum Mol Genet 18: 1323-1331.
- Wang Z, Li VG, Chan CK, Phan T, Nudelman AS, et al. (2009) Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS One 4: e6979.
- Guo DF, Rahmouni K (2011) Molecular basis of the obesity associated with Bardet-Biedl syndrome. Trends Endocrinol Metab 22: 286-293.
- Garza JC, Guo M, Zhang W, Lu XY (2008) Leptin increases adult hippocampal neurogenesis in vitroand in vivo. J Biol Chem 283: 18238-18247.
- Greco SJ, Bryan KJ, Sarkar S, Zhu X, Smith MA, et al. (2010) Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer�?¢â�?¬â�?¢s disease. J Alzheimer�?¢â�?¬â�?¢s Disease 19: 1155-1167.
- P�?�?�?©rez-Gonz�?�?�?¡lez R, Antequera D, Vargas T, Spuch C, Bolos M, et al. (2011) Leptin induces the proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer�?¢â�?¬â�?¢s disease. J Alzheimer�?¢â�?¬â�?¢s Disease 24: 17-25.
- Amador-Arjona A, Elliott J, Miller A, Ginbey A, Pazour GJ, et al. (2011) Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. J Neurosci 31: 9933- 9944.
- Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, et al. (2008) Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11: 277-284.
- Kempermann G (2011) Adult Neurogenesis 2. Oxford University Press, New York.
- Funahashi H, Yada T, Suzuki R, Shioda S (2003) Distribution, function, and properties of leptin receptors in the brain. Int Rev Cytol 224: 1-27.
- Stratigopoulos G, LeDuc CA, Cremona MK, Ghung WK, Lieibel RL (2011) CUT-like homeobox (CUX) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes coordinates leptin receptor signaling. J Biol Chem 286: 2155-2170.
- Rohatgi R, Snell WJ (2010) The ciliary membrane. Curr Opin Cell Biol 22: 541-546.
- Goetz SC, Ocbina PJR, Anderson KV (2009) The primary cilium as a hedgehog signal transduction machine. Methods Cell Biol 94: 199-222.
- Choi SS, Syn WK, Karaca GF, Omenetti A, Moylan CA, et al. (2010) Leptin promotes the myofibroblastic phenotype in hepatic stellate cells by activating the hedgehog pathway. J Biol Chem 285: 36551-36560.
- Mongiat LA, Scnider AF (2011) Adult neurogenesis and the plasticity of the dentate gyrus network. Eur J Neurosci 33: 1055-1061.
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA (2011) A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci 12: 585-601.
- Kenney AM, Rowitch DH (2000) Sonic hedgehog promotes G1 cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol 20: 9055-9067.
- Avraham Y, Davidi N, Lassari V, Vorobiev L, Kabesa M, et al. (2011) Leptin induces neuroprotection neurogenesis and angiogenesis after stroke. Curr Neurovasc Res Oct 10 [Epub ahead of print].
- Paz-Filho G, Wong M-L, Licinio J (2011) Ten years of leptin replacement therapy. Obesity Rev e315-e323.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 14870
- [From(publication date):
March-2012 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 10256
- PDF downloads : 4614