Research Article Open Access
Isolation, Optimisation and Purification of Lipase Production by Pseudomonas Aeruginosa
Benattouche Zouaoui1* and Abbouni Bouziane21Department of biology, Faculty of Science, Mascara University, Algeria
2Department of biology, Faculty of Science, Sidi belabbes University, Algeria
- Corresponding Author:
- Benattouche Zouaoui
Department of biology, Faculty of Science
Mascara University, Algeria
Tel: 00213 557172691
Fax: 0021348646312
E-mail: benattouche_22@yahoo.fr
Received date: November 10, 2011; Accepted date: December 19, 2011; Published date: December 21, 2011
Citation: Zouaoui B, Bouziane A (2011) Isolation, Optimisation and Purification of Lipase Production by Pseudomonas Aeruginosa. J Biotechnol Biomaterial 1:120. doi:10.4172/2155-952X.1000120
Copyright: © 2011 Zouaoui B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Six isolates of lipase producing (Ps1, Ps2, Ps3, Ps4, Ps5 and Ps6) were secreened from wastewater on a selective medium agar that contained tween 80 or olive oil as the only source of carbon. The isolate showed highest lipase activity was Ps5 which later was identified as Pseudomonas aeruginosa. The effect of media composition was analysed to maximize production of lipase. The maximum extracellular lipase present in the broth was purified 11 folds with an overall yield of 65.51 % through purification procedure of ammonium sulphate precipitation and DEAE cellulose chromatography. The purified lipase had maximal activity within the pH range of 6-8, with an optimum PH of 7, and within the temperature range of 25 – 35°C, with optimum temperature for the hydrolysis of olive oil at 35°C. The lipase activity of the enzyme was enchanced by Ca+2 and Mg+2 but strongly inhibited by heavy metals Zn+2, Cu+2and Mn+2.
Keywords
Pseudomonas aeruginosa; Lipase; purification; Biomass; Heavy metals
Introduction
Lipases are glycerol ester hydrolases (EC: 3.1.1.3), which hydrolyze ester linkages of glycerides at water-oil interface. During hydrolysis lipases pick acyl group from glycerides forming lipase-acyl complex, which then transfers its acyl group to OH group of water. However, in non-aqueous conditions, these naturally hydrolytic enzymes can transfer acyl groups of carboxylic acids to nucleophiles other than water [12]. Thus lipases can acylate alcohols, sugars, thiols and amines synthesizing a variety of stereo-specific esters, sugar esters, thioesters and amides [5,16].
Microbial lipases have already established their vast potential regarding to their usage in different industries. The interest in microbial lipase production has increased in the last decades. Due to the versatility of the molecular structure and catalytic properties, these enzymes have potential biotechnological application in different industrial sectors such as food, waste water treatment, cosmetics, oleo chemical, pharmaceutics, detergents and in the fuel sector, which applies lipase as catalyst for synthesis of esters and for trans esterification of the oil for the production of bio diesel [14].
Generally, the enzymes of industrial interest are produced in the presence of inducers in the case of lipases, the presence of triacyl glycerol, surfactants, vegetable oils, oil industry wastes or their hydrolysis products in the culture medium have, in most cases, an inducible effect on lipase production [4]. Most of the well studied microbial lipases are inducible extracellular enzymes [17]. They are synthesized with in the cell and exported to its external surface or environment. Extracellular lipases have been produced from microorganisms, such as fungi, yeast, and bacteria beside from plants, and animals. Commercial lipases from Pseudomonas genus from Pseudomonas cepacia, Pseudomonas alcaligenes, Pseudomonas mendocina [15]. These lipases express different physicochemical properties that depend on metal ions, substrate, pH and temperature. The aim of this study is to analyse the influence of media components on the production of lipase and to purity and characterize lipase produced on a common production medium using Pseudomonas aeruginosa.
Materials and Methods
Isolation of producer microorganism
The bacterial strain Pseudomonas aeruginosa used in this study was isolated from a wastewater at sidi bel abbes, Algeria. The isolates were identified on the basis of various morphological, physico-chemical, and biochemical characteristics. Lipolytic bacteria are typically detected and secreened through the appearance of clearing zones by using a selective medium [11]. Which was containing Tween 80 or olive oil as the only source of carbon? The diameter ratio of clear zone and colony was measured.
Optimization of lipase production
Four different cultivation media were evaluated to the production of lipase enzyme. The composition of the different fermentation media can be summarized as follows: Medium (A): 0.3 % yeast extract, 0.1 % bacto peptone, 1% olive oil, 0.07% K2HPO4, 0.03% KH2PO4, 0.05% MgSO4, 0.01% MnCl2, 0.025 %(NH4)2SO4 and 0.01 CaCl2 [9 ]. Medium (B): 1% Tween 80 which replaced olive oil in medium (A) Medium (C) : 2% dextrose added to medium (A) Medium (D): 2% dextrose added to medium (B) The PH of all the production media was adjusted to 7.2 using 0.5 N NaOH, before autoclaving at 121°C for 15 min. The enzyme assay was carried out in 500 ml Erlenmeyer flasks containing 94 ml of medium production inoculated with 6 ml of the overnight culture.
The flasks were incubated at 35 °C with a constant shaking at 125 rpm for 3 days. The cell was separated by centrifugation at 10000 rpm and 4 °C for 20 min. The supernatants were collected and used to determine the lipase activity. The isolation expressed the highest activity was selected for further studied.
Biomass estimation
Biomass concentration was estimated by measuring the optical density at 600 nm by using a Spectronic instrument and standard curve previously determined (biomass g/l corresponding to 0,270x OD600) [3].
Protein determination
Protein was measured photometrically by the method of Bradford with Coomassie protein Assay reagent [2].
Lipase activity
Lipase activity was determined titrimetrically using olive oil hydrolysis [13]. 1 ml enzyme solution was added to the assay substrate containing 10 ml of 10% homogenized olive oil in 10% gum acacia, 2 ml of 0,6% CaCl2 solution and 5 ml of 0,2 mol/ l phosphate buffer, PH 7.2. The enzyme-substrate was incubated on orbital shaker at 125 rpm at 35°C for 1h. 20 ml ethanol –acetone (1:1) was added to stop the reaction. Liberated fatty acids were titrated with 0.1mol/ l NaOH using phenophtaline as indicator. The reaction mixture without the enzyme was titrated in the same way and used as blank. One lipase unit was defined as the amount of the enzyme that released one μmol fatty acid per min under standard assay conditions [7].
Purification of lipase
Lipase purification was carried out at 4°C. The culture medium was centrifuged at 10000 rpm for 20 min to obtain crude enzyme, the supernatant fluid was subjected to precipitation with ammonium sulphate to 40 % saturation and stirred for 2h. The precipitate was removed by centrifugation. Lipase activity both in the precipitate and supernatant was determined.
Additional ammonium sulphate was added to the supernatant to bring the saturation to 60 %. The precipitate were collected, dissolved in buffer PH 7.2 and dialyzed against same buffer. The enzyme mixture was loaded onto the DEAE cellulose column (15 x1.5 cm) equilibrated with the phosphate buffer. The enzyme was eluted with a linear gradient of 0-1M NaCl in the same buffer at a flow rate of 1 ml/min. The active fractions that contained lipase enzyme were pooled, dialysed and assessed for protein content. The resulting enzyme was utilised for the characterization of the extracellular lipase. The protein content at each stage of enzyme purification was determined accordingly to Bradford et al. with bovine serum albumin as the standard
Determination of PH and temperature optimum
The temperature and PH optimum of purified lipase was determined at different degrees ranging from 20-50°C and PH values from 3 to 10. To determine the effect of temperature on lipase activity, purified enzyme and substrate were incubated at various reaction temperatures before starting the experiment and the enzyme assay was performed to determine the optimal temperature titrimetrically using olive oil as substrate. The optimal PH was determined by incubating the enzymesubstrate at various PH from (3 to 10) and assayed for lipase activity.
Thermo stability and pHstability of lipase
The thermo stability of the lipase fraction was studied by incubating the purified enzyme at various temperatures (20 -50°C) or 1h. The residual lipolytic activities were then determined using olive oil substrate. For PH stability, purified enzyme was incubated using different PH buffers for 1h. The reaction mixtures were incubated as per standard assay and the residual lipolytic activities were then determined using olive oil as substrate.
Effect of metal ions on lipase activity
For determining the effect of metal ions on lipase activity, the purified enzyme were preincubated with 1 mM of Ca+2, Mg+2, Mn+2, Cu+2 and Zn+2 for 1h at 30°C and the residual activity was determined using olive oil as substrate under standard assay conditions.
Results and Discussion
Isolation of lipase –producing micro organisms
Six isolates grown in the selective medium were found to produce lipase which was identified as bacteria (Ps1, Ps2, Ps, Ps4, Ps5 and Ps6). Their growth showed that they can use olive oil as carbon source and showed the lipase producing feasibility. Evaluation of the lipase producing efficiency, based on the clear zone around colony, indicated that all of them could produce lipase enzyme. The result of Ps5 was outstanding (Figure 1). When their produced lipases were applied on the emulsion olive oil- agar with emulsion Tween 80-agar and incubated at 35°C for 96h. Their abilities of lipase producing were evaluated with the diameter ratio of clear zone and colony (Table 1). (Ps5) is the highest with 26 mm with olive oil and 19 mm with Tween 80, whereas of Ps4 is 16 mm with olive oil and 13.6 mm with Tween 80, and Ps6 is 15 mm with olive oil and 12 mm with Tween 80 (Table1)
| Bacterial | Diameter ratio of clear zone and colony (mm) | |
| Olive oil | Tween 80 | |
| Ps1 | 6.5 | 4 |
| Ps2 | 8.4 | 5.8 |
| Ps3 | 12.4 | 11 |
| Ps4 | 16 | 13.6 |
| Ps5 | 26 | 19 |
| Ps6 | 15 | 12 |
Table 1: The diameter ratio of clear zone and colony of the isolated bacterial.
The Ps5 was identified and found that it is a member in Pseudomonas genus. Therefore, the Ps5 was classified as Pseudomonas aeruginosa.
Optimization of lipase production by Pseudomonas sp
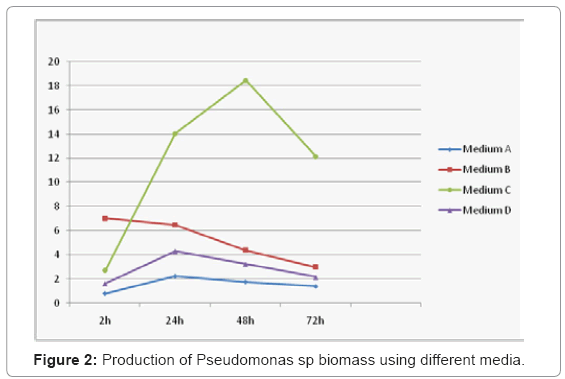
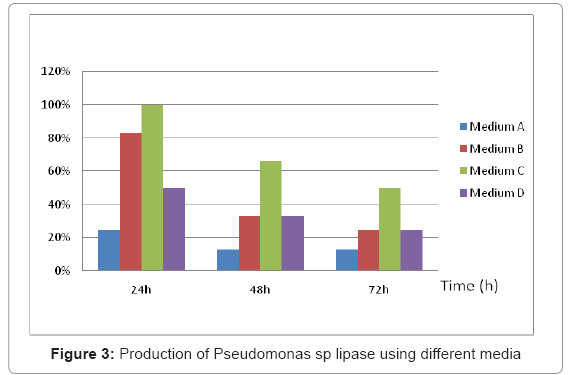
Four different media were used for lipase production by Pseudomonas aeruginosa. The results in (Figure 2) showed that medium (C) with dextrose as carbon, olive oil an inducer of lipase gave the maximum biomass and activity 18.36 g/l after 48 h. In the present study lipase production with different compositions were studied to maximise the production of lipase, the medium (C) presented the highest activity of 41.6 U/ml with specific enzyme activity 48.23 U/mg at 35°C after 24h (Figure 3).Lipase production in medium (A) without dextrose was 16.7% less as can pored to the medium (C) and the medium (D) with Tween 80 and dextrose produced 50% less lipase. Production studies on medium (B) shows a 75 % reduction in the production of lipase. Some Authors stated that lipase production greatly affected by the composition of the medium [10]. Based on these results, dextrose was selected as the main component for the fermentation medium and olive oil by Pseudomonas aeruginosa. Olive oil was better inducer than Tween 80, under the test conditions whereas dextrose was the best carbon source for lipase production. In fact, dextrose has been considered as a growth medium for microorganisms providing a satisfactory supply of nutrients for growth.
In conclusion, the Pseudomonas aeruginosa strain was found to be the best lipase producer. Wheat dextrose and olive oil were the best substrate and inducer, respectively for lipase production by this strain.
Purification of lipase
The crude enzyme was subjected to purification, maximal activity, specific activity, and yield (21.5 U/ml, 25.65 U/mg and 51.68 %) were detected at 40 % ammonium sulphate respectively (Table 2). By increasing the ammonium sulphate concentrations to 60 % a slight decrease in total activity was obtained. The specific activity 204.12 U/mg with yield of 65.51 % was obtained after the single step purification on DEAE cellulose chromatography (Table 2).
| C. Protein(mg) | Lipase Activity | Specific Activity | F. Fold | Yield % | |
|---|---|---|---|---|---|
| Supernatant (crude extract) |
82.46 | 41.6 U/ml | 46.34 U/mg | ||
| (NH4)2SO4 40% | 53.7 | 21.5 U/ml | 25.65 U/mg | 0.55 | 51.68 |
| (NH4)2SO4 60% | 42.6 | 9.8 U/ml | 17.7 U/mg | 0.69 | 45.58 |
| DEAE cellulose | 4.54 | 6.42 U/ml | 204.12 U/mg | 11.53 | 65.51 |
Table 2: Purification of lipase from Pseudomonas sp.
PH and temperature optimum
Initial PH of the culture broth is one of the most critical environmental parameters affecting both growth and lipase production. The results showed that the Pseudomonas aeruginosa was able to grow in the PH range from 6to 8 (Table 3) and reached the maximum lipase activity 42 U/ml at PH 7.0. These data are in agreement with that of Yuzo et al. [18] who reported that maximum lipase activity from Pseudomonas fluorescens HU 380 was detected at PH 7.
| pH | Relative activity (%) |
| 3 | 18 |
| 4 | 26 |
| 5 | 57 |
| 6 | 83 |
| 7 | 100 |
| 8 | 76 |
| 9 | 35 |
| 10 | 16 |
Table 3: Effect of PH on Pseudomonas sp lipase.
The results showed that maximum lipase activity detected at 35°C (Table 4). A decrease in the lipolytic activity were observed above 40°C and completely ended after 45°C. Such results are similar to that reported for many bacterial species [6].
| Temperature | Relative activity (%) |
| 20 | 22 |
| 25 | 53 |
| 30 | 76 |
| 35 | 100 |
| 40 | 39 |
| 45 | 07 |
| 50 | 00 |
Table 4: Effect of temperature on Pseudomonas lipase.
PH and temperature stability
The PH stability of the lipase was determined by the activity retained at different PH from 3 to 10 after 1h of incubation. The PH stability curve showed that the lipase was stable at PH 6 to 8 (Table 5). The stability data showed a decline in lipase activity below 6 and above 8, however 70 and 80% relative activity was retained at these PH [18] stated that the majority of bacterial lipase present optimal PH stability in the range of 6 to 8 and was unstable at PH values above 8.
| pH | Relative activity (%) |
| 3 | 12 |
| 4 | 21 |
| 5 | 32 |
| 6 | 69 |
| 7 | 100 |
| 8 | 78 |
| 9 | 23 |
| 10 | 06 |
Table 5: Stability of lipase in different PH.
Maximal thermo stability of the lipase was observed in the temperature range of 25 to 35°C (Table 6). The enzyme was found to be completely stable at 35°C after 1h. At 30°C, the enzyme maintained 81% after initial activity after 1h.
| Temperature | Relative activity (%) |
|---|---|
| 20 | 28 |
| 25 | 65 |
| 30 | 80 |
| 35 | 100 |
| 40 | 43 |
| 45 | 11 |
| 50 | 00 |
Table 6: Effect of temperature on lipase stability.
Effect of metal ions on lipase activity
Among the metal ions tested, enhancement in the enzyme activity was observed in presence of Ca+2 with 121% and Mg+2 with 112% relative activity when compared to control (Table 7). These ions have been reported to play role as lipase cofactor [8].
| Metal ions used | Concentration used (mM) | Remaining activity (%) |
|---|---|---|
| Control | none | 100 |
| Ca+2 | 1 | 121 |
| Mg+2 | 1 | 112 |
| Mn+2 | 1 | 32 |
| Cu+2 | 1 | 31 |
| Zn+2 | 1 | 31 |
Table 7: Effect of different metal ions on enzyme activity.
Calcium ion, especially, is used in Ca+2 binding processing which impact to position specificity on active site [1]. Same lipases produced by Pseudomonas sp. Have been found to be Ca+2dependent, however Ca+2 exerted inhibitory effect on Pseudomonas sp Strain [13]. However, the hydrolytic activity of the enzyme was inhibited by heavy metals Zn+2, Mn+2 and Cu+2 with 32% relative activity.
Conclusion
Out of 36 bacterial isolates obtained from the wastewater at Sidi Bel abbes, Algeria, 6 exhibited lipase activity. Pseudomonas aeruginosa proved to be the best lipase producer various physicochemical parameters were studied to determine the optimum conditions for its lipase production. The obtained results showed that the medium composition for lipase production was (1 bacto peptone, 3 yeast extract, 20 dextrose, 10 olive oil, 0.7 K2HPO4, 0.3 KH2PO4, 0.5 MgSO4, 0.1 MnCl2 g/l ) at PH 7, 35°C and 125 rpm in 100 ml in 500 ml flask. The purified lipase showed optimal activity in a wide range of temperature and PH values. Moreover, because of its pronounced thermal stability as well as preservation of activity and stability.
Acknowledgements
We are grateful to the technical staff in the department of biology, faculty of science, sidi Bel abbes and Mascara universities, Algeria
References
- Surinenaite B, Bendikiene V, Juodka B, Bachmatova I, Marcinkevichiene L (2002) Characterization and physicochemical properties of a lipase from Pseudomonas. Biotechnol Appl Biochem 36: 47-55.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Camacho RM, Mateos JC, González-Reynoso O, Prado LA, Córdova J (2009) Production and characterization of esterase and lipase from Haloarcula marismortui. J Ind Microbiol Biotechnol 36: 901-909.
- Damaso MCT, Passianoto MA, Loridio difreitas S, Guimaraes freire DM, Celiaraujo lago R, et al. (2008) Utilization of agro industrial residues for lipase production by solid state fermentation. Brazilian J Microbial 39: 676-681.
- Dellamora-ortiz GM, Martins RC, Rocha WL, Dias AP (1997) Activity and stability of a Rhizomucor miehei lipase in hydrophobic media. Biotechnol Appl Biochem 26: 31-37.
- Gilbert JE, Cornish A, Jones CW (1991) Purification and properties of extracellular lipase from Pseudomonas aeruginosa EF2. J Gen Microbiol 137: 2223- 2229.
- Gombert AK, Pinto AL, Castillo LR, Freire DMG (1999) Lipase production by Penicillium restrictum in solid-state fermentation using babassu oil cake as substrate. Process Biochem 35: 85-90.
- Dong H, Gao S, Han S, Cao S (1999) Purification and characterization of a Pseudomona sp. Lipase and its properties in non-aqueous media. Biotechnol App Biochem 30: 251-256.
- Li CY, Cheng CY, Chen TL (2004) Fed- batch production of lipase by Acinetobacter radioresistens using tween 80 as carbon source. Biochem Eng J 19: 25- 31.
- Lin ES, Ko HC (2005) Glucose stimulates production of the alkaline thermostable lipase of the edible Basidiomycete Antrodia Cinnamomea. Enzyme and Microbial Technology 37: 261-265.
- Loo JL, Lai OM, Long K, Ghazali HM (2006) Identification and characterization of a locally isolated lipolytic microfungus Geotrichum candidum. Malaysian J Microbiology 2: 22-29
- Martinelle M, Hult K (1995) Kinetics of acyl transfer reactions in organic media catalysed by Candida Antarctica lipase B. Biochimica Biophysica Acta 1251: 191-197.
- Prita SB, Ragini GB, Srinivasa RR, Khobragade CN (2009) Purification and characterization of extracellular lipase from a new strain-Pseudomonas aeruginosa SRT9. Brazilian J Micobiology 40: 358-366.
- Sharma R, Chisti Y, Banergee UC (2001) Production, purification, characterization, and applications of lipases. Biotechnol Adv 19: 627-662.
- Chigusa k, Hasegawa T, Yamamoto N, Watanabe Y (1996) Treatment of wastewater from oil manufacturing plant by yeasts. Wat Sci Technol 34: 51-58.
- Singh SK, Felse AP, Nunez A, Foglia TA, Gross RA (2003) Regioselective enzyme catalysed synthesis of phospholipid esters, amides, and multifunctional monomers. J Organic Chem 68: 5466-5477.
- Tan T, Zhang M, Wang B, Ying C, Deng I (2003) Screening of high lipase producing Candida sp and production of lipase by fermentation. Process Biochem 39: 459-465.
- Kojima Y, Shimizu S (2003) Purification and characterization of the lipase from Pseudomonas fluorescens HU 380. Journal of bioscience and bioengineering 96: 211-226.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 30386
- [From(publication date):
December-2011 - Nov 28, 2025] - Breakdown by view type
- HTML page views : 23635
- PDF downloads : 6751