Research Article Open Access
An Ultra-Sensitive LC Method for Simultaneous Determination of Rosuvastatin, Alprazolam and Diclofenac Sodium in API, Pharmaceutical Formulations and Human Serum by Programming the Detector
Najma Sultana1, Muhammad Saeed Arayne2 and Saeeda Nadir Ali2*
1Department of Pharmaceutical Chemistry, Faculty of Pharmacy, University of Karachi, Pakistan
2Department of Chemistry, University of Karachi, Pakistan
- *Corresponding Author:
- Saeeda Nadir Ali
Department of Chemistry
University of Karachi
Karachi-75270, Pakistan
Tel: 00923337208120
E-mail: saeeda_khowaja@hotmail.com
Received date: October 21, 2012; Accepted date: November 25, 2012; Published date: December 03, 2012
Citation: Sultana N, Arayne MS, Ali SN (2012) An Ultra-Sensitive LC Method for Simultaneous Determination of Rosuvastatin, Alprazolam and Diclofenac Sodium in API, Pharmaceutical Formulations and Human Serum by Programming the Detector. J Anal Bioanal Tech 3:154. doi: 10.4172/2155-9872.1000154
Copyright: © 2012 Sultana N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Here, we report a rapid and efficient liquid chromatographic method with UV detection for the simultaneous determination of rosuvastatin, alprazolam and diclofenac sodium in API, pharmaceutical formulations and human serum employing a reversed phase Bondapak C18 (25 cm, 0.46 cm, 10 μm) column at room temperature using methanol: water (80:20 v/v) and pH adjusted to 2.9 with 85% o-phosphoric acid. Separation was achieved within a time span of 6 min. The detector response was monitored at 240 nm and the flow rate was set at 1.0 mL.min-1. Various LC parameters were optimized and developed method was validated as per ICH (2006) guidelines. Linear calibration range was determined to be 0.02-0.64, 0.125-4.0 and 0.05-1.6 μg.mL-1 and detection and quantitation limits were found to be 4.0, 17.0, 7.0 and 12.0, 52.0, 22.0 ng.mL-1 for rosuvastatin, alprazolam and diclofenic sodium respectively. Method was linear for all analytes with correlation coefficients >0.998. The intra-day and inter-day accuracy and precision of the assay were in acceptable range of 98.21-101.91% recovery and 0.20-2.07% RSD. The sensitivity of the method was enhanced when the chromatograms were analyzed by programming the detector at individual λmax of 244, 222 and 284 nm for rosuvastatin, alprazolam and diclofenac sodium respectively. High peak intensity was observed on injecting same sample concentration after the detector was programmed. Linearity was obtained even at lower concentrations of 0.02-0.64, 0.05-1.60 and 0.02-0.64 μg.mL-1. LOD and LOQ values shifted down to 3.0, 6.0, 1.0 and 9.0, 19.0, 3.0 ng.mL-1 respectively. Recovery was found to be in the range of 98.32-101.87% and inter-day and intra-day precision was less than 2.10%. The proposed method was found to be robust and successfully employed for the determination of studied drugs in commercial formulations and human serum without interference of excipients or endogenous components of serum.
Keywords
Rosuvastatin; Alprazolam; Diclofenac sodium; RP HPLC; Time program
Introduction
Rosuvastatin (ROS), chemically bis [(e)-7-[4-(4-fluorophenyl)-6- isopropyl-2-[methyl(methylsulfonyl)amino] pyrimidin-5-yl] (3r,5s)-3, 5-dihydroxyhept-6-enoicacid] calcium salt is a member of lipid-lowering drug class commonly referred to as statins. It prevents the biosynthesis of key rate-limiting cholesterol enzyme in liver by inhibition of 3-hydroxy-3-methylglutaryl-coenzyne A (HMG-CoA) reductase. On the other side, statins behave as antitumor [1], immunomodulator [2], anti-malarial [3], anti-oxidative [4], and bone forming agents [5]. Alprazolam (ALP), chemically 8-chloro-1-methyl-6-phenyl-4H-s-triazolo [4,3-I] [1,4] benzodiazepine is mainly used to treat anxiety disorders. Diclofenac sodium (DCL), chemically sodium [o-(2,6-dichloroanilino) phenyl] acetate (also available in potassium salt) is a safe and effective synthetic drug of class non-steroidal anti-inflammatory drug having marked antirheumatic, anti-inflammatory, analgesic and antipyretic properties [6]. It is commonly prescribed for joint diseases and other arthritic conditions [7]. Chemical structures of ROS, ALP and DCL are given in figure 1.
Different LC methods either alone or in combination with other analytes have been reported for the determination of these drugs in pharmaceutical formulations and in body fluids including determination of ROS with fenofibric acid [8], statins [9] and with DCL and timolol [10]. HPLC methods have also been reported for the determination of ROS in pharmaceuticals [9], rat plasma [11] and in human plasma along with gemfibrozil [12]. Similarly, LC methods for the determination of ALP with fluoxetine hydrochloride [13], sertaline [14] and propranolol hydrochloride [15] in pharmaceutical formulation and with oxazepam,and diazepam in human urine samples [16] and DCL alone [17] and in combination with flufenamic acid, indomethacin and ketoprofen [18] and papaverine hydrochloride [17] have been reported in literature.
In the past few years, our research co-workers have developed and validated individual or simultaneous liquid chromatographic methods for the determination of these drugs like determination of ROS with atenolol, spironolactone, glibenclamide and naproxen sodium [19], with pioglitazone, gliquidone and simvastatin [20], prazosine, atorvastatin and simvastatin [21] and with lisinopril, pravastatin and atorvastatin [22] and simultaneous determination of NSAIDs including DCL with captopril [23], metformin [24] and verapamil [25].
This paper reports the development and validation of an efficient and ultra-sensitive isocratic reverse phase LC method for the simultaneous determination of ROS, ALP and DCL at isobestic point and by programming the detector at individual wavelength of each component. The procedure involved easily available low cost reagents and chemicals and did not require installation of any complicated or sensitive detector. Validation was performed following the ICH guidelines [26]. The proposed method can be successfully applied for the determination of these drugs with good percent recovery values in pharmaceutical formulations and human serum without any chromatographic interference from tablet excipients or endogenous components of serum.
Experimental
Materials and reagents
ROS (Pharm Evo Pvt. Ltd), ALP (Genix Phrma Pvt Ltd) and DCL (Brookes Pharmaceutical Laboratories Pakistan limited) were kind gifts from respective pharmaceuticals. Pharmaceutical formulation X-plended® 10 mg, Nerum® 0.5 mg and Phlogin®50 mg were purchased from local pharmacy. HPLC grade methanol, acetonitrile and 85% o-phosphoric acid were purchased from Merck, Darmstadt, Germany. Double distilled de-ionized water was used throughout the analysis which was prepared by using Millipore ultra-pure water system.
Instrumentation
The chromatographic system was facilitated with Shimadzu LC- 20 AT VP solvent delivery pump, rheodyne manual injector fitted with a 20 μL loop and a SPD-20A Shimadzu UV visible detector. Chromatographic separation was performed by using a Bondapak C18 (25 cm, 0.46 cm, 10 μm) column. Data integration was carried out on Shimadzu CBM-102 Communication Bus Module and the data was acquired and quantified on Shimadzu Class-GC 10 software (version 2). Shimadzu 1800 UV-visible spectrophotometer was used for the determination of isosbestic point and individual λmax of analyte.
Calibration curves
The stock solution of 50 μg.mL-1 was diluted to 0.02-0.64, 0.125-4.0, 0.05-1.60 μg.mL-1 (isobestic point) and 0.02-0.64, 0.05-1.60, 0.02-0.64 (time program) for ROS, ALP and DCL respectively. These working standard solutions were prepared once and analyzed daily for interday and intraday precision of the method. 20 μL of degassed and filtered (0.45 μm pore size) sample was injected into the system.
Sample Preparation
Pharmaceutical formulation
Twenty tablets of each formulation were finely triturated into pestle and mortar. The powder equivalent to 1 mg mL-1 was separately dissolved in small volume of diluent and shaken well for proper mixing. All the solutions were allowed to stand for 30 min and then sonicated for complete solubilization of drugs. The contents were filtered to separate the insoluble excipients and volumes were completed with diluent. Aliquot of each drug was transferred in to 25 mL volumetric flask to get the required concentration and further dilutions were prepared for analysis. The solutions were injected into the system after micro filtration through 0.45 μm millipore filter paper.
Drug serum sample
Blood sample was collected from a human donor at Fatmid Foundation Karachi in an ethylenediaminetetraacetic acid (EDTA) glass tube and centrifuged at 1600×g for 10 min at 4°C. The plasma collected was treated with 9.0 mL acetonitrile (per mL plasma) and vortexed for one minute followed by centrifugation for 10 minutes at 10,000 rpm. The clear serum solution obtained in supernatant was spiked with respective concentration of ROS, ALP and DCL for analysis at isobestic point and at individual λmax.
Chromatographic conditions
The chromatographic analysis was performed by using mobile phase consisting of methanol-water (80:20 v/v) adjusted to pH 2.9 with o-phosphoric acid (85%). Prior to delivering into the system it was filtered through a millipore vacuum filter system equipped with 0.45 μm filter and degassed using an LC 30H ultrasonic bath. All the raw materials were eluted isocratically with a flow rate of 1.0 mL.min-1 at room temperature. Initially, the peak response was monitored at isobestic point of 240 nm where the spectra of all drugs cross each other, and then the spectrophotometric detector was operated by programming at 244, 222 and 284 nm for ROS, ALP and DCL respectively.
Method development and experimental condition optimization
In the present study, an LC method with UV detection for the simultaneous determination of ROS, ALP and DCL has been developed and optimized by considering a number of parameters discussed below.
Selection of stationary phase
Three different columns of different specifications, Bondapak C18 column (25 cm, 0.46 cm, 10 μm), Discovery C18 (5 μm, 25×0.46 cm) column and Purospher Star C18 (5 μm, 25×0.46 cm) column for the analysis of ROS, ALP and DCL were tried. The column which resulted in short separation time was selected for analysis.
Mobile phase composition and pH
Different ratios of various solvents including methanol, acetonitrile and water with variable pH were tested at room temperature for simultaneous determination of studied drugs to obtain best separation and good resolution.
Flow rate
The suitable flow rate was evaluated by varying it in the range of 0.8-2.0 in isocratic mode.
Detector wavelength
UV-scan of each analyte was taken on Shimadzu 1800 UV-Vis Spectrophotometer and the cut-off point was determined for the analysis at isobestic point.
Method validation
The proposed method was validated following the ICH 2006 guidelines for system suitability test, specificity and selectivity, linearity, accuracy, precision, detection and quantitation limits and robustness.
System suitability test
The chromatographic system was equilibrated with initial mobile phase composition, followed by ten consecutive injections of same standard. The system suitability was evaluated on each day of method validation and parameters including capacity factors (k’), theoretical plates (N), tailing factor (T), resolution (Rs), and separation factor (α) were calculated.
Specificity
For specificity studies, blank serum sample, placebo, spiked serum sample and solution of pharmaceutical formulation were injected separately after filtration through 0.45 μm millipore filter paper and the possible interference of unwanted species was observed.
Linearity
Linearity of the proposed method was determined from the calibration curve constructed between peak area and concentration of each analyte at six concentration levels. The calibration curves were constructed for API and serum and regression characteristics including intercept, slope, correlation coefficient, standard error and standard error estimate were calculated.
Recovery
Percent recovery of the method was determined for each analyte from the pharmaceutical formulation and spiked serum sample at six and three concentration levels respectively. It was calculated by applying the equation;
% Recovery = [C]/[X]×100
Where, [C] and [X] are the area of analyte in pharmaceutical formulation or spiked sample and in reference standard respectively.
Precision
Precision of the method was analyzed through repeatability and intermediate precision of bulk drug and serum at isobestic point and at individual λmax of analyte. The bulk drug was analyzed at six concentration levels and serum at three concentration levels and %RSD of each analyte was calculated by using formula;
% RSD=(SD/ Mean)×100
Where, SD and Mean are the standard deviation and average of analyte at specific concentration.
Detection and quantitation limits
The LOD and LOQ were determined at the concentration where signal to noise ratio was three times and ten times to the baseline noise respectively. These were calculated from the formulae:
LOD = 3.3×SD/ α
LOQ = 10×SD/ α
Where SD and α are the standard deviation and slope of the calibration curve.
Robustness
Deliberate changes in various chromatographic parameters were carried out like mobile phase composition was changed to ± 2 mL, pH up to ± 2 and flow rate was varied to 0.1 mL.min-1 and their effect was observed on analytical results.
Results and Discussion
Dyslipidemia is a condition in which the lipoprotein metabolism is disordered and elevated or deficient amount of cholesterol is produced. It is very common in women with polycystic ovary syndrome. Overweight women with dyslipidemia have musculoskeletal pain as well. Almost, many of the dyslipidemia patients also suffer from medical condition of stress like anxiety. Medication that is highly recommended to relieve the symptoms of dyslipidemia, anxiety and acute or chronic pain are statins, anti anxiety and NSAIDs. ROS, a competitive HMGCoA reductase enzyme inhibitor, belongs to drug class statin having mechanism of action similar to that of other drugs of this class, is prescribed to treat dyslipidemia. ALP is a most prescribed psychoactive drug, commonly used for panic and anxiety disorder. DCL is effective drug for musculoskeletal pain and inflammation. It was, therefore, the need for simultaneous determination of these co-prescribed drugs in bulk, dosage formulations and in body fluids.
Method development and optimization
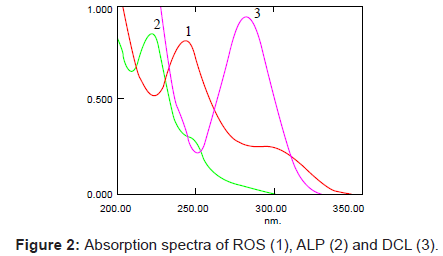
A number of parameters were studied to develop the optimized analytical method for the determination of ROS, ALP and DCL simultaneously in bulk drug, pharmaceutical formulations and human serum. All the studied drugs are soluble in organic solvents like acetonitrile and methanol and also in aqueous solutions. Different ratios of acetonitrile: water and methanol: water with varying pH was used to analyze the efficiency. It has been observed that all the drugs show good resolution and separation in both mobile phases (acetonitrile: water 60:40 and methanol: water 80:20). The use of 80:20 methanol:water mobile phase is advantageous as methanol is low cost solvent and retention times of drugs were short. The pH of mobile phase was adjusted to 2.9 using o-phosphoric acid and the wavelength of detector was set at 240 nm for determination at isobestic point. The representative UV spectra and chromatograms of ROS, ALP and DCL are shown in figures 2 and 3.
Method validation
The newly developed method for the simultaneous determination of ROS, ALP and DCL has been validated at isobestic point of 240 nm following the ICH guidelines [26] for specificity and selectivity, system suitability, linearity, accuracy, precision, detection and quantitation limits and robustness.
System suitability test
The system suitability calculated on each day of validation was found to be in the acceptance criteria. The efficiency of column expressed by means of theoretical plates was greater than 2000 and tailing factor was less than 2 for all the drugs (table 1).
| Drug | tR | K’ | N | T | Rs | α |
|---|---|---|---|---|---|---|
| Isosbestic point | ||||||
| ROS | 3.02 | 3.14 | 2937 | 2.19 | - | 1.09 |
| ALP | 3.69 | 4.06 | 3355 | 1.77 | 2.80 | 1.29 |
| DCL | 5.19 | 6.11 | 4260 | 1.43 | 0.24 | 1.19 |
| Time program method | ||||||
| ROS | 3.03 | 2.32 | 3166 | - | 0.21 | 1.49 |
| ALP | 3.74 | 3.07 | 3472 | 1.24 | 2.90 | 1.32 |
| DCL | 5.31 | 4.77 | 4350 | 0.96 | 0.32 | 1.14 |
Retention time (tR), Capacity factors (k’), Theoretical plates (N), Tailing factor (T), Resolution (Rs), Separation factor (α)
Table 1: System suitability parameters.
Specificity
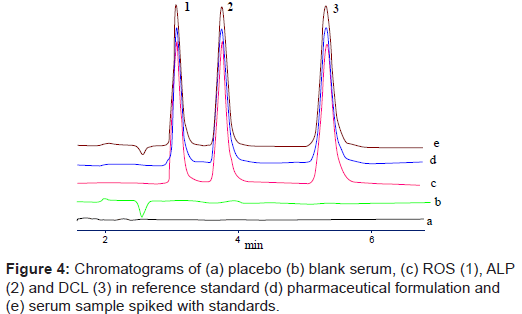
Specificity of the proposed method was demonstrated by means of blank serum and excipients and spiked with samples. From figure 4, it is evident that there was no interfering peak of serum or excipient which interrupts in the determination of ROS, ALP and DCL indicating the specificity of method.
Linearity
Calibration curves between concentration and peak areas of ROS, ALP and DCL were constructed, which were found to be 0.02-0.64, 0.125-4.0 and 0.05-1.60 μg.mL-1 respectively. The correlation coefficient in each case was greater than 0.998. Regression characteristics including slope, intercept, correlation coefficient, standard error and standard error estimate are given in (table 2).
| Drug | Linearityµg mL-1 | Intercept | Slope | R2 | SEa | SEEb | LODng mL-1 | LOQng mL-1 |
|---|---|---|---|---|---|---|---|---|
| Isobestic point | ||||||||
| ROS | 0.02-0.64 | 37209 | 385689 | 0.9980 | 0.0083 | 0.0117 | 4 | 12 |
| ALP | 0.125-4.0 | 28678 | 82038 | 0.9986 | 0.0608 | 0.0942 | 17 | 52 |
| DCL | 0.05-1.60 | 18236 | 229464 | 0.9982 | 0.0169 | 0.0277 | 7 | 22 |
| Time program | ||||||||
| ROS | 0.02-0.64 | 33408 | 428502 | 0.9992 | 0.0050 | 0.0074 | 3 | 9 |
| ALP | 0.05-1.60 | 45080 | 168733 | 0.9986 | 0.0471 | 0.0668 | 6 | 19 |
| DCL | 0.02-0.64 | 27893 | 553467 | 0.9984 | 0.0115 | 0.0180 | 1 | 3 |
| Serum | ||||||||
| ROS | - | 34415 | 393904 | 0.9999 | 0.0053 | 0.0054 | 3 | 9 |
| ALP | - | 24418 | 83294 | 0.9999 | 0.0282 | 0.0311 | 13 | 40 |
| DCL | - | 17455 | 227581 | 0.9999 | 0.0110 | 0.0125 | 10 | 29 |
a=standard error estimate, b= standard error
Table 2: Regression characteristics and sensitivity of the method.
Accuracy
Percent recovery values of ROS, ALP and DCL in X-plended®, Nerum® and Phlogin® capsule at six concentration levels and in human serum at three concentration levels were calculated. These were found to be in the range of 98.21-101.91% for pharmaceutical formulations and human serum. Table 3 illustrates the good percent recovery of studied drugs.
| Conc | %Rec | Conc | %Rec | Conc | %Rec | Conc | %Rec | Conc | %Rec | Conc | %Rec |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isobestic point | Individual λmax | ||||||||||
| ROS | ALP | DCL | ROS | ALP | DCL | ||||||
| Pharmaceutical formulations | |||||||||||
| 0.02 | 99.14 | 0.125 | 100.76 | 0.05 | 99.44 | 0.02 | 101.87 | 0.05 | 100.20 | 0.02 | 98.32 |
| 0.04 | 99.68 | 0.25 | 99.22 | 0.10 | 100.08 | 0.04 | 100.26 | 0.10 | 98.67 | 0.04 | 99.10 |
| 0.08 | 100.76 | 0.50 | 100.45 | 0.20 | 100.83 | 0.08 | 99.93 | 0.20 | 99.49 | 0.08 | 99.19 |
| 0.16 | 100.56 | 1.00 | 100.22 | 0.40 | 101.91 | 0.16 | 100.27 | 0.40 | 99.58 | 0.16 | 100.62 |
| 0.32 | 99.82 | 2.00 | 99.69 | 0.80 | 98.24 | 0.32 | 99.32 | 0.80 | 98.69 | 0.32 | 99.92 |
| 0.64 | 99.76 | 4.00 | 98.21 | 1.60 | 100.77 | 0.64 | 99.83 | 1.60 | 99.82 | 0.64 | 99.85 |
| Serum | |||||||||||
| 0.02 | 98.59 | 0.125 | 100.09 | 0.05 | 99.78 | 0.02 | 98.59 | 0.05 | 98.70 | 0.02 | 100.93 |
| 0.2 | 100.01 | 1.0 | 100.87 | 0.4 | 101.77 | 0.16 | 99.59 | 0.40 | 99.67 | 0.16 | 99.91 |
| 0.6 | 100.61 | 4.0 | 99.58 | 1.6 | 99.90 | 0.64 | 100.18 | 1.60 | 99.95 | 0.64 | 99.81 |
Table 3: Recovery pharmaceutical formulations and in serum.
Precision
The precision of the method was examined with respect to repeatability and intermediate precision in terms of percent RSD. It was found to be 0.13-2.07% for inter-day and 0.16-1.87% for intra-day precision of the method. The %RSD values of each analyte in API and in serum are tabulated in (table 4).
| ROS | ALP | DCL | ||||||
|---|---|---|---|---|---|---|---|---|
| Conc | Inter-day %RSD | Intra-day %RSD | Conc | Inter-day %RSD | Intra-day %RSD | Conc | Inter-day %RSD | Intra-day %RSD |
| Isobestic point | ||||||||
| 0.02 | 2.01 | 0.70 | 0.125 | 1.16 | 0.16 | 0.05 | 1.86 | 1.05 |
| 0.04 | 1.50 | 1.88 | 0.25 | 2.07 | 1.37 | 0.10 | 0.60 | 0.45 |
| 0.08 | 1.29 | 0.39 | 0.5 | 0.71 | 0.70 | 0.20 | 0.97 | 0.51 |
| 0.16 | 0.29 | 0.39 | 1.0 | 1.37 | 1.77 | 0.40 | 1.61 | 1.08 |
| 0.32 | 0.37 | 0.65 | 2.0 | 0.53 | 0.69 | 0.80 | 1.28 | 1.87 |
| 0.64 | 0.20 | 1.01 | 4.0 | 1.15 | 1.31 | 1.60 | 1.01 | 0.89 |
| Time program | ||||||||
| 0.02 | 1.55 | 0.84 | 0.05 | 0.82 | 0.73 | 0.02 | 0.54 | 0.78 |
| 0.04 | 0.37 | 0.80 | 0.10 | 0.35 | 1.26 | 0.04 | 1.02 | 1.28 |
| 0.08 | 0.86 | 1.07 | 0.20 | 0.19 | 0.79 | 0.08 | 0.21 | 0.85 |
| 0.16 | 0.79 | 0.58 | 0.40 | 0.23 | 1.22 | 0.16 | 0.51 | 0.94 |
| 0.32 | 0.69 | 0.68 | 0.80 | 0.62 | 0.95 | 0.32 | 2.08 | 1.11 |
| 0.64 | 0.37 | 0.91 | 1.60 | 0.78 | 0.65 | 0.64 | 1.12 | 0.82 |
| Serum | ||||||||
| 0.02 | 0.62 | 0.82 | 0.125 | 0.13 | 0.13 | 0.05 | 1.80 | 0.56 |
| 0.2 | 0.45 | 1.07 | 1 | 1.22 | 0.82 | 0.4 | 0.32 | 1.83 |
| 0.6 | 1.79 | 0.30 | 4 | 1.20 | 0.47 | 1.6 | 0.93 | 1.16 |
Table 4: Precision of the proposed method.
Detection and quantitation limits
Limit of detection is the concentration of analyte that can be determined by a statistical approach but not necessarily quantitated as an exact value, where as limit of quantitation is the concentration at which the analytical results can be reported with high degree of confidence [27]. These are the concentration of samples that give peak height three times or ten times to the baseline noise and were calculated to be 4.0, 17.0, 7.0 and 12, 52, 22 ng.mL-1 in reference standard and 3.0, 13, 10 and 9.0, 40, 29 ng.mL-1 in human serum for ROS, ALP and DCL respectively (table 2).
Robustness
The mobile phase composition was intentionally altered along with variable pH and subsequent effect in chromatograms was observed. Theoretical plates were in between 2000-8000 and tailing factor was found to be less than 2. The data presented in table 5 signify that there is no much change in theoretical plates and tailing factor, which confirms the robustness of proposed method.
| Parameters | N | T | Res | N | T | Res | N | T | Res | |
|---|---|---|---|---|---|---|---|---|---|---|
| ROS | ALP | DCL | ||||||||
| pH | 3.3 | 3395 | 1.54 | 0.14 | 3886 | 1.51 | 1.96 | 4625 | 1.45 | 1.10 |
| 3.4 | 3369 | 1.51 | 0.24 | 3857 | 1.47 | 1.96 | 4574 | 1.43 | 1.12 | |
| 3.5 | 3234 | - | 0.12 | 3579 | 1.61 | 1.90 | 4403 | 1.37 | 1.09 | |
| Mobile phase (MeOH:H2O) | 78:22 | 2833 | - | 0.39 | 3035 | - | 1.08 | 3678 | 1.21 | - |
| 80:20 | 2630 | - | 0.36 | 2845 | - | 1.04 | 3321 | 1.23 | 1.34 | |
| 82:18 | 2536 | - | 0.48 | 2681 | 0.97 | 1.00 | 3026 | 1.18 | 1.90 | |
| Flow rate (mL min-1) | 0.9 | 3049 | 1.44 | 0.73 | 3387 | 1.37 | 1.14 | 4029 | 1.21 | 1.09 |
| 1.0 | 2761 | - | 0.13 | 3143 | - | 1.03 | 3795 | 1.22 | 1.87 | |
| 1.1 | 2614 | 1.33 | 0.69 | 2779 | - | 1.02 | 3160 | 1.11 | 1.58 | |
N=Theoretical plates, T=Tailing factor, Res=Resolution
Table 5: Robustness.
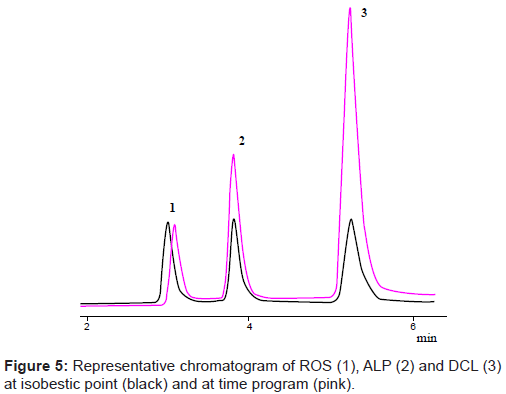
Programming the detector
UV-spectra of ROS, ALP and DCL showed λmax of 244, 222 and 284 nm respectively. When the detector was programmed for studied drugs at their specific wavelengths for the time period of their complete elution, it was observed that the sensitivity of the method becomes high with better separation and good resolution of components. The chromatogram was analyzed for the simultaneous determination of all the studied drugs in API, pharmaceutical formulations and human serum by programming the detector (Figure 5).
The linearity was determined to be 0.02-0.64, 0.05-1.60 and 0.02-0.64 μg.mL-1 with correlation coefficient greater than 0.998. The regression data calculated from calibration curves constructed between peak area and concentration of each analyte including slope, intercept, correlation coefficient, standard error and standard error estimate are given in table 2. The inter-day and intra-day precision was in the range of 0.19-2.08% and percent recovery values were in between 98.32-101.87% confirming that the method is accurate and precise. The detection and quantitation limits shoot down by enhancing the sensitivity of method. The LOD and LOQ values were found to be 3.0, 6.0, 1.0 and 9.0, 19.0 and 3.0 ng.mL-1 respectively.
Application of proposed method
The developed and validated LC method for the simultaneous determination of ROS, ALP and DCL was found to be accurate and precise through statistical results and proved to be reliable and robust and showed non significant results on deliberate changes in chromatographic parameters. The applicability of the proposed method in pharmaceutical formulations and in human serum showed good percent recoveries and relative standard deviation (tables 3 and 4) confirming that method is applicable for routine analysis. Moreover, no interfering species were detected or found to interrupt at the same retention time where the analyte is eluted. It is there for a reliable method for the simultaneous determination of studied drugs in pharmaceutical formulation and in human serum with good % recovery and without interference of excipients or endogenous components of serum.
Conclusion
A rapid, sensitive and least time consuming liquid chromatographic method for the simultaneous determination of ROS, ALP and DCL has been developed and validated for specificity, linearity, accuracy, precision, detection and quantitation limits in bulk drug, human serum and pharmaceutical formulation at isobestic point of 240 nm and by programming the detector at 244, 222 and 284 nm respectively. Results are accurate and precise and are confirmed by the statistical parameters. There was no interference of excipients in pharmaceutical formulation analyses, or endogenous components of serum, thus no additional extraction or separation procedures were required. The advantages which encourage the developed method for routine analyses is the use of very small quantity of sample required for analyses by programming the detector, high sensitivity and less retention time.
Acknowledgements
Mrs Saeeda Nadir Ali (PhD scholar) is deeply indebted to the Higher Education Commission, Pakistan for providing the indigenous scholarship for this research work.
References
- Sassano A, Platanias LC (2008) Statins in tumor suppression. Cancer Lett 260: 11-19.
- Mach F (2002) Statins as immunomodulators. Transpl Immunol 9: 197-200.
- Wong RP, Davis TM (2009) Statins as potential antimalarial drugs: low relative potency and lack of synergy with conventional antimalarial drugs. Antimicrob Agents Chemother 53: 2212-2214.
- Schupp N, Schmid U, Heidland A, Stopper H (2008) Rosuvastatin protects against oxidative stress and DNA damage in vitro via upregulation of glutathione synthesis. Atherosclerosis 199: 278-287.
- Garrett IR, Gutierrez G, Mundy GR (2001) Statins and bone formation. Curr Pharm Des 7: 715-736.
- Small RE (1989) Diclofenac sodium. Clin Pharm 8: 545-558.
- Gostick N, James IG, Khong TK, Roy P, Shepherd PR, et al. (1990) Controlled-release indomethacin and sustained-release diclofenac sodium in the treatment of osteoarthritis: A comparative controlled clinical trial in general practice. Curr Med Res Opin 12: 135-142.
- Trivedi RK, Kallem RR, Mullangi R, Srinivas NR (2005) Simultaneous determination of rosuvastatin and fenofibric acid in human plasma by LC-MS/MS with electrospray ionization: assay development, validation and application to a clinical study. J Pharm Biomed Anal 39: 661-669.
- Gomes FP, Garcia PL, Alves JMP, Singh AK, Kedor-Hackmann ERM, Santoro MIRM (2009) Development and Validation of Stability-Indicating HPLC Methods for Quantitative Determination of Pravastatin, Fluvastatin, Atorvastatin, and Rosuvastatin in Pharmaceuticals. Anal Lett 42: 1784-1804.
- Nasir F, Iqbal Z, Khan A, Ahmad L, Shah Y, et al. (2011) Simultaneous determination of timolol maleate, rosuvastatin calcium and diclofenac sodium in pharmaceuticals and physiological fluids using HPLC-UV. J Chromatogr B Analyt Technol Biomed Life Sci 879: 3434-3443.
- Kumar TR, Shitut NR, Kumar PK, Vinu MC, Kumar VV, et al. (2006) Determination of rosuvastatin in rat plasma by HPLC: validation and its application to pharmacokinetic studies. Biomed Chromatogr 20: 881-887.
- Vittal S, Shitut NR, Kumar TR, Vinu MC, Mullangi R, et al. (2006) Simultaneous quantitation of rosuvastatin and gemfibrozil in human plasma by high-performance liquid chromatography and its application to a pharmacokinetic study. Biomed Chromatogr 20: 1252-1259.
- Patel RB, Patel MR, Shankar MB, Bhatt KK (2009) Development and validation of second-derivative spectrophotometry method for simultaneous estimation of Alprazolam and Fluoxetine hydrochloride in pure powder and tablet formulation and its comparison with HPLC method. Eurasian J Anal Chem 4: 76-86.
- Patel RB, Patel AB, Patel MR, Shankar MB, Bhatt KK (2009) Estimation of alprazolam and sertraline in pure powder and tablet formulations by high-performance liquid chromatography and high-performance thin-layer chromatography. Anal Lett 42: 1588-1602.
- Rani GT, Shankar DG, Kadgapathi P, Satyanarayana B (2011) A Validated RP HPLC Method for Simultaneous Determination of Propranolol hydrochloride and Alprazolam in Bulk and in Pharmaceutical formulations. J Pharm Res 4: 358-360.
- Vardini MT, Mashayekhi HA, Tehrani MS (2012) Dispersive liquid-liquid microextraction followed by high-performance liquid chromatography as an efficient and sensitive technique for the simultaneous determination of alprazolam, oxazepam, and diazepam in human urine samples. J Liq Chromatogr Relat Technol 35: 988-999.
- Kasperek R (2008) Determination of diclofenac sodium and papaverine hydrochloride in tablets by HPLC method. Acta Pol Pharm 65: 403-408.
- Abdel-Hamid ME, Novotny L, Hamza H (2001) Determination of diclofenac sodium, flufenamic acid, indomethacin and ketoprofen by LC-APCI-MS. J Pharm Biomed Anal 24: 587-594.
- Sultana N, Arayne MS, Iftikhar B (2008) Simultaneous determination of atenolol, rosuvastatin, spironolactone, glibenclamide and naproxen sodium in pharmaceutical formulations and human plasma by RP-HPLC. J Chin Chem Soc 55: 1022-1029.
- Arayne MS, Sultana N, Mirza AZ, Shamshad H (2010) High performance liquid chromatographic analysis of pioglitazone, gliquidone, rosuvastatin and simvastatin in formulations and human serum. Chin J Chem 28: 1998-2002.
- Sultana N, Arayne MS Naz S, Shafi N, Naveed S (2010) Simultaneous Determination of Prazosin, Atorvastatin, Rosuvastatin and Simvastatin in API, Dosage Formulations and Human Serum by RP-HPLC. J Chin Chem Soc 57: 1286-1292.
- Sultana N, Arayne MS, Naveed S (2011) Validated Method for the Simultaneous Determination of Lisinopril, Pravastatin, Atorvastatin and Rosuvastatin in API, Formulations and Human Serum by RP-HPLC. Chin J Chem 29: 1216-1220.
- Arayne MS, Sultana N, Mirza AZ, Siddiqui FA (2010) Simultaneous determination of gliquidone, fexofenadine, buclizine, and levocetirizine in dosage formulation and human serum by RP-HPLC. J Chromatogr Sci 48: 382-385.
- Arayne MS, Sultana N, Zuberi H, Haroon U (2010) In vitro studies of Interaction between Metformin and NSAIDS (Non Steroidal Anti-Inflamatory Drugs) using Spectrophotometry and RP-High Performance Liquid Chromatography. J Chil Chem Soc 55: 206-211.
- Sultana N, Arayne MS, Waheed A (2011) Method Development of Verapamil in Presence of NSAIDs using RP-HPLC Technique. Bull Korean Chem Soc 32: 2274-2278.
- International Conference on Hormonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (2005) Validation of Analytical Procedures: Text and Methodology Q2(R1). Complementary Guideline on Methodology incorporated in November 2005, London.
- Armbruster DA, Tillman MD, Hubbs LM (1994) Limit of detection (LQD)/limit of quantitation (LOQ): comparison of the empirical and the statistical methods exemplified with GC-MS assays of abused drugs. Clin Chem 40: 1233-1238.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15422
- [From(publication date):
December-2012 - Nov 17, 2025] - Breakdown by view type
- HTML page views : 10671
- PDF downloads : 4751