Research Article Open Access
Leaf Demography of Some Evergreen and Deciduous Tree and Shrub Species of Kumaun Himalaya, India
Sanjay Kumar1*, Lalit M. Tewari1 and Ashish Tewari21Department of Botany, D. S. B. Campus Kumaun University, Nainital, India
2Department of Forestry, D. S. B. Campus Kumaun University, Nainital, India
- *Corresponding Author:
- Sanjay Kumar
Department of Botany
D. S. B. Campus Kumaun University
Nainital-263002, India
Tel: +91-5942-237764
E-mail: sanjay14_kumar@yahoo.in
Received date: June 04, 2013; Accepted date: June 24, 2013; Published date: June 26, 2013
Citation: Sanjay Kumar, Lalit M. Tewari, Ashish Tewari (2013) Leaf Demography of Some Evergreen and Deciduous Tree and Shrub Species of Kumaun Himalaya, India. J Ecosys Ecograph 3:127. doi:10.4172/2157-7625.1000127
Copyright: © 2013 Sanjay Kumar, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Leaf demography was studied in 9 trees (6 Evergreen (ES), 1 Semideciduous (SD) and 2 Deciduous (DS)) and 10 shrubs (7 Evergreen (ES) and 3 Deciduous (DS)) occurring between 350 to 2500 m elevation in the Kumaun Himalaya, India. Although each species had its own pattern about seasonality of leaf recruitment, it was possible to group the species based on leaf expansion behavior. The result shows the leaf expansion rate was greater for deciduous species compared to Evergreen species. The percent leaf area after four weeks of expansion was also greater for deciduous species.
Keywords
Kumaun Himalaya; Deciduous species; Evergreen species; Semideciduous species; Leaf area; Leaf expansion
Introduction
Leaf is the more sensitive organ, which reacts rapidly to environmental conditions and affects the growth and development of other organs [1]. Timiryazev [2] stressed the significance of leaf in the life of the plant and considered equivalent to a plant. Accurate and precise information with respect to amounts and distribution of foliar surface is requisite for reliable estimation of the primary productivity of forested ecosystems. Evans [3] laid emphasis on the study of leaf expansion and dry matter increase (due principally to accumulation of cellulose and lignin) with time. According to Brouwer and De- Wit [4], the water stress may often check leaf expansion even before affecting the photosynthesis. Watson [5] emphasized that the yield of plants ultimately depends upon the photosynthetic efficiency and the extent of photosynthetic area. Evans and Huges [6] suggested that the environmental factors generally bring out large changes in specific leaf area.
Several workers [7,8] used the Relative Growth Rate (RGR) as a single time-invariant variable to determine the response of plants to environmental factors (light flux, CO2 concentration etc.), or even to account for the ecological behavior of plants [9].
Marked variations are found in the leaf longevity of woody species, at inter-specific level as well as at intra-specific level. Leaf longevity is important both as a nutrient cycling process and as a specific plant adaptation [10]. In the nutrients cycling context, leaf longevity plays a major role in determining the nutrient turnover rate in the ecosystems because a significant portion of the nutrient capital in the ecosystem is in leaves [11]. As an adaptation to varying nutrient availability, leaf longevity might:
a. Determine the photosynthetic carbon return per unit of nutrient invested in leaf production [12],
b. Determine the ability of old new leaves to act as a nutrient source in production of new leaves [13-15] or
c. Determine the ability of old leaves to act as a sink for nutrients taken up during periods of no growth [16].
Several workers addressed the adaptive significance of evergreen vs. deciduous leaves [17-32]
In this study, leaf expansion in 9 trees (6 Evergreen (ES), 1 Semi Deciduous (SD) and 2 Deciduous (DS)) and 10 shrubs (7 Evergreen (ES) and 3 Deciduous (DS)) in Kumaun Himalayan region were recorded.
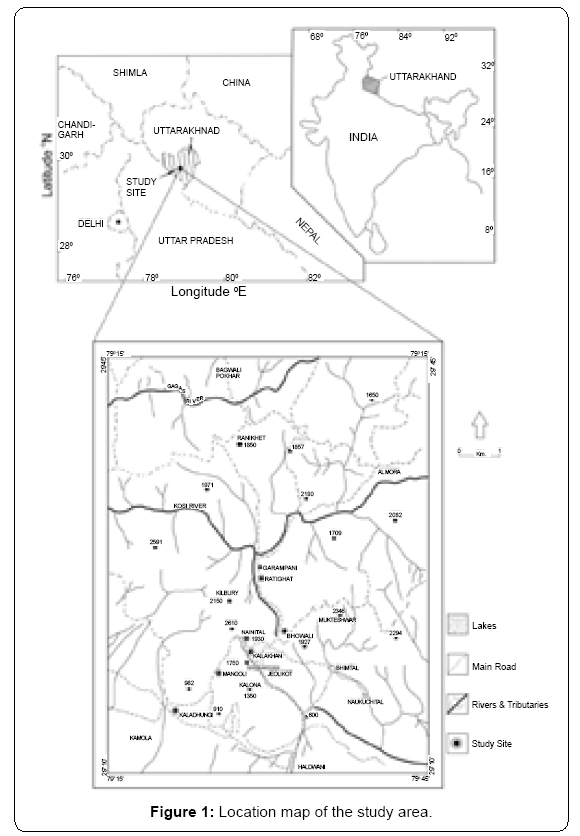
Description of the study area
This study comprises six study sites located between 29°22’ N latitudes and 79° 29’ E longitudes along an elevation transect of 350- 2500 m in Kumaun Himalaya (Table 1 and Figure 1). The degree of evergreeness increases with elevation [33]. In general, from lower to higher elevations domination of following forest prevailed: Sal (Shorea robusta) forests below 1000 m; Chir pine (Pinus roxburghii) forest between 1000 - 100 m; Banj oak (Quercus leucotrichophora) forests between 1600-2200 m.
| Forest type | Elevation (m) | Selected Species | |
|---|---|---|---|
| Trees | Shrubs | ||
| Tilonj-oak forest | 1900-2200 | Ilex dipyerna Wall | Berberis asiatica Roxb. ex DC. |
| Acer oblongum Wall. ex. D.C. | Berberis chitria Edwards | ||
| Myrsine africana L. | |||
| Viburnum cotinifolium D. Don | |||
| Banj-oak forest | 1700-2000 | Quercus leucotrichophora A. Camus | Daphne cannabina Wall. |
| Myrica esculenta Ham. ex D. Don | Rubus ellipticus Smith | ||
| Rhododendron arboreum Smith | Debregeasia salicifolia(D.Don) Rendle | ||
| Oak-mixed broadleaf forest | 1600-1700 | Prunus cerasoides D.Don | |
| Mixed broadleaf forest | 1000-1100 | Bauhinia variegata Linn. | |
| Pine mixed with Sal forest | 900-1200 | Woodfordia fruticosa (L.) Kurz | |
| Sal forest | 400-900 | Shorea robusta Gaertn.f. | Murraya paniculata (L.) Jack. |
| Mallotus philippinensis (Lam.) Muell.-Arg. | Flemingia strobilifera Roxb. ex Aiton. f. | ||
Table 1: List of species selected for study in the Kuamun Himalayan region.
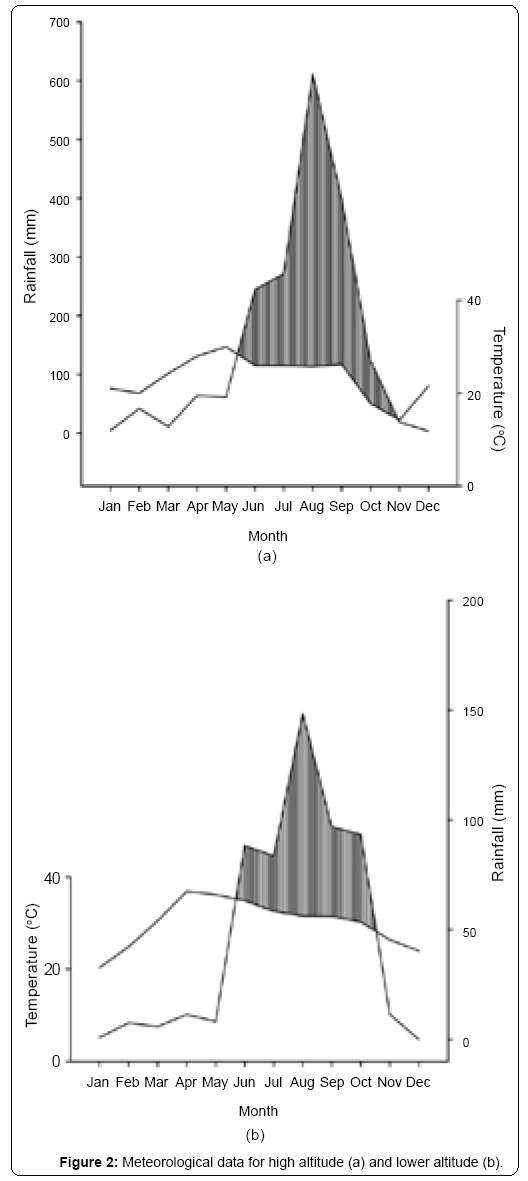
Climate: This region has certain characteristic climatic features. Though it falls under sub-tropical latitude, the abrupt rise in mountains creates a temperature comparable to that of a temperate climate. Data obtained from the State Observatory at Nainital for the study years (2008-2009) indicate that within the elevation transect of 1000-2500 m the mean monthly maximum temperatures range from 14°C in November to 30°C in May; mean monthly minimum temperatures from -2°C in January to 16°C in October and mean monthly rainfall ranges from 4mm in December to 611 mm in August. The winters are severe and frosts are common from December to February. Data obtained from the O/I Agromet Observatory, Pantnagar indicated that within the elevation transect of 400-1200 m the mean monthly maximum temperatures range from 20°C in January to 36°C in May; mean monthly minimum temperatures from 7°C in January to 26°C in July and mean monthly rainfall ranges from 1 mm in January to 148 mm in August. Also, temperatures increase with decreasing elevation.
Soils: The Soil was sandy loam, with sand percentage being highest at 400-900 m elevation and lowest at 1600-1700 m elevation. An increasing trend with elevation was found for soil organic matter content (r=0.67, P<0.05), clay (r=0.89, P<0.01) and water holding capacity (r=0.84, P<0.01). Soil pH ranging between 5.1 and 7.9. Among the soil nutrient the available Nitrogen concentration increases with increasing elevation (r=0.81, P<0.05). Available Phosphorus was maximum in the soil of high elevation sites, occupied by forest of oaks (Quercus spp.) and least in low elevation site occupied by Sal (Shorea robusta) forest. The soil of oak forests was markedly richer in available Potassium than those of other forest types such as Sal forest and Chir pine forest (Pinus roxburghii) [34].
Materials and Methods
Species and its selection: The selection of evergreen and deciduous species was based on their natural co-occurrence. The species investigated were described in Table 1. To determine the demography of leaves for each of the 9 tree species, 10 average sized mature trees (dbh >31.5 cm), having a similar degree of crown development and 10 shrubs were selected within 1 ha permanent plot of each of the selected forest sites. In each of the selected individuals, 30 vegetative buds were marked in spring 2008. 15 new shoots (originating from marked buds) were tagged and observed for number of leaves randomly at monthly intervals. In this way it was possible to record the periodical changes in number of leaves precisely. Leaves for each of the tree and shrub species were collected randomly on each of the sampling date (at weekly intervals from bud-break to full leaf expansion, and at monthly intervals during the leaf senescence) from the marked twigs. All collected leaves of individual species were sketched on graph paper to measure the leaf area [35,36]. Mean leaf life-span was adapted from Negi [36] who has calculated it by considering the time interval (days) between peak leafing and peak leaf drop on a leaf population basis in a forest stand.
Results
The species investigated (Table 1) can be divided into three groups based on leaf habit:
(i) evergreen- leaf fall completed only after substantial development (>80%) of new foliage, thus the trees never become leafless,
(ii) semideciduous- as above, but leaf fall completed when new foliage development is limited (<20%), rendering some branches of a tree leafless for a few days or weeks, and
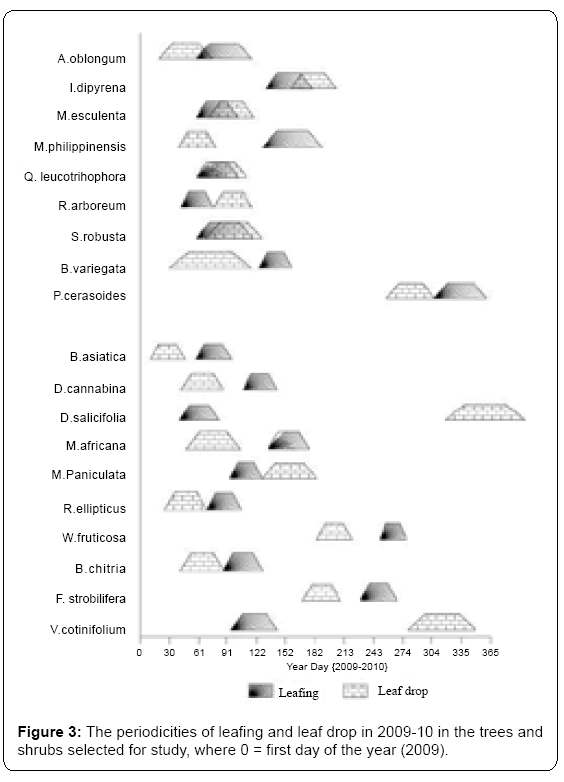
(iii) Deciduous- with time gap between leafing and leaf fall, rendering the whole trees leafless for some time in an annual cycle. The periodicity of leafing and leaf drop for the tree and shrub species is depicted in Figure 3.
Leaf demography
Trees: In all the species leaf initiation occurred between mid of March to first half of May. However, leaf initiation was recorded earliest in February in M. esculenta (ES). Three tree species i.e. M. philippinensis (SD), B. variegata (DS) and I. dipyrena (ES) were last to produced leaves in May. However, in P. cerasoides (DS) leaf initiation occurred during autumn season. The leafing period varied from species to species. In majority of species it was completed within 6 - 9 weeks except A. oblongum (ES) in which it was completed in 7-10 weeks. Variations in leaf initiation time were pronounced in year 2008 and 2009. In A. oblongum (ES) it was five weeks earlier, in I. dipyrena (ES) it was three weeks earlier and in one M. esculenta (ES) it was two weeks earlier in 2008 compared to 2009. In S. robusta (SD) and R. arboreum (ES) it was delayed by five weeks, in B. variegata (DS) and Q. leucotrichophora (ES) it was delayed by two weeks time and in M. phillipensis (SD) and P. cerasoides (DS) it was delayed by one week in 2008 compared to 2009 (Figure 3).
The concentrated spring/summer leaf drop in the evergreen species accounted for 80% of the total leaves produced in a year; the remaining 20% leaves were retained till rainy season in M. esculenta; till winters in Q. leucotrichophora and I. dipyrena (all ES). A. oblongum (ES) differed from rest of the evergreen species in respect of shedding all the old leaves in a single summer leaf drop episode. A minor leaf drop activity occurred in most of the deciduous species during rainy season. The DS i.e., B. variegata retained some leaves until the late winter (January and February). This species showed lengthy leaf drop (average 12 weeks), and became leafless only for about one month in spring season. The leaf drop period varied from one species to another. In majority of species it ranged from 5-7 weeks. However, in B. variegata (DS), A. oblongum (ES) and in S. robusta (SD) it was a longer activity (average=10 weeks), ranging from 8 weeks in I. dipyrena (ES) to 12 weeks in B. variegata (DS).
The peak leaf area was found smallest in I. dipyrena (18.65 cm2) and largest in S. robusta (179.14 cm2). On an average the peak leaf area for DS (87.71) was greater than for the ES (33.67 cm2). Accomplishment of peak leaf area in ES was rather slow and accomplished one month later than the DS. Per cent leaf area per shoot accomplished after one month of leaf emergence was 76.59% for ES and 90.70% for DS (Table 2 and Figure 4).
| Species | Leaf area at full expansion (cm2) | Leaf expansion rate (cm2 day-1) | % leaf area after two weeks of expansion | % leaf area after four weeks of expansion |
|---|---|---|---|---|
| Evergreen: | ||||
| A. oblongum | 30.65 | 0.72 | 30.15 | 76.41 |
| I. dipyrena | 18.65 | 0.44 | 49.01 | 82.84 |
| M. esculenta | 33.98 | 0.81 | 42.32 | 68.63 |
| M. philippinensis | 34.56 | 0.71 | 51.68 | 67.07 |
| Q. leucotrichophora | 31.90 | 0.65 | 30.14 | 86.78 |
| R. arboreum | 52.27 | 1.24 | 49.97 | 77.81 |
| Average | 33.67 | 0.76 | 42.21 | 76.59 |
| Semi deciduous: | ||||
| S. robusta* | 179.14 | 3.66 | 12.93 | 77.68 |
| Deciduous: | ||||
| B. variegata | 136.58 | 3.25 | 48.78 | 89.64 |
| P. cerasoides | 38.84 | 0.92 | 39.93 | 91.76 |
| Average | 87.71 | 2.09 | 44.36 | 90.70 |
*excluding S. robusta, a SD that has 179.14 cm2 leaf area
Table 2: Leaf area and leaf expansion rate of the studied tree species.
Shrubs: In majority of shrubs leaf initiation started from mid - March to first half of April. Early leafing was recorded in one ES (D. salicifolia) i.e., February. Three ES species i.e. M. africana (May-June), W. fruticosa (September - October) and F. strobilifera produced leaves in August-September and were last to produced leaves. The leaf initiation time of first leaf emergence varied significantly among the species. In M. paniculata (ES) and V. cotinifolium (DS) it was three weeks earlier, in B. chitria (DS) it was two weeks earlier and in three ES (D. cannabina, D. salicifolia, F. strobilifera) it was one week earlier in 2008 compared to 2009. In B. asiatica (ES) it was delayed by three weeks, in two ES (W. fruticosa and M. africana) it was delayed by two weeks time and in R. ellipticus (ES) it was one week delayed in 2008 compared to 2009 (Figure 3). The average leafing period for ES was about to 5 weeks for DS species it was 6 weeks.
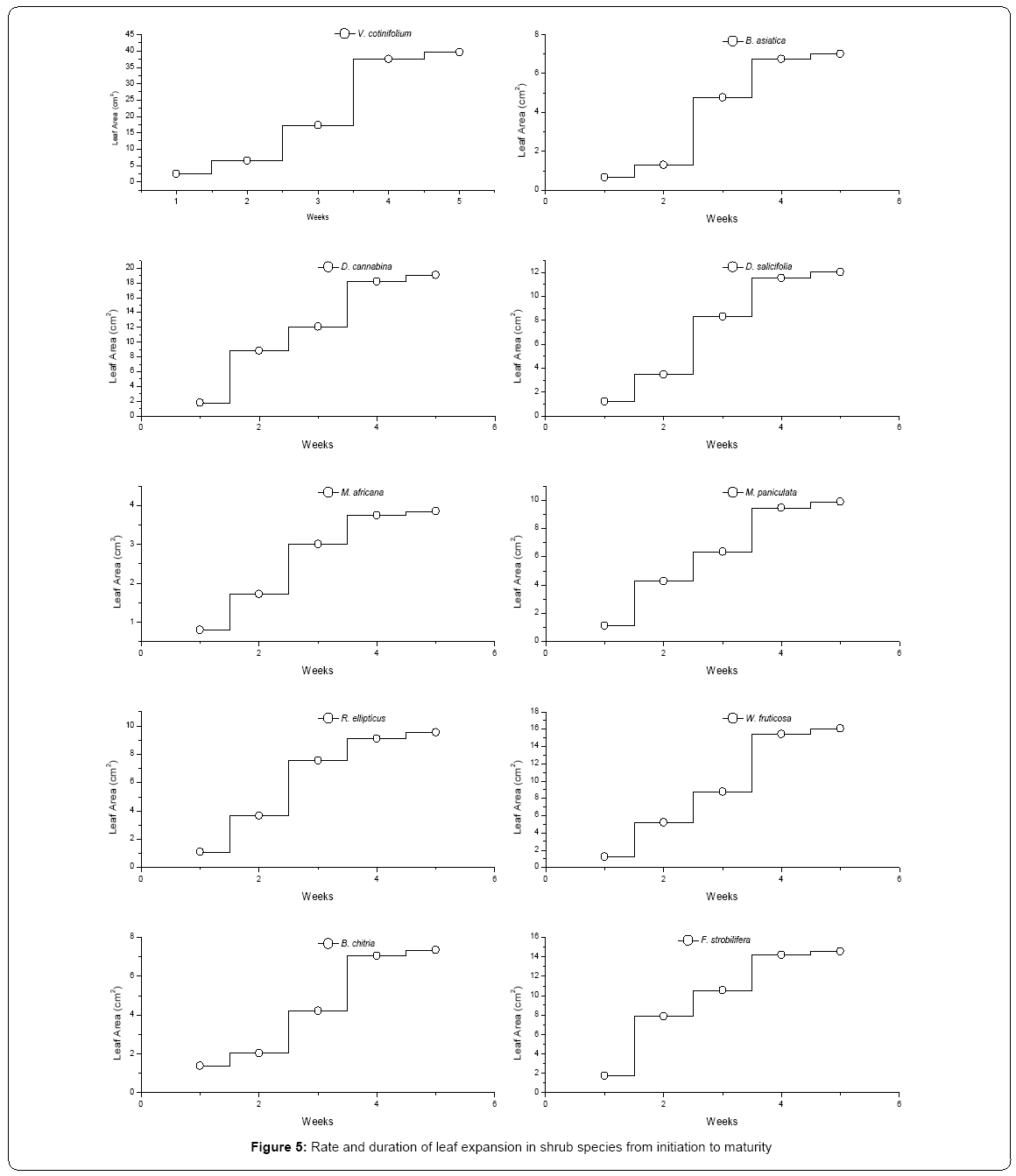
In three DS leaf drop was confined to autumn and winter seasons (cool to cold, dry part of the year). The major period of leaf drop for seven ES was spring through summer season (warm-dry part of the year). In majority of species leaf drop period ranged from 5-8 weeks. Longest leaf drop period (9-10 weeks) was measured for three ES (D. salicifolia, M. paniculata and M. africana). The DS (V. cotinifolium) retained some leaves until late winter (January and February). This species showed lengthy leaf drop (average 10 weeks), and became leafless only for about one month in winter-spring season. The average leaf drop period in DS (7.2 weeks) is greater than ES (6.6 weeks) (Table 3 and Figure 5).
| Species | Leaf area at full expansion (cm2) | Leaf expansion rate (cm2 day-1) | % leaf area after two weeks of expansion | % leaf area after four weeks of expansion |
|---|---|---|---|---|
| Evergreen: | ||||
| B. asiatica | 7.01 | 0.20 | 18.65 | 96.35 |
| D. cannabina | 19.04 | 0.54 | 46.27 | 95.43 |
| D. salicifolia | 12.04 | 0.34 | 28.65 | 95.85 |
| M. africana | 3.85 | 0.11 | 44.68 | 97.14 |
| M. paniculata | 9.87 | 0.28 | 43.06 | 95.74 |
| R. ellipticus | 9.56 | 0.27 | 38.39 | 95.29 |
| W. fruticosa | 16.08 | 0.46 | 32.23 | 95.82 |
| Average | 11.06 | 0.32 | 35.99 | 95.95 |
| Deciduous: | ||||
| B. chitria | 7.34 | 0.21 | 27.87 | 96.03 |
| F. strobilifera | 14.56 | 0.42 | 53.99 | 97.47 |
| V. cotinifolium | 39.67 | 1.13 | 16.49 | 94.63 |
| Average | 20.52 | 0.59 | 32.78 | 96.04 |
Table 3: Leaf area and leaf expansion rate of the studied shrub species.
Discussion
As in most areas of the Indian subcontinent, the monsoon pattern of rainfall is characteristic climatic feature of this region. The period of mid-June to mid-September when about three-fourth of the annual rainfall occurs is the most favorable season for plant growth, for it is also warm. The majority of tree species presently studied were evergreen. A characteristics feature of trees was that the concentrated leaf drop activity immediately followed the spurt of leafing. In present study, the leaf initiation in tree species occurredbetween mid-March to first half of May, however, it was recorded earliest i.e. in February in M. esculenta and last i.e. in May in M. philippinensis, B. variegata and I. dipyrena. In case of shrubs, majority of species started leaf initiation from mid-March to first half of April. Early leafing was recorded in D. salicifolia i.e. February. Three shrub species i.e. M. africana (May-June), W. fruticosa (September-October) and F. strobilifera were last to produced leaves in August- September. There existed a positive relationship (r=0.470, df=19, P<0.05) between leafing period (days) of the tree and shrub species and elevation in which they occur. In a majority of species including those which form most of the forests the bud-break occurred within a month from mid-March to mid-April, when temperature and day length began to rise (Figure 3). A significant correlation was found between the leaf initiation day and mean temperature (°C) of particular day for tree (r=0.468, df=17, p<0.05) and shrub species (r=0.456, df=19, p<0.05) of two study years.
Studies [37,38] have implicated day length and air temperature [39] increase as the inducer of leaf flushing, which holds true for the present study area where peak activity of bud break and leafing takes place during March-April when photoperiod and temperatures are increasing [34]. In the study sites, though the soil moisture continues to be low from October to mid-June, the long dry spells are broken by isolated rain showers (average monthly rainfall is 14.8 and 70.1 mm around 2000 m elevation, and 5.95 and 11.4 mm in March and April, respectively in lower altitudes), possibly facilitating leaf emergence during March-April. Reich and Borchert [40] reported that a storm of 20 mm rainfall could revive the water potential sufficiently to support growth in Tabebuia neochrysantha.
In B. variegata the bud-break occurred in May, i.e. long after, it occurred in other species. In May photoperiods were close to the maximum within the annual cycle and the mean temperature was about twice as high as in January and February. Young leaves of these species are likely to be more adapted to water stress than the majority of the species which produced leaves earlier. For, because of higher temperatures water stress must be more severe at the end of the drought period [36].
In most species, leaf drop also occurred during the dry seasons immediately after rainy season in deciduous species and towards the end of dry period (mid-March to mid-April) in evergreen species. While in case of deciduous species photoperiod and temperature were declining at the time of leaf drop, in case of evergreen species both were increasing. The short flushing behavior is characteristic to temperate trees [41,42]. Some of the species of lower elevations (e.g. S. robusta) showed longer leafing period, thus suggesting the retention of a. character, attributed to tropical species [39,43,44]. Earlier and rapid leaf expansion in deciduous species than in evergreen species is considered typical of northern temperate trees and shrubs [45-48]. Gill and Mahall [49] demonstrated that in a chaparral shrub community of California, the deciduous species (Salvia mellifera) did not initiate growth significantly earlier and at faster rate than the evergreen species (Ceanothus megacarpus) co-occurring at a set of same microsites. Though in general, a majority of the leaves were recruited during spring/summer season and additional recruitments, generally on terminal portion of shoots occurred during rainy season or/and autumn season. However, leaves produced in each of these seasons were shed at the same time, indicating that those recruited during rainy and autumn seasons had shorter life - spans than those recruited during spring/summer season.
Of special interest is the leaf phenology of evergreen species which dominate the forests of this region, out competing deciduous species in mature communities of most habitats [33]. Evergreen species retain leaves throughout the winter and exhibit simultaneous leaf fall an emergence in summer resembling the “leaf-exchanging type” species of tropical forests [50]. Retaining leaves throughout a year enables the Evergreen species to utilize the same unit of nutrients to support the new growth [51] and maintain some photosynthesis throughout the winter [52]. This strategy makes a tight circulation of nutrients in the ecosystem, a characteristic feature of late successional communities [53] occupied by the evergreen species in this region. Although the Deciduous species of this region has significantly greater area compared to Evergreen species (87.71 vs 33.67 cm2, trees; and 20.52 vs 11.06 cm2, shrubs), and they are known to have higher photosynthetic efficiency [54,55].
References
- Pravdin LF (1969) Scot pine variation, Intra specific taxonomy and selection. Israel Prog. for Scientific translation. IPST. Jerusalem.
- Timiryazev KA (1938) Zhizn’ rasteniya (Plant Biology). Sobrainie Sochinent 4, Moskia selkhoziz
- Evans GC (1972) The quantitative analysis of plant growth. Blackwell Scientific Pulblications Oxford.
- Brouwer R, De-Wit CT (1969) Over net groeritime van populieren. Comm. Inst. Forest Res. Wageningen.
- Watson, DJ (1958) The dependence of net assimilation rate of leaf area index. Ann Bot 22: 37-54.
- Evan GC, Huges AP (1962) Plant growth and aerial environment III. On the computation of unit leaf rate. New Phytol 61: 322-327
- Blackman GE, Wilson GL (1951) Physiological and ecological studies in the analysis of plant environment. VII. An analysis of the differential effects of light intensity on the net assimilation rate, leaf area ratio, and relative growth rate of different species. Ann Bot London New Series 15: 373-408.
- Gottlieb LD (1978) Allocation, growth rates and gas exchange in seedlings of Stephanomeris exigns ssp: Coronaria and its recent derivatives. Am J Bot 65: 970-977.
- Grime JP, Hunt R (1975) Relative growth rate: its range and adaptive significance in a local flora. J Ecol 63: 393-422.
- Shaver GR (1981) Mineral nutrition and leaf longevity in an evergreen shrub, Ledum palustre spp Decumbens. Oecologia 49: 362-365.
- Whittaker RH, Likens GE, Bormann FH, Eaton JE, Siccama TG (1979) The Hubbard Brook study: nutrient cycling and element behavior. Eco 60: 203-220.
- Small E (1972) Photosynthetic rates in relation to nutrient recycling as an adaptation to nutrient deficiency in peat bog plants. Can J Bot 50: 2227-2233.
- Small E (1972) Ecological significance of four critical elements in plants of raised sphagnum peat bogs. Eco 53: 498-503.
- Turner J, Olson PR (1976) Nitrogen relations in a Douglas fir plantation. Ann Bot 40: 1185-1193.
- Reader RJ (1980) Effects of nitrogen fertilizer, shade and the removal of new growth on longevity of overwintering bog ericad leaves. Can J Bot 58: 1737-1743.
- Mooney HA, Rundel PW (1979) Nutrient relations of the evergreen shrub, Adenostoma fasciculatum in the California chaparral. Bot Gazette 140: 109-113.
- Chabot BF, Hicks DJ (1982) The Eco of leaf life spans. Ann Rev Eco Sys 13: 229-259.
- Goldberg DE (1982) The distribution of evergreen and deciduous trees relative to soil type: an example from the Sierra Madre, Mexico, and a general model. Eco 63: 942-951.
- Sarmiento G, Goldstein G, Meinzer F (1985) Adaptive strategies of woody species in neotropical savannas. Bio Rev 60: 315-355.
- Givnish TJ (1986) On the economy of plant form and function. Cambridge University Press, New York p 25-55.
- Woodward FI (1987) Climate and plant distribution. Cambridge University Press England.
- Arris LL, Eagleson PS (1989) Evidence of a physiological basis for the boreal-deciduous forest ecotone in North America. Vegetatio 82: 55-58.
- Arris LL, Eagleson PS (1994) A water-use model for locating the boreal-deciduous forest ecotone in eastern North America. Water Res Res 30: 1-9.
- Gower ST, Richards JH (1990) Larches: deciduous conifers in an evergreen world. Biosci 40: 818-826.
- Kikuzawa K (1995) Leaf phenology as an optimal strategy for carbon gain in plants. Canadian J Bot 73: 158-163.
- Hollinger DY (1992) Leaf and simulated whole-canopy photosynthesis in 2 co-occurring tree species. Eco 73: 1-14.
- Sobrado MA (1997) Embolism vulnerability in drought-deciduous and evergreen species of a tropical dry forest. Acta Oecologica 18: 383-391.
- Salleo S, Nardini A, Logullo MA (1997) Is sclerophylly of Mediterranean evergreens an adaptation to drought? New Phy 135: 603-612.
- Damesin C, Rambal S, Joffre R (1998) Co-occurrence of trees with different leaf habit: a functional approach on Mediterranean oaks. Acta Oecologica 19: 195-204.
- Walters MB, Reich PB (1999) Low-light carbon balance and shade tolerance in the seedlings of woody plants: do winter deciduous and broadleaved evergreen species differ? New Phy 143: 143-154.
- Kloeppel BD, Gower ST, Vogel JG, Reich PB (2000) Leaf-level resource use for evergreen and deciduous conifers along a resource availability gradient. Functional Eco 14: 281-292.
- Namikawa K, Okamoto S, Sano J (2000) Edaphic controls on mosaic structure of the mixed deciduous broadleaf/conifer forest in northern Japan. Forest Eco and Manag 127: 169-179.
- Singh JS, Singh SP (1987) Forest vegetation of the Himalaya. Bot Rev 52: 80-192.
- Kumar S (2011) Studies on phenology and seedling dynamics of major tree and shrub species along an altitudinal gradient in Kumaun Himalaya. Kumaun University, Nainital.
- Ralhan PK (1985) c. Ph.D. Thesis, Kumaun University, Nainital
- Negi GCS (1989) Phenology & Nutrient dynamics of tree leaves in Kumaun Himalaya. Ph.D. Thesis, Kumaun University, Nainital.
- Njoku E (1963) Seasonal periodicity in the growth and development of some forest trees in Nigeria. I. Observations on mature trees. J Ecol 51: 617-624.
- Lawton JRS, Akpan EEJ (1968) Periodicity in Plumeria. Nature 218: 384-386.
- Frankie GW, Baker HG, Opler PA (1974) Comparative phenological studies of trees in tropical wet and dry forests in the lowlands of Casta Rica Ecol 62: 881-919.
- Reich PB, Borchert R (1982) Phenology and ecophysiology of the tropical tree. Tabebuia neoochrysantha. Eco 63: 294-299.
- Carnell MGR, Last FT (1976) Tree Physiology and Yield Improvement. Academic Press. New York 223-243.
- Rook DA, Corson MJ (1978) Temperature and irradiance and the total daily photosynthesis production of a Pinus radiata tree. Oecologia 36: 371-383.
- Opler PA, Frankie CW, Baker HC (1980) Comparative phenological studies of treelet and shrub species in tropical wet and dry forests in the lowlands of Costa Rica. J Ecol 68: 167-188.
- Shimizu Y (1983) Phenological studies of the subtropical broad-leaved evergreen forests at Chichijima island in the Bonin islands. Jap J Ecol 33: 135-147.
- Kozlowski TT (1971) Growth and Development of Trees. Vol I Academic Press New York.
- Kozlowski TT (1971) Growth and Development of Trees. Vol II Academic Press New York.
- Chapin FS, Johnson DA, McKendrick JD (1980) Seasonal movement of nutrient in plants of differing growth form in an Alskan tundra ecosystem: implication for herbivory. J Ecol 68: 189-210.
- Chapin FS III, Tryon PR (1983) Habitat and leaf habit as determinants of growth, nutrient absorption and nutrient use by Alaskan taiga forest species. Can J For Res 13: 818-826.
- Gill DS, Mahall BE (1986) Quantitative phenology and water relations of an evergreen and a deciduous chaparral shrub. Ecol Monogr 56: 127-143.
- Longman KA, Jenik J (1974) Tropical Forest and its Environment. Longman. London and New York. 96.
- Fife DN, Nambiar EKS (1982) Accumulation and retranslocation of mineral nutrients in developing needles in relation to seasonal growth of young radiate pine trees. Ann Bot 50: 817-829.
- Saeki T, Nomoto N (1958) On the seasonal change of photosynthetic activity of some deciduous and evergreen broadleaf trees. Bot Mag 71: 235-241.
- Vitousek PM, Reiners WA (1975) Ecosystem succession and nutrient relation: a hypothesis. Bio Sci 25: 376-384.
- Murphy PG, Lugo AE (1986) Ecology of tropical dry forest. Ann Rev Ecol Syst 17: 67-88
- Bhadula SK, Joshi SC, Purohit AN (1995) Seasonal variation in photosynthetic characteristics of some mountain tree species from Garhwal Himalaya. Physiol Mol Biol Pl 1: 151-160.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 15883
- [From(publication date):
September-2013 - Oct 19, 2025] - Breakdown by view type
- HTML page views : 11167
- PDF downloads : 4716