Research Article Open Access
Kappa Opioid Receptor-Mediated Disruption of Novel Object Recognition: Relevance for Psychostimulant Treatment
Jason J. Paris, Kate J. Reilley and Jay P. McLaughlin*Torrey Pines Institute for Molecular Studies, 11350 SW Village Parkway, Port St. Lucie, FL 34987, USA
- *Corresponding Author:
- Jay P. McLaughlin
Torrey Pines Institute for Molecular Studies
11350 SW Village Parkway
Port St. Lucie, FL 34987, USA
Tel: (772) 345-4715
Fax: (772) 345-3649
E-mail: jmclaughlin@tpims.org
Received November 16, 2011; Accepted December 20, 2011; Published December 24, 2011
Citation: Paris JJ, Reilley KJ, McLaughlin JP (2011) Kappa Opioid Receptor- Mediated Disruption of Novel Object Recognition: Relevance for Psychostimulant Treatment. J Addict Res Ther S4:007. doi:10.4172/2155-6105.S4-007
Copyright: © 2011 Paris JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Kappa opioid receptor (KOR) agonists are potentially valuable as therapeutics for the treatment of psychostimulant reward as they suppress dopamine signaling in reward circuitry to repress drug seeking behavior. However, KOR agonists are also associated with sedation and cognitive dysfunction. The extent to which learning and memory disruption or hypolocomotion underlie KOR agonists’ role in counteracting the rewarding effects of psychostimulants is of interest. C57BL/6J mice were pretreated with vehicle (saline, 0.9%), the KOR agonist (trans)-3,4-dichloro-N-methyl-N-[2-(1- pyrrolidinyl)-cyclohexyl] benzeneacetamide (U50,488), or the peripherally-restricted agonist D-Phe-D-Phe-D-lle-D-Arg- NH 2 (ffir-NH 2 ), through central (i.c.v.) or peripheral (i.p.) routes of administration. Locomotor activity was assessed via activity monitoring chambers and rotorod. Cognitive performance was assessed in a novel object recognition task. Prolonged hypolocomotion was observed following administration of 1.0 and 10.0, but not 0.3 mg/kg U50,488. Central, but not peripheral, administration of ffir-NH 2 (a KOR agonist that does not cross the blood-brain barrier) also reduced motor behavior. Systemic pretreatment with the low dose of U50,488 (0.3 mg/kg, i.p.) significantly impaired performance in the novel object recognition task. Likewise, ffir-NH 2 significantly reduced novel object recognition after central (i.c.v.), but not peripheral (i.p.), administration. U50,488- and ffir-NH 2 -mediated deficits in novel object recognition were prevented by pretreatment with KOR antagonists. Cocaine-induced conditioned place preference was subsequently assessed and was reduced by pretreatment with U50,488 (0.3 mg/kg, i.p.). Together, these results suggest that the activation of centrally-located kappa opioid receptors may induce cognitive and mnemonic disruption independent of hypolocomotor effects which may contribute to the KOR-mediated suppression of psychostimulant reward.
Keywords
Cocaine; Conditioned place preference; Locomotion; Memory; Cognition.
Introduction
Kappa opioid receptor (KOR)-specific agonists demonstrate antinociception [1] without the respiratory suppression or addictive liabilities produced by established opioid analgesics such as morphine [2], but they do generate dysphoria and sedation attributed to the activation of KOR in the CNS [3-5]. While these properties of KOR agonists can complicate pain management, they are potentially beneficial as a therapeutic intervention in psychostimulant addiction [5,6]. In rats, acute systemic administration of a KOR agonist attenuated amphetamine-induced psychomotor behavior [7] and prevented behavioral sensitization to cocaine-induced motor behaviors [8]. The central mechanisms that underlie these effects are thought to involve suppression of mesolimbic dopaminergic signaling in the brain [8-10], as KOR agonists prevent basal and Cocaine induced dopamine signaling in the nucleus accumbens of cocainenaïve, or cocaine-exposed, rats [9-14]. Thus, despite the soporific and dysphoric effects of KOR agonists, their therapeutic potential for psychostimulant addiction is of interest.
Animal models dependent on locomotor activity, such as drug self-administration paradigms, have difficulty distinguishing the influence of soporific drug effects (which can be peripherally and/or centrally-mediated) and cognitive dysfunction. These properties raise the suggestion, lesser studied, that KOR agonists may counteract drug reinforcement through additional, behavioral means [15,16] such as mnemonic disruption. Indeed, KOR agonists impair cognition and memory performance in a number of animal models, suggesting that KOR activation may be important in the disruption of Hippocampus sand/ or amygdala-mediated tasks involved with the acquisition and consolidation of mnemonic information [17-21]. Consistent with this, polymorphisms in alleles encoding for genes related to dynorphin function are associated with variations in mnemonic performance in an aged patient sample [22].
We have previously observed that systemic administration of the KOR agonist, (trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)- cyclohexyl] benzeneacetamide (U50,488), to C57BL/6J mice can reduce novel object recognition [21], a cognitive behavioral assay that is frontal-cortex- and hippocampus-dependent [23,24]. However, whether KOR agonists can attenuate learning and memory performance and psychostimulant-seeking behavior at doses independent of soporific effects remains to be determined. Accordingly, we conducted four experiments with C57BL/6J mice. First, we examined the locomotor effects of the KOR agonist U50, 488 and a highly selective peptidergic KOR agonist, D-Phe-D-Phe-D-lle- D-Arg-NH2 (ffir-NH2) [25] which is not thought to cross the Blood brain barrier (BBB) [26], to determine dosing that did not produce soporific effects. Second, we hypothesized that the administration of KOR agonists with central activity (ffir-NH2 administered i.c.v, or U50, 488 regardless of route of administration) would attenuate novel object recognition in mice. Third, we confirmed the role of central nervous system (CNS) KOR activation in the suppression of learning and memory performance by pretreating animals with the nonselective opioid receptor antagonist, naloxone methiodide (NXM), which cannot readily cross the BBB, or the selective KOR antagonist, nor-binaltorphimine (nor-BNI). Finally, hypothesizing that KORAbstract mediated inhibition of learning and memory processes would also impair the perception of drug reward, we anticipated that systemic administration of U50, 488 at a dose that did not attenuate locomotor behavior, but did attenuate cognitive performance, would inhibit cocaine-induced conditioned place preference (CPP).
Materials and Methods
Animals and housing
Subjects were 221 adult (approximately 70 days of age), male C57BL/6J mice purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were maintained in a temperature- and humidity- controlled room at the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL) vivarium on a 12:12 h light / dark cycle (lights off at 19:00 h) with ad libitum access to food and water.
All mice used in this study were housed and cared for in accordance with the 2002 National Institutes of Health Guide for the Care and Use of Laboratory Animals. All methods were pre-approved by the Institutional Animal Care and Use Committee at the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL) and were conducted in accordance with ethical guidelines defined by the National Institutes of Health (NIH Publication No. 85-23).
Intracerebroventricular administration technique
Intracerebroventricular injections were made directly into the lateral ventricle according to the modified method of Haley and McCormick [27]. The volume of all i.c.v. injections was 5 μL, using a10 μl Hamilton microliter syringe. Mice were lightly anesthetized with isoflurane, an incision was made in the scalp, and the injection was made 2 mm lateral and 2 mm caudal to bregma at a depth of 3 mm.
Chemicals
Cocaine, naloxone methiodide (NXM), nor-binaltorphimine (nor-BNI), and (trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)- cyclohexyl] benzeneacetamide (U50,488) were obtained from Sigma- Aldrich (St. Louis, MO). As it is not thought to cross the BBB [26], the highly-selective KOR agonist, ffir-NH2 [25] was obtained from the Torrey Pines Institute for Molecular Studies (Port Saint Lucie, FL) to assess the sufficiency of central KOR activation. This agonist was chosen from given that an analog, D-Phe-D-Phe-D-NIe-D-Arg-NH2 (ff(nle)r), is presently in clinical trial testing and its KOR selectivity exceeds that of other KOR agonists presently in clinical trials [28-31]. Moreover, recent findings demonstrate ffir-NH2 as a nor-BNI-sensitive KOR agonist that is efficacious in animal models of visceral pain [32].
Experimental schedule and drug dosing
In Experiment 1, following habituation for assessment of locomotor behavior (described in detail below), mice (n = 8 / group) were administered saline (0.1 ml / 10 g body weight), U50,488 (0.3, 1.0 or 10.0 mg / kg, i.p.), ic.v. ffir-NH2 (3 or 10 nmol), or i.p. ffir-NH2 (1 or 10 mg / kg, i.p.) and immediately assessed for ambulation in the CLAMS apparatus. Another cohort of mice (n = 6 / group) were habituated to the rotorod task, administered saline (0.1 ml / 10 g body weight), U50, 488 (0.3 or 1.0 mg / kg, i.p.), or ffir-NH2 (3 nmol i.c.v. or 1 mg / kg i.p.), and immediately assessed for rotorod performance.
In Experiment 2, prior to Phase I of the novel object recognition task, mice were administered (5 μl infusate volume) saline or the non-specific opioid receptor antagonist NXM (30 nmol i.c.v.) [33,34]. Fifteen min later, mice were administered (i.p.) saline (0.1 ml / 10 g body weight) or U50,488 (0.3 mg / kg). After a 15 min incubation, mice were assessed in the novel object recognition task (described below), yielding 4 groups (n = 12 / group): (1) saline (i.c.v.) / saline (i.p.), (2) saline (i.c.v.) / U50,488 (0.3 mg / kg, i.p.), (3) NXM (30 nmol, i.c.v.) / saline (i.p.), and (4) NXM (30 nmol, i.c.v.) / U50,488 (0.3 mg / kg, i.p.).
In Experiment 3, prior to Phase I of the novel object recognition task, mice were administered i.c.v. saline or ffir-NH2 (3 nmol). Five min later, mice were administered an i.p. injection of saline (0.1 ml / 10 g body weight) or ffir-NH2 (1 mg / kg). After 5 min incubation, mice were assessed in the novel object recognition task as described below. As a negative control, a group of mice were administered the KOR antagonist nor-BNI (5 mg / kg, i.p.) 20 h prior to behavioral assessment. (Note that i.p. administration of a KOR antagonist that crosses the BBB minimized confounds that would arise from multiple i.c.v. infusions in this control group). Thus, this experiment consisted of 4 groups (n = 12 / group): (1) saline (i.c.v.) / saline (i.p.), (2) saline (i.c.v.) / ffir-NH2 (1 mg / kg, i.p.), (3) ffir-NH2 (3 nmol, i.c.v.) / saline (i.p.), and ffir-NH2 (3 nmol, i.c.v.) / nor-BNI (5 mg/kg). We have previously demonstrated that nor-BNI does not have cognition- enhancing effects on its own [21].
In Experiment 4, mice were assessed for conditioned place preference (described in detail below) following subcutaneous (s.c.) treatment with cocaine (10 mg / kg) with (n = 15) or without (n = 24) a 15-min pretreatment of U50,488 (0.3 mg / kg, i.p) or saline with a 15-min pretreatment of U50,488 (0.3 mg / kg, i.p.; n = 24).
Behavioral assays
Locomotor activity: In Experiment 1, locomotion was recorded using the automated, computer-controlled Comprehensive Lab Animal Monitoring System (CLAMS) apparatus (Columbus Instruments, Columbus, OH) [35]. The CLAMS apparatus consists of a cage (23.5 × 11.5 × 13 cm) that is equipped with 32 photocells to detect horizontal and vertical ambulation of mice. Briefly, mice were placed in a closed apparatus and allowed to habituate for 30 min. Following habituation, mice were administered saline, U50, 488 or ffir-NH2 and returned to the apparatus. Five minutes later, ambulatory behavior was recorded for a 30 min period.
While CLAMS assesses ambulatory behavior, rotorod performance was used as an additional measure to assess sedative/ hypolocomotor effects of drugs, as modified from previous protocols [36]. The rotorod (San Diego Instruments, San Diego, CA) is 3 cm in diameter and suspended approximately 46 cm high. Once mice could maintain 30 s standing on the immobile rotorod, they were habituated to the task via three fixed speed trials (30 s max. latency at 10 rpm), two fixed speed trials (180 s max. latency at 10 rpm), and two accelerated speed trials (180 s max. latency at 0-20 rpm), and the latency to fall from the rotorod was measured. The last of these habituation trials was utilized as a baseline measure of rotorod performance. Following a one hour interval, mice were administered drug treatment and assessed in accelerated speed trials (180 s max. latency at 0-20 rpm) over a 30 min period. Increased latencies to fall indicate increased motor performance. Data are expressed as the percent change from baseline performance based on prior methods [37].
Novel object recognition: In Experiments 2 and 3, mice were assessed in the novel object recognition task, a cognitive behavioral assay that is frontal-cortex- and hippocampus-dependent [23,24], as previously described [21]. In brief, the testing paradigm consisted of 3 phases (two 10 min acquisition trials followed by one 10 min retention trial; each separated by a 10 min inter-trial interval). All trials were conducted on the same day and drug treatments occurred prior to testing in Phase I so that the influence of manipulations on associative learning could be assessed. In Phase I, objects for mouse exploration were two small square objects (standard playing dice; each 16 × 16 × 16 mm) centralized at opposite ends of a rectangular cage (16 × 24 × 12 cm). In Phase II, the placement of one object was altered while the other die remained centralized. In Phase III, the target object (previously displaced in Phase II) was replaced with a round object of approximate equal dimension (a marble; 1.25 cm diameter). In between subjects and trials, objects were cleaned with quatricide (60 ml / gallon) in order to minimize carryover of olfactory stimuli. During each phase, the amount of time mice spent exploring the constant object and the target object was recorded. For each phase, a recognition index was derived wherein the percentage of time that the subject spent investigating the target object was calculated as a function of the total amount of time that the subject spent investigating: [(time spent investigating target object / time spent investigating constant object + time spent investigating target object) × 100]. An increased percentage of time spent with the novel object in Phase III is considered an index of enhanced learning and memory performance [21].
Cocaine-conditioned place preference: In Experiment 4, C57BL/6J mice were conditioned based on an established cocaine CPP paradigm [38,39]. A counterbalanced design was used in this study. Briefly, the amount of time subjects spent in each of three compartments (two cue-differentiated outer compartments: 25 × 25 × 25 cm, separated by a middle compartment: 8.5 × 25 × 25 cm, outfitted with infrared beams to track animal progress; San Diego Instruments, San Diego, CA) was measured over a 30 min testing period. Prior to place conditioning, animals did not significantly differ in their preference to explore the left (521 ± 18 s) versus right (579 ± 24 s) compartments (p = 0.11; Student’s t-test). Each day on the next two days, mice were administered vehicle (0.9% saline) and consistently confined in the pre-determined outer compartment; half of each group in the right chamber, half of each group in the left chamber. Four hours later, mice were administered cocaine, or cocaine preceded by U50,488 (0.3 mg / kg, i.p.), and confined to the opposite compartment for 30 min. Cocaine (10 mg / kg, s,c,, dissolved in sterile 0.9 % saline) was used for place conditioning as it was previously demonstrated to produce reliable CPP in C57BL/6J mice [40,41]. Data are expressed as the difference in time (s) spent on the treatment-, vs. vehicle-, associated chamber. Thus, positive values indicate a greater preference for treatment compared to vehicle, whereas negative values indicate an aversion to the treatment compared to vehicle.
Statistical analysis
Locomotor (CLAMS or rotorod) and novel object recognition data from Experiments 1-3 were analyzed via repeated measures analysis of variance (ANOVA) with drug treatment condition as a Between groups factor, and time (Expt. 1) or trial phase (Expts. 2 and 3) as within-groups factors. For all repeated measures ANOVAs, simple main effects and simple main effect contrasts are presented following significant interactions. Conditioned place preference data obtained in Experiment 4 were analyzed via one-way ANOVA with the difference in time spent on the treatment-, vs. vehicle-, associated side as the dependent measure and conditioning status as the Between groups factor. Where appropriate, Tukey’s Honestly Significant Difference post-hoc tests were used to assess group differences. Effects were considered significant when p < 0.05. All effects are expressed as mean ± SEM.
Results
Experiment 1- Locomotor activity is dose-dependently suppressed by activation of central kappa opioid receptors
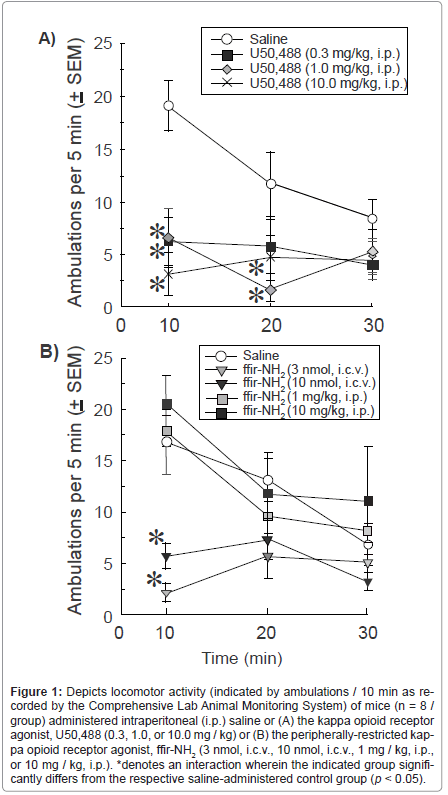
Systemic administration of saline or U50,488 (0.3, 1.0, or 10.0 mg / kg, i.p.) influenced the ambulation of mice in the CLAMS apparatus. U50,488 dosing and time-course following injection interacted to significantly influence motor behavior [F(6,56) = 2.36, p < 0.05]. Initially (within 10 min post administration), all doses of U50,488 significantly [F(3,28) = 8.75, p < 0.05] reduced locomotor behavior compared to vehicle (pU50,488 (1.0 mg/kg) = .0007; pU50,488 (1.0 mg/kg) = .0009; pU50,488 (1.0 mg/kg) < .0001; Figure 1A). However, after 10 min, only 1.0 (p = 0.005) and 10.0 (p = 0.04), but not 0.3 mg/kg U50,488 significantly differed from vehicle administration [F(3,28) = 3.25, p < 0.05] (Figure 1A). At 30 min post injection, significant group differences were no longer observed among U50,488 (0.3, 1.0, or 10.0 mg / kg)- or Vehicle treated mice (Figure 1A).
Figure 1:Depicts locomotor activity (indicated by ambulations / 10 min as recorded by the Comprehensive Lab Animal Monitoring System) of mice (n = 8 / group) administered intraperitoneal (i.p.) saline or (A) the kappa opioid receptor agonist, U50,488 (0.3, 1.0, or 10.0 mg / kg) or (B) the peripherally-restricted kappa opioid receptor agonist, ffir-NH2 (3 nmol, i.c.v., 10 nmol, i.c.v., 1 mg / kg, i.p., or 10 mg / kg, i.p.). *denotes an interaction wherein the indicated group significantly differs from the respective saline-administered control group (p < 0.05).
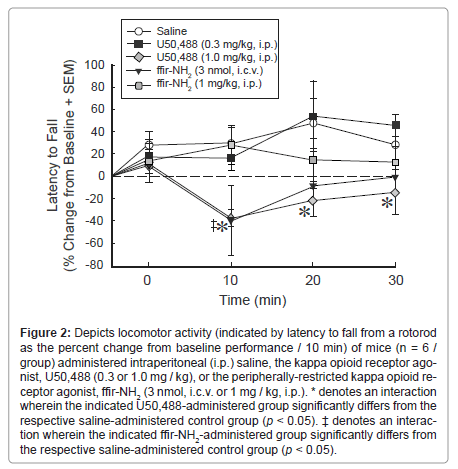
Figure 2:Depicts locomotor activity (indicated by latency to fall from a rotorod as the percent change from baseline performance / 10 min) of mice (n = 6 / group) administered intraperitoneal (i.p.) saline, the kappa opioid receptor agonist, U50,488 (0.3 or 1.0 mg / kg), or the peripherally-restricted kappa opioid receptor agonist, ffir-NH2 (3 nmol, i.c.v. or 1 mg / kg, i.p.). * denotes an interaction wherein the indicated U50,488-administered group significantly differs from the respective saline-administered control group (p < 0.05). ΓΆΒ?Β΅ denotes an interaction wherein the indicated ffir-NH2-administered group significantly differs from the respective saline-administered control group (p < 0.05).
The duration and locomotor effects of ffir-NH2 following central or peripheral administration were also assessed. Route of administration and time-course following injection significantly interacted to influence ambulation in the CLAMS apparatus [F(8,70) = 4.03, p < 0.05]. Central administration of ffir-NH2 (3 nmol, i.c.v.; p < 0.01 or 10 nmol, i.c.v.; p < 0.05), but not administration of vehicle or peripheral ffir-NH2 (1 mg / kg, i.p. or 10 mg / kg, i.p.), significantly reduced locomotor behavior initially [F(4,35) = 14.69, p < 0.05] (Figure 1B). However, after 10 min, significant differences were not observed between vehicle or either route of ffir-NH2 administration (Figure 1B).
Rotorod performance was also influenced by U50,488 or ffir- NH2 administration. Dose/route of administration and time-course following injection significantly interacted to influence the latency to fall from the rotorod, relative to baseline performance [F(12,75) = 2.18, p < 0.05]. Compared to saline administration, U50,488 (1.0 mg / kg, i.p.) significantly reduced the relative latency for mice to fall from the rotorod 10 (p = 0.01). 20 (p = 0.03), and 30 (p = 0.05) min following administration (Figure 2). As well, ffir-NH2 (3 nmol, i.c.v.) significantly reduced the relative latency for mice to fall 10 min (p = 0.02) following administration (but not at later timepoints; Figure 2). Systemic (i.p.) administration of neither U50,488 (0.3 mg / kg), nor ffir-NH2 (1 mg / kg) significantly altered the relative latency for mice to fall from the rotorod (Figure 2).
Experiment 2- Agonist-induced activation of central kappa opioid receptors disrupts novel object recognition
| Experiment 2 | Constant Object | Novel Object |
|---|---|---|
| Saline (i.c.v.) / Saline (i.p.) | 11 ± 2 | 18 ± 3 |
| Saline (i.c.v.) / U50,488 (0.3 mg/kg, i.p.) | 16 ± 3 | 14 ± 2 |
| NXM (30 nmol, i.c.v.) / Saline (i.p.) | 8 ± 1† | 16 ± 2 |
| NXM (30 nmol, i.c.v.) /
U50,488 (0.3 mg/kg, i.p.) |
7 ± 1† | 16 ± 3 |
| Experiment 3 | Constant Object | Novel Object |
| Saline (i.c.v.) / Saline (i.p.) | 10 ± 2 | 22 ± 3 |
| Saline (i.c.v.) / ffir-NH2 (1 mg/kg, i.p.) | 12 + 3 | 22 ± 4 |
| ffir-NH2 (3 nmol, i.c.v.) / Saline (i.p.) | 8 ± 1 | 6 ± 1‡ |
| ffir-NH2 (3 nmol, i.c.v.) /
nor-BNI* (5 mg/kg, i.p.) |
11 ± 2 | 19 ± 3 |
*nor-BNI = nor-binaltorphimine
Table 1: Presents the amount of time (sec ± SEM) that mice (n = 12 / group) spent investigating the constant and novel objects in the novel object recognition task. † indicates a main effect wherein naloxone methiodide (NXM)-infused mice significantly differed from saline-infused mice on exploration of the constant object, irrespective of peripheral injection condition (p < 0.05). ‡ indicates a significant interaction wherein mice infused with ffir-NH2 significantly differed from salineinfused controls on exploration of the novel, but not constant, object (p < 0.05).
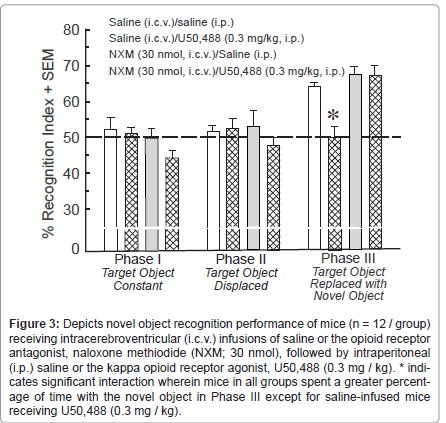
Systemic administration of U50,488 (0.3 mg / kg, i.p.), suppressed novel object recognition. A significant three-way interaction between testing phase, central infusate, and peripheral injection was observed [F(2,88) = 5.45, p < 0.05]. Simple main effects revealed that mice spent a significantly greater percentage of time investigating the novel object in Phase III compared to familiar or displaced objects in Phase I (p < 0.0001) or Phase II (p < 0.0001), respectively (Figure 3). However, within Phase III, there was a simple main effect [F(3,44) = 12.368, p < 0.05] for saline-infused mice that were administered systemic U50,488 to spend a significantly reduced percentage of time investigating the novel object compared to saline administered/infused controls (p < 0.0001; Figure 2). No other group significantly differed from saline administered/infused controls in any phase of testing. Pretreatment with NXM (30 nmol, i.c.v.) prevented U50,488-induced suppression of novel object recognition in Phase III (p < 0.0001; Figure 3). Notably, the amount of time mice spent investigating the constant object was lower among NXM-infused mice compared to saline-infused mice, irrespective of U50,488 administration [F(1,44) = 8.86, p < 0.05] (Table 1, top). No significant differences in investigation of the novel object were observed (Table 1, top).
Figure 3:Depicts novel object recognition performance of mice (n = 12 / group) receiving intracerebroventricular (i.c.v.) infusions of saline or the opioid receptor antagonist, naloxone methiodide (NXM; 30 nmol), followed by intraperitoneal (i.p.) saline or the kappa opioid receptor agonist, U50,488 (0.3 mg / kg). * indicates significant interaction wherein mice in all groups spent a greater percentage of time with the novel object in Phase III except for saline-infused mice receiving U50,488 (0.3 mg / kg).
| Experiment 2 | Constant Object | Novel Object |
|---|---|---|
| Saline (i.c.v.) / Saline (i.p.) | 11 ± 2 | 18 ± 3 |
| Saline (i.c.v.) / U50,488 (0.3 mg/kg, i.p.) | 16 ± 3 | 14 ± 2 |
| NXM (30 nmol, i.c.v.) / Saline (i.p.) | 8 ± 1† | 16 ± 2 |
| NXM (30 nmol, i.c.v.) /
U50,488 (0.3 mg/kg, i.p.) |
7 ± 1† | 16 ± 3 |
| Experiment 3 | Constant Object | Novel Object |
| Saline (i.c.v.) / Saline (i.p.) | 10 ± 2 | 22 ± 3 |
| Saline (i.c.v.) / ffir-NH2 (1 mg/kg, i.p.) | 12 + 3 | 22 ± 4 |
| ffir-NH2 (3 nmol, i.c.v.) / Saline (i.p.) | 8 ± 1 | 6 ± 1‡ |
| ffir-NH2 (3 nmol, i.c.v.) /
nor-BNI* (5 mg/kg, i.p.) |
11 ± 2 | 19 ± 3 |
*nor-BNI = nor-binaltorphimine
Table 1: Presents the amount of time (sec ± SEM) that mice (n = 12 / group) spent investigating the constant and novel objects in the novel object recognition task. † indicates a main effect wherein naloxone methiodide (NXM)-infused mice significantly differed from saline-infused mice on exploration of the constant object, irrespective of peripheral injection condition (p < 0.05). ‡ indicates a significant interaction wherein mice infused with ffir-NH2 significantly differed from salineinfused controls on exploration of the novel, but not constant, object (p < 0.05).
Experiment 3- Central kappa opioid receptors modulate novel object recognition
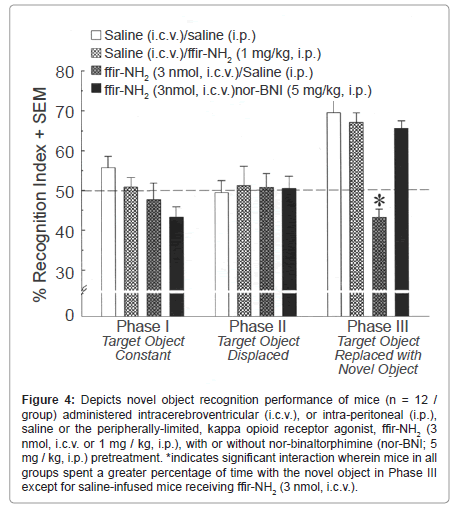
Central, but not peripheral, administration of the highly selective kappa opioid receptor agonist ffir-NH2 suppressed novel object recognition. There was a significant interaction between testing phase and ffir-NH2 condition [F(6,88) = 6.32, p < 0.05]. Simple main effects revealed that mice spent a significantly greater percentage of time [F(2,141) = 13.60, p < 0.05] investigating the novel object in Phase III, compared to either the familiar or displaced objects in Phases I (p < 0.0001) or II (p < 0.0001; Figure 4). However, in Phase III, mice receiving central infusions of ffir-NH2 (3 nmol) combined with systemic saline injections spent a significantly reduced percentage of time investigating the novel object compared to either saline infused/ injected controls (p = 0.0005) or mice receiving saline-infusions combined with an analgesically equipotent systemic administration of ffir-NH2 (1 mg / kg; p = 0.003; Figure 4). The amount of time mice spent investigating the novel (but not constant) object was also lower among ffir-NH2-infused mice compared to saline-infused mice [F(3,44) = 6.15, p < 0.05] (Table 1, bottom). Notably, prior administration of the KOR antagonist, nor-BNI, attenuated these effects (Figure 4).
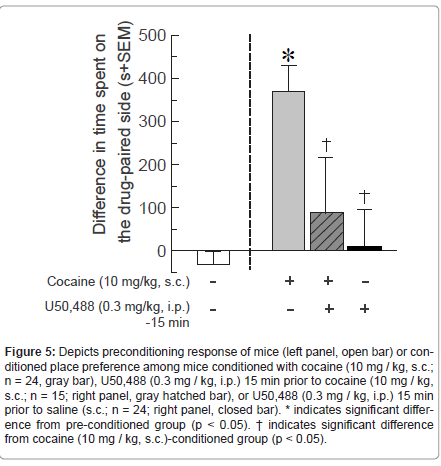
Experiment 4- Kappa opioid receptor agonism that suppresses novel object recognition, without prolonged motor effects, can reduce cocaine-conditioned place preference
Activating KOR via systemic administration of U50,488 (0.3 mg / kg, i.p.) impaired cocaine-induced CPP [F(3,122) = 9.22, p < 0.05] (Figure 5). Mice conditioned with cocaine (10 mg / kg) spent significantly more time on the cocaine-paired side, compared to pre- conditioning responses (p < 0.0001). Those pretreated with U50,488 (0.3 mg / kg) 15 min prior to cocaine (10 mg / kg) administration spent significantly less time on the treatment-associated side than did those treated with cocaine in the absence of U50,488 (p < 0.001), and did not significantly differ from pre-conditioning responses (Figure 5). Notably, treatment with this dose of U50,488 (0.3 mg / kg) in the absence of cocaine did not result in significant place preference or aversion compared to the pre-conditioning response (Figure 5).Comparison of total beam breaks in CPP boxes did not reveal statistically-significant difference between activity of mice administered cocaine without (922 ± 348), or with (640 ± 302), U50,488 on-board [t(37) = 0.56, p > 0.05, n.s.].
Figure 4:Depicts novel object recognition performance of mice (n = 12 / group) administered intracerebroventricular (i.c.v.), or intra-peritoneal (i.p.), saline or the peripherally-limited, kappa opioid receptor agonist, ffir-NH2 (3 nmol, i.c.v. or 1 mg / kg, i.p.), with or without nor-binaltorphimine (nor-BNI; 5 mg / kg, i.p.) pretreatment. *indicates significant interaction wherein mice in all groups spent a greater percentage of time with the novel object in Phase III except for saline-infused mice receiving ffir-NH2 (3 nmol, i.c.v.).
Figure 5:Depicts preconditioning response of mice (left panel, open bar) or conditioned place preference among mice conditioned with cocaine (10 mg / kg, s.c.; n = 24, gray bar), U50,488 (0.3 mg / kg, i.p.) 15 min prior to cocaine (10 mg / kg, s.c.; n = 15; right panel, gray hatched bar), or U50,488 (0.3 mg / kg, i.p.) 15 min prior to saline (s.c.; n = 24; right panel, closed bar). * indicates significant difference from pre-conditioned group (p < 0.05). ΓΆΒ?Β indicates significant difference from cocaine (10 mg / kg, s.c.)-conditioned group (p < 0.05).
Discussion
The present investigation supported the hypothesis that a centrally- active KOR agonist (U50,488) would impair novel object recognition in a NXM-dependent manner at a dose independent of prolonged sedative effects. Compared to saline administration, dosing with U50,488 (0.3, 1.0, 10.0 mg / kg, i.p.) revealed 0.3 mg / kg to be the only dose that did not significantly alter locomotor behavior beyond the initial ambulatory reaction to the injection. When mice were assessed for unprompted ambulation in the CLAMS apparatus, U50,488 reduced ambulatory behavior (albeit, effects were least observed at 0.3 mg / kg dosing). Given that the saline group also decreased activity over time with habituation to this task, rotorod performance of mice was also assessed. When mice were required to perform a goal- oriented motor task in the rotorod paradigm, U50,488 (0.3 mg / kg) was not observed to influence motor performance; however, a greater U50,488 dose (1.0 mg / kg) significantly decreased the latency for mice to fall from the rotorod, relative to baseline performance. These data are consistent with findings that demonstrate dose-dependent soporific effects of U50,488 [42]. Similar effects were observed with ffir-NH2 to reduce both initial ambulation in the CLAMS apparatus and rotorod performance when administered centrally, but not when administered systemically, supporting the notion that ffir-NH2 does not readily cross the BBB. Here, we find that systemic administration of U50,488 (0.3 mg / kg) attenuated associative learning in the novel object recognition task. Pretreatment with a centrally-limited, non-selective opioid antagonist, NXM, blocked this effect. These data support the theory that central KOR activation contributes to opioid- mediated impairment of mnemonic acquisition. Consistent with these findings, ffir-NH2 attenuated novel object recognition of mice when administered centrally (3 nmol, i.c.v.), but not peripherally (1 mg / kg, i.p.). As nor-BNI attenuated the effect of centrally-mediated ffir-NH2, these data together extend those of prior investigations [21], suggesting that central KOR activation may be necessary and sufficient to impair associative memory.
Evidence suggests that KOR activation may impair memory via multiple mechanisms. Although some investigations have reported that the KOR agonist, U50,488, improved cognitive performance in a Y-maze task [43-45], these enhancements occurred following mnemonic impairment via scopolamine administration, and may be mediated by non-KOR systems. Indeed, convergent evidence spanning multiple learning and memory models across species, suggests that KOR agonists impair cognition and memory. Administered dynorphin or the selective KOR agonist U50,488, chicks demonstrate dose-dependent memory impairment when tested 24 h after training in a one-trial avoidance task [18]. In the amygdala-mediated passive avoidance task, U50,488 is observed to reduce retention among CD-1 mice [17]. Likewise, infusion of U50,488 to the dorsal aspect of the hippocampus of C57BL/6J mice attenuates contextual (but not cued) fear conditioning and spatial mnemonic performance in a water maze, an effect blocked by co-infusion of a KOR-selective antagonist [20]. Together, these data suggest that KOR activation may be important in the disruption of hippocampus-and/or amygdala-mediated tasks that require acquisition and consolidation of mnemonic information. Learning is thought to involve the consolidation of information into a stable representation; a process that takes time [46-50]. Formation of short-term memories, occurring over minutes to hours, may be susceptible to environmental factors that influence mnemonic consolidation to enhance or impair learning [51]. Consistent with this, long-term potentiation (LTP) has been demonstrated to be inhibited by KOR activation in vitro [52-55], although the mechanism(s) underlying mnemonic impairment in vivo require further investigation. In the present study, testing in the novel object recognition paradigm occurred over approximately 65 min, a time- frame which is consistent with associative learning in mice, and may be a particularly sensitive bioassay for detecting KOR-mediated changes in associative learning.
The detrimental consequences of KOR activation on associative learning could conceivably contribute to the KOR agonist-induced suppression of psychostimulant addiction. A number of studies have demonstrated that co-administering U50,488 with cocaine can attenuate cocaine-induced CPP in rats [56,57]; albeit, these investigations utilized dosing that are expected to have sedative effects (= 5 mg / kg). Here we observed that a low dose of U50,488 (0.3 mg/kg) administered in the absence of cocaine did not produce significant place conditioning (i.e., either conditioned place preference or aversion). Notably, place conditioning with U50,488 alone can produce conditioned place aversion at higher doses [32,57- 59]. This effect is dose-dependent and centrally mediated [60]. In the present investigation, we find that a dose of U50, 488 that is relatively devoid of soporific and place-conditioning effects can disrupt associative memory, also reducing cocaine-CPP. These data support the notion that KOR-mediated influences on associative learning may be important for developing drug-seeking behavior. Given that U50,488 was administered during conditioning in the present investigation, effects of KOR agonism on cocaine-CPP may be interpreted as both (1) reducing rewarding substrate actions (such as prevention of dopaminergic response to cocaine) and (2) by blocking the contextual association of the cocaine-paired side of the box. KOR agonists can inhibit psychostimulant-induced dopaminergic signaling [9-14], an effect closely paired to the suppression of cocaine conditioned place preference [61]. However, these counter-mnemonic effects of KOR agonists may act in an integrated manner with the dopaminergic suppression. As is well-studied in the hippocampus, psychostimulants induce NMDAR-mediated LTP in the ventral tegmental area (VTA) of the midbrain [62,63], a primary brain region in the mesolimbic dopamine pathway that is activated in response to all known drugs of abuse [64]. Provoking mesocorticolimbic dopamine release via electrical stimulation or via administration of a psychostimulant drug, such as cocaine or amphetamine, is found to enhance memory [65-71]. It stands to reason that KOR- mediated processes in this region mediate the learned associations of psychostimulant reinforcement that are more traditionally associated with dopaminergic activation [72-74]. Indirect inhibition of LTP in the midbrain may present as one mechanism by which KOR activation can dampen psychostimulant associations with reward. In support, KOR agonists inhibit presynaptic glutamate release and post-synaptic NMDAR potentials [53,75-77] and infusion of KOR agonists to the VTA inhibit midbrain dopaminergic cells [78,79]. Moreover, intra- VTA KOR activation influences psychostimulant effects, decreasing sensitization to cocaine seeking [80] and attenuating cocaine-induced repetitive motor behavior [81]. Recently, cocaine-reinstatement was found to be dampened among squirrel monkeys administered a KOR agonist in a nor-BNI-dependent manner [82]. Thus, KOR-mediated influence on acquisition of associative learning may have important implications for drug seeking and psychostimulant sensitization that can be mediated via modulation of rewarding dopaminergic signaling as well as impairments to associative learning.
Beyond pharmacological investigation, the KOR compounds used in the present study may be important clinical tools. In particular, the KOR agonist ffir-NH2 may serve as an important opioid therapeutic for pain management that is relatively devoid of abuse potential given its ability to be administered in a peripherally-restricted manner [26,83]. The non-peptidergic ligand asimadoline, a potent KOR agonist with ~500-fold selectivity over mu or delta opioid receptors [28,30] that poorly penetrates the blood brain barrier [84] is presently in clinical trials. Among patients with irritable bowel syndrome, asimadoline significantly alleviates visceral pain compared to placebo, [85,86]. Likewise, peripheral-restricted antinociception has been demonstrated in preclinical work using D-Phe-D-Phe-D-Nle-D-Arg- NH2, a highly selective KOR agonist that is a close analog of ffir-NH2, in the acetic acid writhing and formalin-induced flinching assays [31]. Selectivity for human KOR over mu or delta receptors was over 30,000 and 68,000-fold, respectively [31]. Unlike U50,488, systemic administration of ffir-NH2 did not alter associative learning among mice in the novel object recognition task, suggesting this compound may offer analgesia with fewer liabilities. Future investigations will characterize the aversive, dysphoric and antinociceptive properties of this compound.
The present data must be presented with some caveats. Foremost, the central ffir-NH2 dosing utilized did demonstrate some soporific effects in the rotorod task. These effects did not appear to influence the amount of time spent investigating the constant target in the novel object recognition task and were diminished by 20 min post- infusion; however, the amount of time spent investigating the novel object was reduced. Future investigations will aim to assess the motor and cognitive effects of ffir-NH2 dosing between 0 and 3 nmol (i.c.v.). As well, it is notable that the present experimental design utilized a CPP paradigm wherein U50,488 was administered prior to cocaine administration. While, it is important to have demonstrated prophylactic effects of KOR agonism on cocaine-CPP, it is also important to note that drug-related associations generally form before any treatment intervention. Administration of U50,488 30 min after daily cocaine place conditioning has been performed previously [39], but found to have no effect on eventual cocaine-CPP. Administration of U50,488 immediately prior to final preference testing might be expected to suppress the place preference response either due to the sedative effects or disruption of memory processes by the KOR agonist. The inability of U50,488 treatment following daily place conditioning might be interpreted to suggest that a disruption of memory recall (with low dose KOR agonists) may not be enough to suppress an established cocaine place preference response, although this bears further investigation. Interestingly, administration of KOR agonists actually *induced* reinstatement of extinguished drug seeking behavior [87], suggesting the outcome of this experiment may not be so simple as initially supposed. Indeed timing and dosage of U50,488 have been demonstrated to influence KOR-modulation of cocaine-CPP. U50,488 (5 mg /kg) administration 60 min prior to cocaine potentiates; whereas administration 15 min prior to cocaine suppresses, cocaine-induced CPP [39]. While the potentiation of cocaine CPP was shown to be independent of associative learning mechanisms [88], their involvement in the suppression of the rewarding valence of cocaine-paired environment was not determined. As such, future investigations of the effects of KOR agonists on drugrelated associations after their establishment would be of interest. Moreover, further studies utilizing microinjection of KOR agonists into hippocampus and other brain regions associated with learning and memory performance, as well as studies of U50,488 with mice possessing cite-specific gene-disruption of KOR in the dopamine reward pathway (e.g., VTA and nucleus accumbens), would be of value in further parsing out the contribution of associative learning mechanisms to drug seeking behavior, and the therapeutic benefit disrupting such mechanisms would have for treating drug abuse.
In summary, the present study demonstrates that agonist-induced activation of CNS KOR at a dose that is sub-threshold for sedation prevents novel object recognition in an NXM-dependent manner and also significantly reduces cocaine-CPP. These data extend prior work demonstrating memory impairments induced by KOR agonists, suggesting that central KOR activation is necessary and sufficient for effects on associative learning impairment, and may contribute to the therapeutic mediation of psychostimulant-seeking behavior.
Acknowledgements
This work was funded by the National Institutes of Mental Health (MH085607) and Drug Abuse (DA031370) and funds from the State of Florida, Executive Office of the Governor’s Office of Tourism, Trade, and Economic Development. Technical assistance by Shainnel O. Eans is appreciated.
References
- Dykstra LA, Gmerek DE, Winger G, Woods JH (1987) Kappa opioids in rhesus monkeys. I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther 242: 413-420.
- Aldrich JV, McLaughlin JP (2009) Peptide kappa opioid receptor ligands: potential for drug development. AAPS J 11: 312-322.
- Pfeiffer A, Brantl V, Herz A, Emrich HM (1986) Psychotomimesis mediated by kappa opiate receptors. Science 233: 774-776.
- Bowdle TA (1988) Clinical pharmacology of antagonists of narcotic-induced respiratory depression. A brief review. Acute Care 1: 70-76.
- Mello N, Negus SS (2000) Interactions between kappa opioid agonists and cocaine. Preclinical studies. Ann N Y Acad Sci 909: 104-132.
- Shippenberg TS, Zapata A, Chefer VI (2007) Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther 116: 306-321.
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF (1999) The kappa-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem 73: 1066-1074.
- Chefer VI, Morón JA, Hope B, Rea W, Shippenberg TS (2000) Kappaopioid receptor activation prevents alterations in mesocortical dopamine neurotransmission that occur during abstinence from cocaine. Neuroscience 101: 619-627.
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, et al. (2005) Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci 25: 5029-5037.
- Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85: 5274-5278.
- Spanagel R, Herz A, Shippenberg TS (1992) Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci U S A 89: 2046-2050.
- Heidbreder CA, Shippenberg TS (1994) U-69593 prevents cocaine sensitization by normalizing basal accumbens dopamine. Neuroreport 5: 1797-1800.
- Heidbreder CA, Goldberg SR, Shippenberg TS (1993) The kappaopioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res 616: 335-338.
- Shippenberg TS, LeFevour A, Heidbreder C (1996) kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther 276: 545-554.
- Negus SS, Mello NK, Portoghese PS, Lin CE (1997) Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 282: 44-55.
- Wang YH, Sun JF, Tao YM, Chi ZQ, Liu JG (2010) The role of kappaopioid receptor activation in mediating antinociception and addiction. Acta Pharmacol Sin 31: 1065-1070.
- Castellano C, Ammassari-Teule M, Libri V, Pavone F (1988) Effects of kappaopioid receptor agonists on locomotor activity and memory processes in mice. Pol J Pharmacol Pharm 40: 507-513.
- Colombo PJ, Martinez JL, Bennett EL, Rosenzweig MR (1992) Kappa opioid receptor activity modulates memory for peck-avoidance training in the 2-dayold chick. Psychopharmacology (Berl) 108: 235-240.
- Shannon HE, Eberle EL, Mitch CH, McKinzie DL, Statnick MA (2007) Effects of kappa opioid receptor agonists on attention as assessed by a 5-choice serial reaction time task in rats. Neuropharmacology 53: 930-941.
- Daumas S, Betourne A, Halley H, Wolfer DP, Lipp HP, et al. (2007) Transient activation of the CA3 Kappa opioid system in the dorsal hippocampus modulates complex memory processing in mice. Neurobiol Learn Mem 88: 94-103.
- Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP (2009) Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J Neurosci 29: 4293-4300.
- Kölsch H, Wagner M, Bilkei-Gorzó A, Toliat MR, Pentzek M, et al. (2009) Gene polymorphisms in prodynorphin (PDYN) are associated with episodic memory in the elderly. J Neural Transm 116: 897-903.
- Ennaceur A, Neave N, Aggleton JP (1997) Spontaneous object recognition and object location memory in rats: the effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res 113: 509-519.
- Broadbent NJ, Squire LR, Clark RE (2004) Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A 101: 14515-14520.
- Dooley CT, Ny P, Bidlack JM, Houghten RA (1998) Selective ligands for the mu, delta, and kappa opioid receptors identified from a single mixture based tetrapeptide positional scanning combinatorial library. J Biol Chem 273:18848-18856.
- Binder W, Machelska H, Mousa S, Schmitt T, Riviere PJM, et al. (2001) Analgesic and antiinflammatory effects of two novel ?-opioid peptides. Anesthesiology 94: 1034-1044.
- Haley TJ, McCormick WG (1957) Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother 12: 12-15.
- Barber A, Bartoszyk GD, Bender HM, Gottschlich R, Greiner HE, et al. (1994) A pharmacological profile of the novel, peripherally-selective kappa-opioid receptor agonist, EMD 61753. Br J Pharmacol 113: 1317-1327.
- Gardell LR, Spencer RH, Chalmers DT, Menzaghi F (2008) Preclinical profile of CR845: A novel, long-acting peripheral kappa opioid receptor agonist. Poster Presentation at the International Association for the Study of Pain, Glasgow, Scotland.
- Gottschlich R, Krug M, Barber A, Devant RM (1994) Kappa-opioid activity of the four stereoisomers of the peripherally selective kappa-agonist, EMD 60400 and EMD 61753. Chirality 6: 685-689.
- Vanderah TW, Largent-Milnes T, Lai J, Porreca F, Houghten RA, et al. (2008) Novel D-amino acid tetrapeptides produce potent antinociception by selectively acting at peripheral ?-opioid receptors. Eur J Pharmacol 583: 62- 72.
- Negus SS, O’Connell R, Morrissey E, Cheng K, Rice KC (2011) Effects of Peripherally Restricted Kappa Opioid Receptor Agonists on Pain-Related Stimulation and Depression of Behavior in Rats. J Pharmacol Exp Ther.
- Bertalmio AJ, Woods JH (1987) Differentiation between mu and kappa receptor-mediated effects in opioid drug discrimination: apparent pA2 analysis. J Pharmacol Exp Ther 243: 591-597.
- Harris RA (1980) Interactions between narcotic agonists, partial agonists and antagonists evaluated by schedule-controlled behavior. J Pharmacol Exp Ther 213: 497-503.
- Reilley KJ, Giulianotti M, Dooley CT, Nefzi A, McLaughlin JP, et al. (2010) Identification of two novel, potent, low-liability antinociceptive compounds from the direct in vivo screening of a large mixture-based combinatorial library. AAPS J 12: 318-329.
- Frye CA, Sumida K, Dudek BC, Harney JP, Lydon JP, et al. (2006) Progesterone’s effects to reduce anxiety behavior of aged mice do not require actions via intracellular progestin receptors. Psychopharmacology (Berl) 186: 312-322.
- Beaudry H, Proteau-Gagné A, Li S, Dory Y, Chavkin C, et al. (2009) Differential noxious and motor tolerance of chronic delta opioid receptor agonists in rodents. Neuroscience 161: 381-391.
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP (2007) Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol 569: 84-89.
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C (2006) Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropsychopharmacology 31: 787-794.
- Brabant C, Quertemont E, Tirelli E (2005) Influence of the dose and the number of drug-context pairings on the magnitude and the long-lasting retention of cocaine-induced conditioned place preference in C57BL/6J mice. Psychopharmacology 180: 33-40.
- Kreibich AS, Blendy JA (2004) cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci 24: 6686-6692.
- Kuzmin A, Sandin J, Terenius L, Ogren SO (2000) Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J Pharmacol Exp Ther 295: 1031-1042.
- Hiramatsu M, Hyodo T, Kameyama T (1996) U-50488H, a selective kappaopioid receptor agonist, improves carbon monoxide-induced delayed amnesia in mice. Eur J Pharmacol 315: 119-125.
- Hiramatsu M, Kameyama T (1998) Roles of kappa-opioid receptor agonists in learning and memory impairment in animal models. Methods Find Exp Clin Pharmacol 20: 595-599.
- Hiramatsu M, Hoshino T (2004) Involvement of kappa-opioid receptors and sigma receptors in memory function demonstrated using an antisense strategy. Brain Res 1030: 247-255.
- Izquierdo I (1989) A game with shifting mirrors. Trends Pharmacol Sci 10:473-476.
- Lynch G (2002) Memory enhancement: the search for mechanism-based drugs. Nat Neurosci 5: 1035-1038.
- McGaugh JL (2000) Memory--a century of consolidation. Science 287: 248- 251.
- Nadel L, Bohbot V (2001) Consolidation of memory. Hippocampus 11: 56-60.
- Nader K, Schafe GE, LeDoux JE (2000) The labile nature of consolidation theory. Nat Rev Neurosci 1: 216-219.
- White NM, Milner PM (1992) The psychobiology of reinforcers. Annu Rev Psychol 43: 443-471.
- Caudle RM, Chavkin C, Dubner R (1994) Kappa 2 opioid receptors inhibit NMDA receptor-mediated synaptic currents in guinea pig CA3 pyramidal cells. J Neurosci 14: 5580-5589.
- Caudle RM, Mannes AJ, Iadarola MJ (1997) GR89, 696 is a kappa-2 opioid receptor agonist and a kappa-1 opioid receptor antagonist in the guinea pig hippocampus. J Pharmacol Exp Ther 283: 1342-1349.
- Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C (2000) Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J Neurosci 20: 4379-4388.
- Terman GW, Wagner JJ, Chavkin C (1994) Kappa opioids inhibit induction of long-term potentiation in the dentate gyrus of the guinea pig hippocampus. J Neurosci 14: 4740-4747.
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP (1995) The effects of the kappa agonist U-50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology (Berl) 120: 392-399.
- Suzuki T, Shiozaki Y, Masukawa Y, Misawa M, Nagase H (1992) The role of mu- and kappa-opioid receptors in cocaine-induced conditioned place preference. Jpn J Pharmacol 58: 435-442.
- Mucha RF, Millan MJ, Herz A (1985) Aversive properties of naloxone in non-dependent (naive) rats may involve blockade of central beta-endorphin. Psychopharmacology (Berl) 86: 281-285.
- Skoubis PD, Lam HA, Shoblock J, Narayanan S, Maidment NT (2005) Endogenous enkephalins, not endorphins, modulate basal hedonic state in mice. Eur J Neurosci 21: 1379-1384.
- Bals-Kubik R, Herz A, Shippenberg TS (1989) Evidence that the aversive effects of opioid antagonists and kappa-agonists are centrally mediated. Psychopharmacology (Berl) 98: 203-206.
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ (2004) Effect of the kappa opioid agonist R-84760 on cocaine-induced increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 173: 146-152.
- Bonci A, Malenka RC (1999) Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci 19: 3723-3730.
- Argilli E, Sibley DR, Malenka RC, England PM, Bonci A (2008) Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J Neurosci 28: 9092-9100.
- Nestler EJ (2005) Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445-1449.
- Blaiss CA, Janak PH (2006) Post-training and post-reactivation administration of amphetamine enhances morphine conditioned place preference. Behav Brain Res 171: 329-337.
- Cestari V, Castellano C (1996) Caffeine and cocaine interaction on memory consolidation in mice. Arch Int Pharmacodyn Ther 331: 94-104.
- Introini-Collison IB, McGaugh JL (1989) Cocaine enhances memory storage in mice. Psychopharmacology (Berl) 99: 537-541.
- Packard MG, White NM (1989) Memory facilitation produced by dopamine agonists: role of receptor subtype and mnemonic requirements. Pharmacol Biochem Behav 33:511-518.
- Routtenberg A (1979) Anatomical localization of phosphoprotein and glycoprotein substrates of memory. Prog Neurobiol 12: 85-113.
- Simon NW, Setlow B (2006) Post-training amphetamine administration enhances memory consolidation in appetitive Pavlovian conditioning: Implications for drug addiction. Neurobiol Learn Mem 86: 305-310.
- Warburton DM (1992) Nicotine as a cognitive enhancer. Prog Neuropsychopharmacol Biol Psychiatry 16: 181-191.
- Ettenberg A, Pettit HO, Bloom FE, Koob GF (1982) Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology 78: 204-209.
- Gerrits MA, Van Ree JM (1996) Effect of nucleus accumbens dopamine depletion on motivational aspects involved in initiation of cocaine and heroin self-administration in rats. Brain Res 713: 114-124.
- Pettit HO, Ettenberg A, Bloom FE, Koob GF (1984) Destruction of dopamine in the nucleus accumbens selectively attenuates cocaine but not heroin selfadministration in rats. Psychopharmacology (Berl) 84: 167-173.
- Caudle RM, Finegold AA, Mannes AJ, Tobias MD, Kenshalo DR , et al. (1998) Spinal kappa1 and kappa2 opioid binding sites in rats, guinea pigs, monkeys and humans. Neuroreport 9: 2523-2525.
- Wagner JJ, Caudle RM, Chavkin C (1992) Kappa-opioids decrease excitatory transmission in the dentate gyrus of the guinea pig hippocampus. J Neurosci 12: 132-141.
- Wagner JJ, Terman GW, Chavkin C (1993) Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature 363: 451-454.
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL (2003) Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci 23: 9981-9986.
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, et al. (2006) Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A 103: 2938-2942.
- Sun W, Xue Y, Huang Z, Steketee JD (2010) Regulation of cocaine-reinstated drug-seeking behavior by kappa-opioid receptors in the ventral tegmental area of rats. Psychopharmacology (Berl) 210: 179-188.
- Cortez AM, Charntikov S, Der-Ghazarian T, Horn LR, Crawford CA, et al. (2010) Age-dependent effects of kappa-opioid receptor stimulation on cocaine-induced stereotyped behaviors and dopamine overflow in the caudate-putamen: an in vivo microdialysis study. Neuroscience 169: 203- 213.
- Rüedi-Bettschen D, Rowlett JK, Spealman RD, Platt DM (2010) Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys: kappa opioid and serotonergic mechanisms. Psychopharmacology (Berl) 210: 169- 177.
- Rivière PJ (2004) Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol 141: 1331-1334.
- Bender HM, Dasenbrock J (1998) Brain concentrations of asimadoline in mice: the influence of coadministration of various P-glycoprotein substrates. Int J Clin Pharmacol Ther 36: 76-79.
- Delvaux M, Beck A, Jacob J, Bouzamondo H, Weber FT, et al. (2004) Effect of asimadoline, a j-opioid, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 20: 237-246.
- Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, et al. (2008) Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther 28: 239-249.
- Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, et al. (2009) Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci USA 106: 19168-19173.
- Schindler AG, Li S, Chavkin C (2010) Behavioral stress may increase the rewarding valence of cocaine-associated cues through a dynorphin/kappaopioid receptor-mediated mechanism without affecting associative learning or memory retrieval mechanisms. Neuropsychopharmacology 35:1932-1942.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15584
- [From(publication date):
specialissue-2011 - Dec 06, 2025] - Breakdown by view type
- HTML page views : 10902
- PDF downloads : 4682