Research Article Open Access
Isolation and Structural Elucidation of Novel Isomeric Process Related Impurities of Zolmitriptan
Neelakandan K1,2*, Chaudhari Ashok1, Manikandan H2, Santosha N1, Prabhakaran B1 and Mukund Gurjar1
1API Research Centre, Emcure Pharmaceutical Limited, Hinjawadi, Pune, 411057, India
2Department of chemistry, Annamalai University, Chidhambaram, India
- *Corresponding Author:
- Neelakandan K
API Research Centre
Emcure Pharmaceutical Limited
Hinjawadi, Pune, 411057, India
Fax: +91 20 39821445
E-mail: Neelakandan.K@emcure.co.in
Received date: March 11, 2013; Accepted date: April 05, 2013; Published date: April 08, 2013
Citation: Neelakandan K, Ashok C, Manikandan H, Santosha N, Prabhakaran B, et al. (2013) Isolation and Structural Elucidation of Novel Isomeric Process Related Impurities of Zolmitriptan. J Anal Bioanal Tech 4:165. doi: 10.4172/2155-9872.1000165
Copyright: © 2013 Neelakandan K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
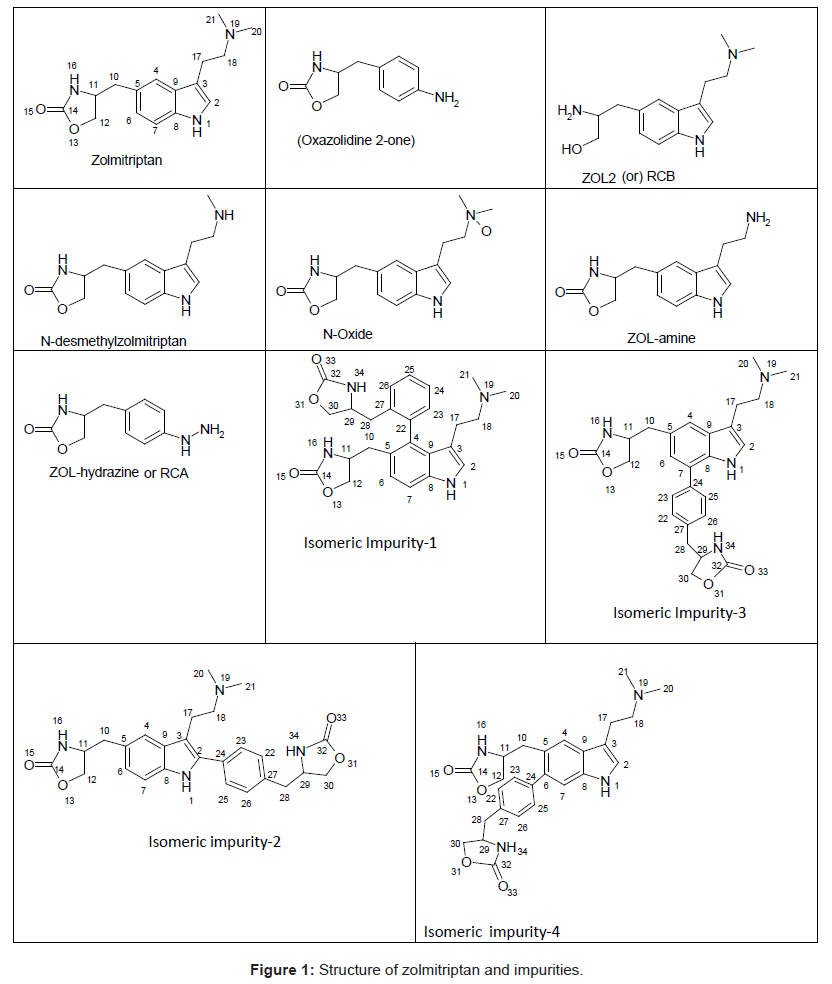
Four isomeric unknown impurities ranging from 0.08-0.12% were found in the purified sample of Zolmitriptan during the batch analysis by gradient reverse phase ultra performance liquid chromatography (UPLC) and their molecular weights determined by liquid chromatography mass spectroscopy (LC-MS) analysis. Subsequently, all the four impurities were isolated by flash chromatography followed by semi-preparative HPLC and characterized by 1H NMR, 13C NMR, 1H-1H COSY, HMBC, HSQC, MS spectroscopy and HPLC. The structures for these four impurities were assigned to be following
Isomeric Impurity-1: 4-((3-(2-(dimethylamino)ethyl)-4-(2-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one,
Isomeric Impurity-2: 4-((3-(2-(dimethylamino)ethyl)-2-(4-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one-,
Isomeric Impurity-3: 4-((3-(2-(dimethylamino)ethyl)-7-(4-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one,
Isomeric Impurity-4: 4-((3-(2-(dimethylamino)ethyl)-6-(4-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one
Isolation and characterization of impurities has helped us in improving the purity of API by removing these impurities using crystallization.
Keywords
Zolmitriptan; LCMS; HPLC; Chromatography; Isolation; Isomeric; NMR
Introduction
HThe impurity profile of a drug substance is a critical parameter for its safety assessment and is inherent to its manufacturing process. As per the ICH guidelines the impurities that exceed 0.1% in a drug must be identified prior to the clinical trials. Since the associated impurities are usually process related, they are invariably, structurally similar to the synthesized target drugs.
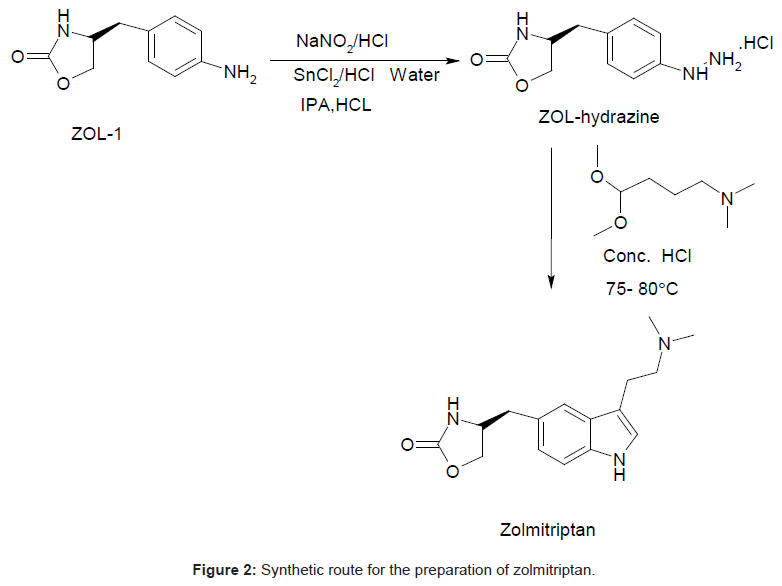
Zolmitriptan, chemically known as 4(S)-4-[3-(2- dimethylaminoethyl)-1H-5-indolylmethyl]-1,3-oxazolan-2-one belongs to a group of medicines known as Serotonin 5-HT1D receptor agonist [1,2]. Zolmitriptan (ZOL) is well absorbed in liver and undergoes extensive metabolism to give major metabolites such as 4(S)-4-[3-(2-methylaminoethyl)-1H-5-indolyl-methyl]-1,3-oxazolan- 2-one (N-desmethylzolmitriptan; impurity A in USP draft 2011) and (4S)-4-[3-[2-(dimethyloxidoamino)ethyl]-1H-indol-5-yl] methyl-2-oxazolidinon(zolmitriptan N-oxide) [3]. Several HPLC methods for their determination have been reported. Quantification of zolmitriptan in human plasma using mass [4], coulometric [5] or fluorescence detection [6] is well described. LC methods are reported for the determination of zolmitriptan and its related substances known as (S)-4-(4-aminobenzyl)-1,3-oxazolidin-2-one (ZOL1), (2S)- 2-amino-3-[3-(2-dimethylaminoethyl)-1H-5-indolyl]-propan-1-ol (RCB; impurity B in USP draft 2011), (S)-4-(4-hydrazinobenzyl)-1,3- oxazolidin-2-one (ZOL-hydrazine) and (4S)-4-[3-(2-aminoethyl)- 1H-indol-5-ylmethyl]-1,3-oxazolidon-2-one (ZOL-amine) (Figure 1) [7,8]. Another unknown impurity was isolated from zolmitriptan tablets using preparative chromatography and characterized to be 4-4-[3-[2-(dimethyloxidoamino) ethyl]-1H-indol-5-yl] methyl-2- oxazolidinone by mass and NMR spectroscopy [9]. A new impurity was identified, isolated, prepared and characterized as (4S,4S’)-4,4’- (2,2’-(4-(dimethylamino)butane-1,1-diyl)bis(3-(2-(dimethylamino) ethyl)-1H-indole-5,2-diyl))bis(methylene)di(oxazolidin-2-one) (ZOLdimer) [10]. Many associated organic impurities may arise as a result of side reactions or degradation of product during the manufacturing processes and or during storage of the drug substances. The criterion for their acceptance up to certain limits are based on pharmaceutical studies or known safety data. As per regulatory guidelines, pharmaceutical studies using a sample of isolated impurity can be considered for safety assessment [11]. It is therefore essential to isolate and characterize unidentified impurities present in Active Pharmaceutical Ingredients (APIs). Many synthetic protocols for Zolmitriptan have been reported in literature [12-20]. The present work describes isolation, identification and characterization of the four isomeric process related impurities 4-((3-(2-(dimethylamino)ethyl)-4-(2- ((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl)oxazolidin- 2-one,4-((3-(2-(dimethylamino)ethyl)-2-(4-((oxazolidin-4-yl)methyl) phenyl)-1H-indol-5-yl)methyl)oxazolidin-2-one-, 4-((3-(2-(dimethylamino) ethyl)-7-(4-((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl) methyl)oxazolidin-2-one and 4-((3-(2-(dimethylamino)ethyl)-7-(4- ((oxazolidin-4-yl)methyl)phenyl)-1H-indol-5-yl)methyl)oxazolidin- 2-one which were found in the drug substance Zolmitriptan during manufacturing. Isolation and characterization of these impurities helped to improve the purity of the final API.
Experimental
Samples and chemicals
The investigated sample of Zolmitriptan was synthesized in Emcure Pharmaceuticals Ltd, Hinjwadi Pune India. All the reagents were of analytical grade unless stated otherwise. acetonitrile (HPLC grade), Milli-Q-grade water, Methanol HPLC grade.
Ultra performance liquid chromatography (UPLC)
A Waters Ultra performance liquid chromatography system equipped with UV detector having empower pro software system was used. The analysis was carried out on Acquity CSH phenyl-hexyl (2.1 mm×100 mm) 1.7 μ. by using mobile phase A (ammonium dihydrogen phosphate (2.72 gm), potassium dihydrogen phosphate (1.2 gm) and hexane-1-sulphonic acid sodium salt (1.0 gm) in 1.0 liter of HPLC grade water and pH was adjusted to 2.0 and mobile phase-B (homogeneous mixture of acetonitrile and methanol, 85:15 ratio) along with diluents such as acetonitrile and water in the ratio of 50:50. The LC gradient program was set as: time (min)/% solution B: 0/10, 3/10, 10/32, 11/32, 11.1/10 and 16/10. UV detection was carried out at 220 nm and flow rate was kept as 0.4 ml/min. This analytical method was able to detect the process related impurities with good resolution.
Liquid Chromatography-Mass Spectroscopy (LC-MS)
The LC-MS analysis was carried out on YMC-Triart C18 (4.6 mm×150 mm) 3 μ or equivalent by using mobile phase A (0.01 M ammonium bicarbonate in HPLC grade water), mobile phase B (methanol and HPLC grade water, 90:10 ratio) with flow rate 1.0 ml/ min. The LC gradient program was set as: time (min)/, % solution B: 0/10, 15/35, 30/40, 60/70, 61/10 and 70/10. The column temperature was maintained at 27°C and the detection was monitored at a wavelength of 225 nm. The mass spectral analysis of the impurities was carried out on a triple quadruple mass spectrometer (MDS Sciex model API 2000) performed in the positive (+ve) ion mode with electron spray ionization technique (ESI) interface using following conditions: declustering potential at 40 V, entrance potential at 10 Volts, focusing potential at 325 Volts, curtain gas 20 liter per minute, ion spray voltage 4500 Volts and temperature 450°C.
Preparative liquid chromatography
A waters auto purification system equipped with binary gradient module (Waters 2545), system fluidics organizer (waters SFO), photodiode array detector (Waters 2998) and a sample manager (Waters 2767) with Mass lynx data handling system was used along with Modcol column hard ware (50×700 mm) packed with diasogel C-18, 10 μm silica gel having bed length 300 mm was employed for the isolation of impurities. Mobile phase-A (0.5% (v/v) acetic acid in water) and Mobile phase-B (acetonitrile) was chosen with flow rate at 25 ml/min and UV detection was carried out at 254 nm. The gradient program was as follows. Time (min)/A (v/v):B (v/v), T0.01/100:00, T10.0/100:00, T11.0/90:10, T25/90:10, T26.00/80:20, T40.0/80:20, T41.00/75:25, T55.0/75:25, T56.0/70:30, T70/70:30, T71.0/50:50, T80.0/50:50.
Flash chromatography
A grace Revelers flash chromatographic system equipped with quaternary gradient module, UV-VIS detector. Grace make flash cartridge having 80 gm silica and 40 micron practical size was employed for the isolation of the impurities. Mobile phase-A consists of dichloromethane and Mobile phase-B consists of 0.1% triethyl amine in methanol. Flow rate was kept at 35 ml/min and UV detection was carried out at 225 nm. The gradient program was as follows. Time (min)/A (v/v): B (v/v), T0.01/100:00, T10.0/100:00, T11.0/95:05, T21/95:05, T22.00/92:08, T32.0/92:08, T33.00/90:10, T43.0/90:10, T44.0/85:15, T54.00/85:15, T55.0/80:20, T65.00/80:20, T66.0/75:25, T76.0/75:25, T77/70:30, T87.0/70:30.
NMR spectroscopy
The NMR experiments were performed on Varian NMR spectrometer at 400 MHZ. The 1H chemical shift values were reported on the δ scale in ppm, relative to TMS (δ=0) as internal standard, 13C NMR, DEPT, HMBC and HSQC spectra were also recorded.
Isolation of impurities
2.0 liter of mother liquor obtained after Zolmitriptan purification was concentrated on rotavapour to obtain a thick residue. 8.0 gm of this residue was loaded on flash chromatography using the conditions mentioned in the section Flash chromatography. Eluted fractions from flash chromatography were monitored by HPLC. Zolmitriptan was completely separated from the isomeric impurities. The enriched fraction of the isomeric impurities was further purified by semipreparative HPLC.
In case of each impurity, fractions having more than 90% HPLC purity were combined, concentrated on rotavapour and extracted with dichloromethane. All the four impurities were analyzed by HPLC, 1H NMR, 13C NMR, and MS.
Results and Discussion
Detection of the impurities
During the process development of Zolmitriptan (Figure 2 Scheme), four unknown impurities were found in the range of 0.08- 0.15% by reverse phase liquid chromatography. The sample was subjected to LC-MS using the conditions mentioned in section Liquid Chromatography-Mass Spectroscopy (LC-MS). The LC-MS study revealed that all the four impurities were having the same mass. These were enriched by flash chromatography up to 10% and purified by preparative HPLC, using the conditions described in the section NMR Spectroscopy. For each impurity, fractions having purity more than 90% were combined, concentrated under reduced pressure and extracted with dichloromethane. These impurities were analyzed by HPLC, 1H NMR, 13C NMR, DEPT, HMBC, HSQC and MS.
Structure elucidation of the process impurities
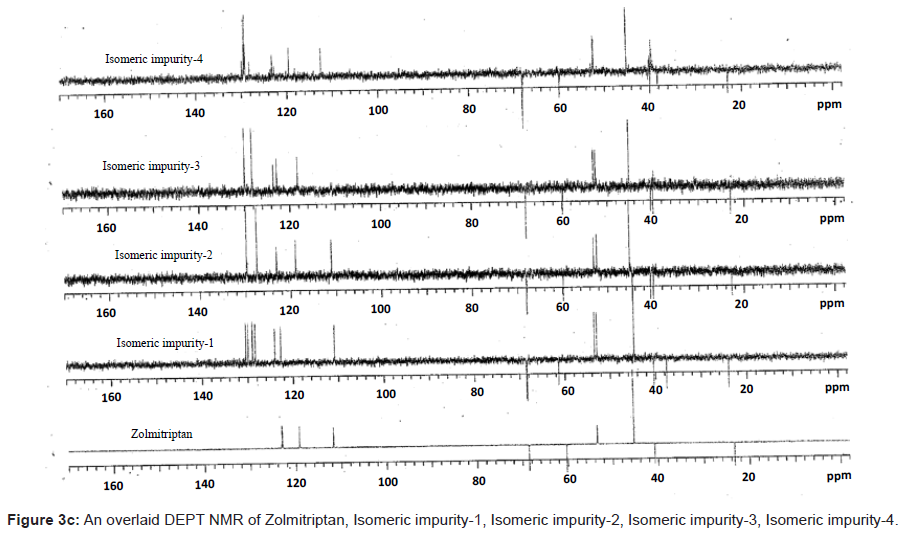
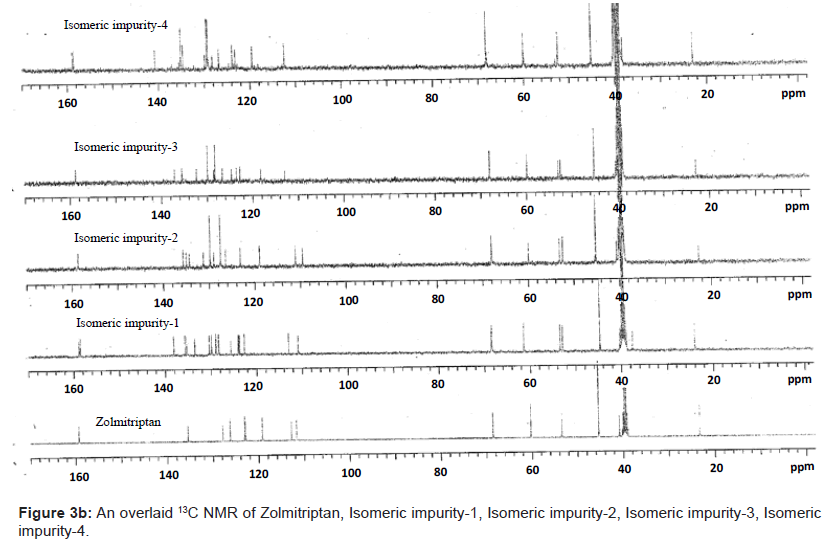
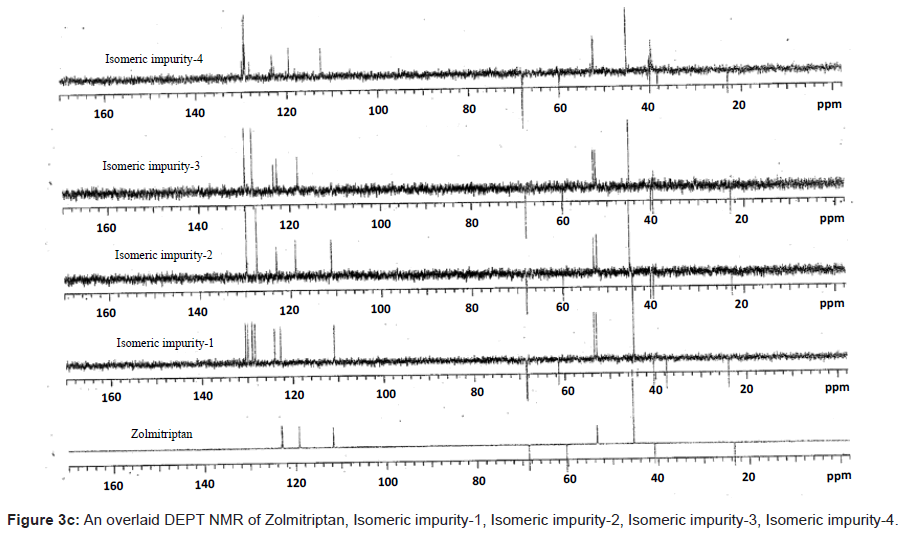
The ESI-MS (positive ion) spectrum of the impurities showed a protonated ion at m/z 462.9 and zolmitriptan showed at m/z 288 amu. The difference of the mass between zolmitriptan and impurities was 175 amu, which indicated the presence of additional benzyl oxazolidinone moiety. The benzyl oxazolidinone could be attached anywhere on the indole ring to form number of isomers. As the MS/MS fragmentation pattern of the impurities could not give information regarding the possible structure of the impurities, the NMR experiments were carried out to confirm the structures. A comparison of 1H NMR spectra of the impurities and Zolmitriptan indicated the presence of 9 additional protons in each case. Three were in aromatic region, four in aliphatic region and one additional exchangeable proton was found. This proved that additional benzyl oxazolidinone moiety substituted on indole was present in these impurities. 13C NMR spectra of impurities showed that 10 additional carbons were present in the impurities as compared to zolmitriptan. Out of the 10 carbon six were aromatic, three were aliphatic and one was carbonyl. DEPT spectral analysis of the impurities showed the presence of six CH2, nine CH and two CH3 carbons. The position of the substitution was confirmed by comparing the 1HNMR and 13CNMR spectra of Zolmitriptan and corresponding isomeric Impurities. The assignment of protons was confirmed by 1H- 1H COSY experiment and the attachments of the protons and carbons were confirmed by HSQC and HMBC experiments in case of each impurity.
Structural elucidation of Impurity-1: In the 1H NMR spectrum of Impurity-1, H-2 exhibited a doublet (7.05 ppm, J=1.6 Hz), where as H-6 (7.29 ppm, J=8.3 Hz) and H-7 (7.1 ppm, J=8.3 Hz) appeared as mutually coupled doublets and H-4 was absent. This indicated that the benzyl oxazolidinone was substituted on C-4. Also a complex splitting pattern was observed for H-22, H-23, H-25 and H-26 indicating presence of o-di-subtituted phenyl group. In 13C NMR spectrum of zolmitriptan C-4(CH) resonated at 119 ppm which was absent in Impurity-1. Also the chemical shift of C-22, C-23, C-25 and C-26 confirmed that benzyl oxazolidine was ortho-sustituted on C-4. From the above spectral information, the structure of the impurity was confirmed as 4-((3-(2-(dimethylamino)ethyl)-4-(2-((oxazolidin-4-yl) methyl)phenyl)-1H-indol-5-yl)methyl) oxazolidin-2-one.
Structural elucidation of Impurity-2: In the 1H NMR spectrum of Impurity-2, H-6 appeared as a double doublet (6.97 ppm, J=8.3, Hz), H-7 as a doublet (7.26 ppm, J=8.3 Hz), H-4 as a singlet (7.37 ppm) and H-2 was absent. This indicated that the benzyl oxazolidine was substituted on C-2. A typical p-di-substituted phenyl pattern was observed with ortho coupling between H-22, C-23 and H-25, H-26 (7.38 and 7.56 ppm, J=8.1 Hz). In 13C NMR spectra of Zolmitriptan, C-2(CH) appeared at 122.86 ppm which was absent in the Impurity-2, also the C-22, C-23, C-25 and C-26 were showing a typical p-disubstituted pattern. This data confirmed that benzyl oxazolidine was substituted on C-2, and the substitution was at para position. From the above spectral information, the structure of the impurity was confirmed as 4-((3-(2-(dimethylamino) ethyl)-2-(4-((oxazolidin-4-yl) methyl) phenyl)-1H-indol-5-yl) methyl) oxazolidin-2-one.
Structural elucidation of Impurity-3: In the 1H NMR spectrum of Impurity-3, H-2 appeared as a doublet (7.1 ppm, J=2 Hz) where as H-6 (6.98 ppm) and H-4 (7.36 ppm) appeared as singlets and H-7 was absent. This indicated that the benzyl oxazolidine was substituted on C-7. Also, a typical p-di-subtituted pehyl pattern was observed with ortho coupling between H-22, H-23 and H-25, H-26 (7.38 and 7.59 ppm, J=7.8 Hz). A comparison with 13C NMR spectra of Zolmitriptan indicated that the C-7(CH) at 111.61 ppm was absent in Impurity-3. Also, the C-22, C-23, C-25 and C-26 were showing a typical p-disubstituted phenyl pattern. This confirmed that benzyl oxazolidine was substituted on C-7 and the substitution was at para position. From the above spectra information, the structure of the impurity was confirmed as 4-((3-(2-(dimethylamino)ethyl)-7-(4-((oxazolidin-4-yl) methyl)phenyl)-1H-indol-5-yl)methyl)oxazolidin-2-one.
Structural elucidation of Impurity-4: In the 1H NMR spectrum of Impurity-4, H-2 appeared as a doublet (7.15 ppm, J=1.6 Hz) while H-7 (7.10 ppm) and H-4 (7.43 ppm) appeared as singlets and H-6 was found to be absent. This indicated that the benzyl oxazolidine was substituted on C-4. A typical p-di-substituted phenyl pattern was observed with ortho coupling between H-22, H-23 and H-25, H-26 (7.23 and 7.30 ppm, J=7.9 Hz). In 13C NMR spectra of Zolmitriptan, C-6(CH) signal at 122.97 ppm was found to be absent in the spectrum of Impurity-4. Also, the C-22, C-23, C-25 and C-26 were showing a typical p-disubstituted phenyl pattern. This confirmed that benzyl oxazolidine was substituted on C-7 and the substitution was at para position. From the above spectral information, the structure of the impurity was confirmed as 4-((3-(2-(dimethylamino)ethyl)-6-(4-((oxazolidin-4-yl) methyl) phenyl)-1H-indol-5-yl)methyl)oxazolidin-2-one.
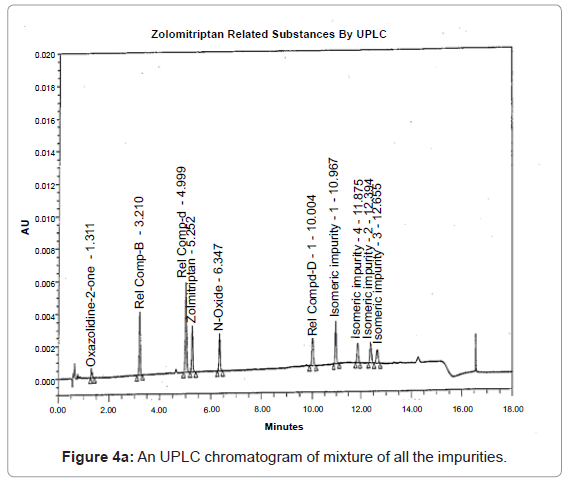
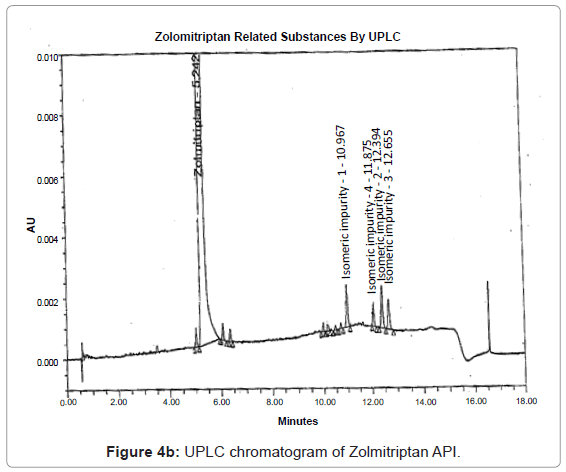
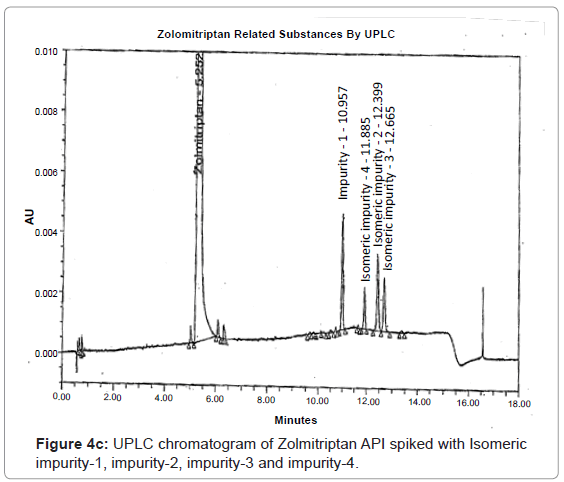
The 1H and 13C NMR chemical shift values of zolmitriptan, isomeric impurity-1, isomeric impurity-2, isomeric impurity-3 and impurity-4 are given in table 1. An overlaid 1H, 13C NMR and DEPT spectra’s of zolmitriptan, isomeric impurity-1, isomeric impurity-2, isomeric impurity-3 and impurity-4 were presented in figures 3a-3c. An UPLC chromatogram of zolmitriptan API and zolmitriptan API spiked with isomeric impurity-1, impuity-2, impurity-3 and impurity-4 are presented in figures 4a-4c.
| Positiona | Zolmitriptan | Isomeric Peak-1 | Isomeric Peak-2 | Isomeric Peak-3 | Isomeric Peak-4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1H, (ppm)(Hz) | 13C | DEPT | 1H, (ppm)(Hz) | 13C | DEPT | 1H, (ppm)(Hz) | 13C | DEPT | 1H, (ppm)(Hz) | 13C | DEPT | 1H, (ppm)(Hz) | 13C | DEPT | |
| 1 | 1H, 10.68 (brs) | - | - | 1H, 10.87 (s) | - | - | 1H, 11.06(s) | - | - | 1H, 10.58(s) | - | - | 1H, 10.72(s) | - | - |
| 2 | 1H, 7.10 (dj=2.08) | 122.97 | CH | 1H, 7.00-7.06(m) | 122.64 | CH | - | 134.28 | Q | 1H, 7.10(d) | 122.76 | CH | 1H, 7.15(d) | 123.19 | CH |
| 3 | - | 112.72 | Q | - | 112.95 | Q | - | 109.45 | Q | - | 112.92 | Q | - | 112.42 | Q |
| 4 | 1H, 7.34(s) | 119.13 | CH | - | 123.7 | Q | 1H, 7.38-7.40(m) | 118.9 | CH | 1H, 7.36-7.40(m) | 118.19 | CH | 1H, 7.44(s) | 119.48 | CH |
| 5 | - | 126.17 | Q | - | 135.26 | Q | - | 128.96 | Q | - | 128.5 | Q | - | 135.08 | Q |
| 6 | 1H, 6.91-6.93(dd) | 122.86 | CH | 1H, 7.00-7.06(m) | 123.96 | CH | 1H, 6.97(d) | 123.15 | CH | 1H, 7.00(s) | 123.54 | CH | - | 123.83 | Q |
| 7 | 1H, 7.24 (d,j=8.24) | 111.61 | CH | 1H,7.20-7.34(m) | 110.82 | CH | 1H, 7.27(d) | 111.02 | CH | - | 124.59 | Q | 1H, 7.09(s) | 112.48 | CH |
| 8 | - | 135.39 | Q | - | 135.62 | Q | - | 134.96 | Q | - | 135.42 | Q | - | 135.08 | Q |
| 9 | - | 127.79 | Q | - | 125.56 | Q | - | 126.35 | Q | - | 126.61 | Q | - | 126.76 | Q |
| 10 | 4H, 2.75-2.91(m) | 40.75 | CH2 | 2H, 2.52-2.93(m) | 40.35 | CH2 | 2H, 2.79-2.97(m) | 40.54 | CH2 | 2H, 2.80-2.95(m) | 40.41 | CH2 | 2H, 2.70-3.01(m) | 40.12 | CH2 |
| 11 | 3H, 4.00-4.249m) | 53.37 | CH | 1H, 3.79-4.39(m) | 53.39 | CH | 1H, 4.02-4.33(m) | 53.09 | CH | 1H, 4.04-4.34(m) | 53 | CH | 1H, 3.69-4.34(m) | 52.47 | CH |
| 12 | 3H, 4.00-4.24(m) | 68.5 | CH2 | 2H, 3.79-4.39(m) | 68.37 | CH2 | 2H, 4.02-4.33(m) | 67.97 | CH2 | 2H, 4.04-4.34(m) | 68.04 | CH2 | 2H, 3.69-4.34(m) | 68.18 | CH2 |
| 14 | - | 159-26 | -C=O | - | 158.47 | -C=O | - | 158.59 | -C=O | - | 158.58 | -C=O | - | 158.45 | -C=O |
| 16 | 1H, 7.75(s) | - | - | 1H, 7.52(s) | - | - | -1H, 7.76(s) | - | - | 1H, 7.80(s) | - | - | 1H, 7.80(s) | - | - |
| 17 | 2H, 2.48-2.52(m) | - | - | 2H, 1.80-2.07(m) | 23.96 | CH2 | 2H, 2.79-2.97(m) | 22.7 | CH2 | 2H, 2.80-2.95(m) | 22.98 | CH2 | 2H, 2.70-3.01(m) | 23.01 | CH2 |
| 18 | 4H, 2.75-2.91(m) | - | - | 2H, 1.80-2.07(m) | 61.38 | CH2 | 2H, 2.50-2.53(m) | 59.89 | CH2 | 2H, 2.53(t) | 59.85 | CH2 | 2H, 2.54(t) | 59.99 | CH2 |
| 20 | 6H, 2.21(s0 | - | - | 3H, 1.80-2.07(m) | 44.65 | CH3 | 3H, 2.23(s) | 45.12 | CH3 | 3H, 2.23(s) | 45.12 | CH3 | 3H, 2.24(s) | 45.12 | CH3 |
| 21 | 6H, 2.21(s) | - | - | 3H, 1.80-2.07(m) | 44.65 | CH3 | 3H, 2.23(s) | 45.12 | CH3 | 3H, 2.23(s) | 45.12 | CH3 | 3H, 2.24(s) | 45.12 | CH3 |
| 22 | - | - | - | - | 138.06 | Q | 1H, 7.38-7.40(m) | 129.75 | CH | 1H, 7.36-7.40(m) | 129.83 | CH | 1H, 7.31(d) | 129.15 | Q |
| 23 | - | - | - | 1H, 7.20-7.34(m) | 129-84 | CH | 1H, 7.56(d) | 127.48 | CH | 1H, 7.59(d) | 128.21 | CH | 1H, 7.25(d) | 129.4 | CH |
| 24 | - | - | - | 1H, 7.20-7.34(m) | 128.27 | CH | - | 135.65 | Q | - | 137.05 | Q | - | 140.69 | CH |
| 25 | - | - | - | 1H, 7.20-7.34(m) | 128.87 | CH | 1H, 7.56(d) | 127.48 | CH | 1H, 7.59(d) | 128.21 | CH | 1H, 7.25(d) | 129.4 | Q |
| 26 | - | - | - | 1H, 7.20-7.34(m) | 130.32 | CH | 1H, 7.38-7.40(m) | 129.75 | CH | 1H, 7.36-7.40(m) | 129.83 | CH | 1H, 7.31(d) | 129.15 | CH |
| 27 | - | - | - | - | 133.48 | Q | - | 131.24 | Q | - | 132.25 | Q | - | 134.61 | CH |
| 28 | - | - | - | 2H, 2.52-2.93(m) | 37.53 | CH2 | 2H, 2.79-2.97(m) | 39.92 | CH2 | 2H, 2.80-2.95(m) | 39.92 | CH2 | 2H, 2.70-3.01(m) | 38.3 | CH2 |
| 29 | - | - | - | 1H, 3.79-4.39(m) | 52.85 | CH | 1H, 4.02-4.33(m) | 52.36 | CH | 1H, 4.04-4.34(m) | 52.53 | CH | 1H, 3.69-4.34(m) | 52.36 | CH |
| 30 | - | - | - | 2H, 3.79-4.39(m) | 68.49 | CH2 | 2H, 4.02-4.33(m) | 68.03 | CH2 | 2H, 4.04-4.34(m) | 68.04 | CH2 | 2H, 3.69-4.34(m) | 68.18 | CH2 |
| 32 | - | - | - | - | 158.79 | -C=O | - | -158.64 | -C=O | - | 158.69 | -C=O | - | 158.74 | -C=O |
| 34 | - | - | - | 1H, 7.67(s) | - | - | -1H, 7.83(s) | - | - | 1H, 7.82(s) | - | - | 1H, 7.54(s) | - | - |
s: singlet; d: doublet; brs: broad singlet; J: coupling constant; Q: quaternary carbon.
aRefer structures for numbering (Figure 1)
Table 1: 1H, 13C NMR, and DEPT data of Zolmitriptan, Impurity-1, Impurity-2, Impurity-3, Impurity-4.
Conclusion
In conclusion, we have successfully identified impurities which were detected during the batch analysis of final API. Subsequent experiments have enabled us to identify the critical parameters to control of the impurities below the acceptable level at laboratory scale well as commercial scale.
Acknowledgement
The author gratefully acknowledges the management of the Emcure Pharmaceuticals Ltd for providing the facility/instruments for the research work. Authors would also like to thank colleagues of Analytical Research Department (ARD) for their support.
References
- Johnson KW, Schaus JM, Durkin MM, Audia JE, Kaldor SW, et al. (1997) 5-HT1F receptor agonists inhibit neurogenic dural inflammation in guinea pigs. Neuroreport 8: 2237-2240.
- Whale R,Bhagwagar Z,Cowen PJ (1999) Zolmitriptan induced growth hormone release in humans mediated by 5-HT1D receptors. Psychopharmacology (Berl) 145: 223-226.
- Dixon R, Warrander A (1997) The clinical pharmacokinetics of zolmitriptan. Cehpalalgia 18: 15-20.
- Zhang Z, Xu F, Tian Y, Li W, Mao G (2004) Quantification of zolmitriptan in plasma by high-performance liquid chromatography-electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 813: 227-233.
- Clement EM, Franklin M (2002) Simultaneous measurement of zolmitriptan and its major metabolites N-desmethylzolmitriptan and zolmitriptan N-oxide in human plasma by high-performance liquid chromatography with coulometric detection. J Chromatogr B Analyt Technol Biomed Life Sci 766: 339-343.
- Chen J, Jiang XG, Jiang WM, Mei N, Gao XL, Zhang QZ, et al. (2004) High performance liquid chromatographic analysis of zolmitriptan in human plasma using fluorescence detection. J Pharm Biomed Anal 35: 639-645.
- Vijayakumar EK, Samel MA, Bhalekar SB, Pakhale SM (2010) A new stability Indicating HPLC method for related substances in zolmitriptan. Ind J Pharm Sci 72: 119-122.
- Rao BM, Srinivasu MK, Sridhar G, Kumar PR, Chandrasekhar KB, et al. (2005) A stability indicating LC method for zolmitriptan. J Pharm Biomed Anal 39: 503-509.
- Miglani A, Kumar P, Negi B, Gautam HD (2010) Isolation and characterization of the zolmitriptan unknown impurity by chromatographic and mass spectroscopy. Anal Chem 9: 12-16.
- Dousa M, Gibala P, Radl S, Klecan O, Mandelova Z, et al. (2012) Identification, Preparation and UHPLC determination of process-related impurity in zolmitriptan. J Pharm Biomed Anal 58: 1-6.
- ICH guideline (2002) Guidance for Industry-Q3A Impurities in New Drug Substances. FDA
- Robertson AD, Hill AP, Glen RC, Martin GR (1995) Indolyl compounds for treating migraine. US Patent 5: 466-699.
- Rajnikant P (1997) One Pot Synthesis Of 2-Oxazolidinone Derivatives. World patent 97/06162.
- Aminul I, Chandan B, Sahadev K (2007) Process for preparing optically pure zolmitriptan. EP Patent EP1799675 A1.
- Bhattacharya S (1994) Titanium(IV) isopropoxide and sodium borohydride : A reagent of choice for reductive amination. Tetrahedron Lett 35: 2401-2404.
- Van Der Schaaf PA, Blatter F, Szelagiewicz M, Berens U, Susan DP (2007) Crystalline forms of zolmitriptan. US Patent US2007/0173536A1.
- Volsar M, Zatopkova M, Ridvan L, Pekarek T (2010) Method for the preparation of zolmitriptan. US Patent US2010/0105919A1.
- Barjoan PD,Asparo MA (2004) Process for Preparing Zolmitriptan Compounds. Montserrat WO2004/014901A1.
- Bhujang Rao, Nannapanen Venkaiah Chowdary (2004) A Novel And An Improved Process For The Preparation Of (S)-4-(4-Aminobenzyl)-2- Oxazolidinone. WO2004/063175A1.
- Aminul I, Chandan B, Sahdev K (2005) Process For Preparing Optically Pure Zolmitriptan. WO2005/105792 A1.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 19360
- [From(publication date):
April-2013 - Oct 25, 2025] - Breakdown by view type
- HTML page views : 14493
- PDF downloads : 4867