Research Article Open Access
IPX-750, A Dopamine Gluconamine That Binds D1/D5 Receptors and Has Anti-Parkinsonian Effects in Three Animal Models, is Transported across the Blood Brain Barrier
Roger Laine1 and M. Tino Unlap2*1Department of Biological Sciences, Louisiana State University, USA
2Departments of Clinical and Diagnostic Sciences, Biochemistry and Molecular Genetics, University of Alabama at Birmingham, Birmingham, AL 35294
- Corresponding Author:
- M. Tino Unlap
Department of Clinical and Diagnostic Sciences
University of Alabama at Birmingham, Birmingham, USA
Tel: 205-934-7382
E-mail: unlap@uab.edu
Received date: July 09, 2012; Accepted date: July 25, 2012; Published date: July 28, 2012
Citation: Laine R, Unlap MT (2012) IPX-750, A Dopamine Gluconamine That Binds D1/D5 Receptors and Has Anti-Parkinsonian Effects in Three Animal Models, is Transported across the Blood Brain Barrier. J Biotechnol Biomater 2:142. doi:10.4172/2155-952X.1000142
Copyright: © 2012 Laine R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Dopamine does not penetrate the blood-brain barrier and IPX 750, a dopamine glycoconjugate, is designed to improve dopamine transport across the blood brain barrier. IPX-750 binds to D1/D5 receptors in vitro and eliminates Parkinson’s disease symptoms in three animal models. These studies were conducted to confirm the bioavailability of IPX-750 following intraperitoneal and gavage administration in rats by detecting IPX-750 in plasma, blood cell, liver and brain extracts. The signature mass ions detected by mass spectrometry in blood, brain and liver confirmed the presence of IPX 750 at 90 min post-administration. Blood levels, (cells + plasma) recovered from samples at 30-90 min after intraperitoneal or gavage administration of 200mg/kg, were 1.4-6.2 μM (600-3200 pg/ml). Blood levels achieved at 30-90 min post-gavage administration were 31-96% of those achieved following intraperitoneal injection. IPX-750 associated with blood cells was 40-130% of that in plasma 60-90 min after intraperitoneal or gavage administration. Brain and liver levels of IPX-750 were 59-195 pg/mg tissue at 30-90 min post-gavage and 900-4700 pg/mg tissue post-ip administration. Following gavage administration brain levels were 55%, 331% and 90% of the levels in the liver at 45, 60 and 90 min. Post-administration with calculated brain penetration indices of 0.6, 3.3 and 0.9.
Keywords
IPX-750; Dopamine Glycoconjugate; Parkinson’s disease; Dopamine Receptors; Blood Brain Barrier
Background
Parkinson’s disease involves degeneration of the dopaminergic neurons in the substantia nigra. Replacement therapy with levodopa/ carbidopa is the most common treatment for Parkinson’s disease [1-3]. Levodopa has multiple side-effects [4] and crosses the blood-brain barrier (BBB) poorly [5]. Glycon LLC has developed a dopamine gluconamine (IPX-750) that is designed to cross the BBB [6]. Since dopamine itself does not cross the BBB [7], the gluconamine conjugation should increase its transport via glycosyl transporters (GLUT), dopamine transporters (DAT) and dopamine receptors in luminal cells in the GI tract, endothelial cells in the BBB, and dopaminergic neural cells in the substantia nigra. In our preliminary studies, IPX-750 exhibits biological activity at D1 and D5 receptors in vitro with potent anti-parkinsonian effects in three different rodent animal models.
Dopamine (DA) does penetrate the blood-brain barrier and the brain and central nervous system are dependent upon endogenous dopamine synthesis [7-10]. While levodopa (L-dopa) has been used in treatments of PD since the 1960s, chronic adaptation to drug and possible levodopa-induced dyskinesia constitute significant limitations [11]. Dopaminergic agonist therapies do not address the metabolic defect in Parkinson’s disease and so there is a critical need for the development of better drugs with greater efficacies and lesser side effects.
We have described synthesis of a novel class of glycosyl N-linked pro-drug compounds designed to access blood brain barrier glycosyl transporter (GLUT) systems while retaining stereospecificity of binding at intestinal Na+/glucosyl co-transporters (SGLT1), dopamine transporter (DAT) and dopaminergic receptors of the D1 and/or D2 families. Subsequently, related compounds and GLUT delivery approaches have confirmed, in principle, both the utility of this chemical design concept and certain of the inherent structural limitations [12-14]. In this regard, pharmacologic studies conducted over the past 20 years have suggested relatively stringent structural requirements for binding and activation at D1 and D2 receptors, particularly with regard to substitutions in and around the dopamine amine nitrogen. Similarly, DAT transporter studies have suggested sensitivity to both N substitution and conformational ring structure. Stereospecificity has also been evidenced in glycosyl transporter studies, e.g. for SGLT, even with vesicle transport and exocytosis net rates estimated to be in the range of 10 thousand to 1 million per second, studies suggest substitution specificity for pyranose sugars that is apparently discriminatory between hundreds of different monosaccharide types and mutarotational.
Our preliminary studies in three animal models of Parkinson’s disease show that IPX-750 has potent anti-parkinsonian effects [15]. The dopaminergic neurotoxin 1,2,3,6-methyl-phenyl-tetrahydropyridine (MPTP) is a chemical that produces Parkinson’s-like neuropathological changes and clinical features in man, monkey and mice [16]. In Sprague Dawley rats, Parkinson’s-like symptoms similar to the human condition are produced by treating these animals with 6-OHDA, a dopamine-depleting drug. The nigral dopamine depletion is induced by using unilateral injections of 6-OHDA directly into the nigrostriatal system (AP: -4.4 mm, ML: 1.1 mm, DV: 7.5 mm, AP: -4.0 mm, ML: 0.8 mm, DV: 7.8 mm). As a result of the 6-OHDA dopamine depletion dopaminergic receptors in post-synaptic terminals are ‘hyper-sensitized’ to the action of dopaminergic compounds. Thus, animals treated with 6-OHDA having the unilateral lesion will exhibit an uncontrolled ‘spinning’, circular wandering and other rotational behaviors after the administration of apomorphine and certain other dopaminergic agonists [17,18]. Finally, genetic knockout mice having defective expression of the Nurr1 gene exhibit age-related selective agenesis at dopaminergic neurons in the midbrain, i.e., similar to the genetic defects observed in patients with Parkinson’s disease [19]. Nurr1 has recently been discovered to act as a transcription regulator of tyrosine hydroxylase gene expression and cohorts of Parkinson’s patients with Nurr1 mutations have been identified [20]. Using the Rota-Rod performance test, our preliminary studies show that IPX- 750 at 20 mg/kg possesses anti-Parkinson’s effects in MPTP-lesioned mice, 6-OHDA-lesioned rats and Nurr1 +/- knockout mice and all three models exhibit similar efficacy to when administered at 80 mg/ kg [15,21].
Therefore, because of the positive in vitro and in vivo data obtained for IPX-750 studies, the current studies were conducted in order to assess the ability of this dopamine glycoconjugate to cross into the brain.
Materials and Methods
Animals and animal dosing
Out-bred rats of about 100-300 g and about 3-4 months age were acquired in both sexes from a local source in Baton Rouge, LA. Calculations used in defining animal dosages were done in order to administer 200 mg IPX-750/kg of animal via intraperitoneal or gavage administration.
Veterinary assistance
IPX-750 was skillfully administered by gavage under a light isofluorane anesthesia by C.B. Hackett, DVM in his veterinary surgical suite at 4803 Perkins Rd, Baton Rouge, LA 70808.
IPX-750
IPX-750 was synthesized as described in US Patent 6,548,484. The same lot of compound used in animal model efficacy testing at Baylor College of Medicine was used for all the analyses described in the present report.
Pharmacokinetic studies
IPX-750 levels in blood, liver and brain were evaluated by administering IPX-750 in sterile PBS, pH 7.1 (10 mM sodium phosphate buffered, pH 7.1, 0.14 M saline) at one of the two different test doses, i.e., either 50 mg/ml (intraperitoneal) or 100 mg/ml (gavage). Animals were weighed and the volume of dose was adjusted to achieve 200 mg/ kg. Blood and tissue samples were collected at 30, 60 and 90 min for intraperitoneal dosing or, (for logistical reasons), 45, 60 and 90 min for gavage dosing.
Collection of samples
Under ether anesthesia, blood was collected by cardiac puncture into a 10 ml syringe containing a volume of sterile 20 mM EDTA sufficient to achieve a final EDTA concentration of 2-4 mM. Cardiac puncture was achieved by surgical cut-down under ether anesthesia. Blood was stored at 2-4°C in an ice water bath until processing. Liver tissue was removed and immediately frozen on dry ice. The cranium of test animals was removed and frozen on dry ice for later surgical removal of brain tissues. Tissue samples were stored at -20°C until processing.
Processing of blood and tissue samples
Blood was separated into cell and plasma fractions by centrifugation 10,500 rpm for 10 min. in an Eppendorf microfuge. Plasma was carefully removed and it and the packed red cell pellet were stored frozen -20°C until use. For GC/MS analysis, the frozen samples were lyophilized (Labconco freeze dry apparatus) overnight and the resultant powders were stored at 20°C until GC/MS analysis.
For GC/MS analysis samples were partially thawed, the brain right hemisphere and portions of the liver were removed and placed in a Petri dish. Twenty to forty milligram portions of these tissues were clipped, weighed, placed in microfuge tubes and lyophilized overnight.
GC/MS Analysis
Levels of IPX-750 in blood and tissue samples were quantified by preparing trimethylsilyl derivatives and measuring the levels of signature mass ions in lyophilized aliquots of plasma, packed blood cells and tissues and by comparing those levels against internal and external reference standards.
Trimethylsilyl (TMS) derivatives were prepared by adding 100 μl of BS-TFA/1% TMCS reagent (Pierce, #38833; N.O.-bis(trimethylsilyl) trifluoroacetamide/1% trimethylchlorosilance) directly to the dried lyophilized tissue or to the lyophilized blood fractions, i.e., constituting about 500 μl of lyophilized plasma and 500 μl of lyophilized blood cells in each of the two separate samples. For initial qualitative studies, sample TMS derivatization was accomplished at 70-100°C/10-15 min resulting in both N-derivatized and non-N-derivatized test compound. For quantitative studies, derivatization was at room temperature resulting in different proportions of N-derivatization, i.e., referred to as Peak “A” (lacking the N-derivatization) and Peak “B” (having the -N-TMS derivatization). Operationally, 100 μl TMS was added to the dry powder samples in microfuge tubes; 1μl of PAGP standard was carefully added to the solution phase; samples were then mixed with the TMS reagent in a water bath sonicator for 3-4 minutes and the dry insoluble tissue powder was removed by centrifugation (microfuge, 10 K rpm, 6 min). For GC/MS analysis, 1μl aliquots of the centrifugal supernatant were carefully removed and injected onto the GC column.
GC/MS sample:For GC/MS analysis 1μl of the TMS-derivatized test sample was injected onto the GC column.
Quantitative analyses:Temperature conditions for quantitative analysis of IPX-750 levels in tissue and blood were: 10 minutes at 60°C; followed by, a temperature ramp from 60°C to 300°C over the next 15 min; followed by, 10 min at 300°C before cooling for the next run. Under these conditions of analysis, IPX-750 mass ions were detected in GC peaks eluting at 12.3-13.1 min (Peaks “A”) and at 13.8 14.6 min (Peaks “B”). IPX-750 exhibited a complete TMS-derivatized molecular ion having a mass of 822, as well as, a major ion at 805-806, i.e., the complete TMS-derivatized molecular ion minus one methyl group (822-15=807; the 1 mass unit difference being within the normal operational specifications of the instrument). Mass spectrometry was used to select the GC peaks containing ions having a mass of 805.5- 806.5. The values for the integrated peak area of each eluted Peak “A” and Peak “B” were determined and the values were added together to calculate the total concentration of IPX-750 in each test sample.
IPX-750 concentration was determined by adding a reference standard to each test sample, i.e., 1μg/1μl PAGP (phenyl-2-acetamidodeoxy- αD-glucopyranoside)/100 μl test sample to achieve a final concentration of 10 ng/1μl in the GC/MS injection volume.
Results and Discussion
Lack of acute toxicity
At the test dose of 200mg/kg no obvious signs of toxicity were observed following intraperitoneal or gavage administration: i.e., respiration appeared normal; no panting was observed; recovery from ether anesthesia was normal; no changes in grooming behavior were observed; animal activity appeared normal without any obvious evidence of lethargy or staggering; and, the heart, lungs, intestines and liver appeared normal on gross necropsy examination.
Confirmation of IPX-750 in blood cells, plasma and tissue samples
Standards: IPX-750 standards when heated (110-120°C) in the presence of trimethyl silylating reagent (BS-TMCS) formed both –O-TMS and –N-TMS derivatives, i.e., involving optional derivatization of the nitrogen atom in the alkyl linker chain between the dopamine ring and the glucosyl sugar. Under the conditions of initial assay, (i.e., a steady 60ºC for 12 min and then a 60°C to 300°C temperature ramp at a rate of 30°C/min; followed by a steady 10 min at 300°C), the –O-TMS derivatized IPX-750 ran in a GC peak having a retention time of about 15.7-15.99 min. (Peak “A”) and the –N-TMS derivatized IPX-750 ran in a GC peak having a retention time of about 17.62-17.72 (Peak “B”). Each of these discrete GC peaks contained IPX- 750 signature mass ions. Therefore, prior to substantive quantitative analysis, the presence of IPX-750 was confirmed in tissue and blood samples by searching these samples for the “A” and “B” GC peaks and confirming the presence of the peaks by examining the constituent mass ions.
Qualitative studies to detect IPX-750 in blood cells, plasma and brain: IPX-750 exhibited prominent degradative mass ions of 554 (the sugar + N-bridge minus the dopamine ring); 310 (the sugar minus the N-bridge and dopamine ring); and, 281 (the dopamine ring minus the N-bridge and sugar). The combination of all three mass ions within a single GC peak served to identify IPX-750 in the peak. Specificity of the analysis was confirmed with (a) the finding of both “A” and “B” GC peaks in all of the samples tested; and, (b) the finding of all major signature IPX-750 mass ions in each of the “A” and the “B” peaks. These qualitative results confirm the presence of intact IPX-750 in the plasma fraction, the red blood cell fraction and in brain tissue of animals receiving 200 mg/kg by either the intraperitoneal or gavage routes of administration, respectively.
IPX-750 Initial quantification in test samples
Quantitative studies: IPX-750 exhibited a major compound ion having a mass of 805-807. The complete TMS derivatized (trimethyl silyl) compound has a calculated molecular mass of 822; TMS easily loses one or more methyl groups; and, a methyl group has a calculated mass of 15; thus, it is assumed that the major 805-807 mass ion of IPX-750 represents the complete derivatized compound minus one methyl group. Relatively few GC peaks in rat tissue and blood extracts contained the 805-807 mass ion; thus, selecting for this ion served as a good marker for identifying IPX-750 GC peaks in tissue and blood samples. Similarly, the TMS-derivatized internal standard PAGP (phenyl-2-acetamido-deoxy-αD-glucopyranoside) had a calculated molecular mass of 497; exhibited a major molecular mass ion of 498; and the GC peaks with this mass ion had retention times in the range of 10.12-10.26 min. Operationally for IPX-750, GC peaks containing the 805.5-806.5 mass ions were selected; the retention time of the selected GC peaks was compared to that of IPX-750 standard (i.e., 12.3-13.2 min and 13.8-14.6 min); and, the peak area of the GC peaks with the appropriate mass ions and the proper retention time were determined and recorded. For the PAGP internal standard, GC peaks containing the 497.5-498.5 mass ions were selected; the retention time of the selected GC peaks was compared to that of PAGP standard (i.e., 10.12-10.26 min.); and, the peak area of the GC peaks with the appropriate mass ions and the proper retention time were determined and recorded.
Calculations: Different amounts of IPX-750 were added to a constant amount of PAGP internal standard and the sum of the GC peak heights was determined. The total signal increased in direct linear relation to the amount of IPX-750 added, e.g. addition of an equal amount of IPX 750 resulted in a doubling of the total GC peak height. Therefore, concentrations were determined in test samples by spiking each sample with PAGP (10ng/1μl injection volume); determining the area under the PAGP and IPX-750 GC peaks; dividing the IPX- 750 peak area by the PAGP peak area to achieve a “peak ratio” value; multiplying the peak ratio times 10ng (the amount of PAGP in a 1μl GC injection volume); adjusting the values for the total volume of tissue/ blood extract (i.e., 100 μl); and finally, adjusting the values for the total blood volume (i.e., 0.5 ml of packed blood cells; 0.5 ml of plasma) or wet weight of tissue (i.e., 20-40mg).
5.3.3. Sample preparation: Lyophilization was selected as the method of choice for processing samples for GC/MS because of the propensity of IPX-750 for associating with cells and proteins which we previously discovered in our preliminary studies. It has been reported previously that during lyophilization, the slow formation of ice crystals concentrates salts into the residual fluid volume of the ice matrix so that the microenvironment can have salt concentrations approaching 6-8M. Theoretically, proteins within the ice matrix should be gradually exposed to increasing salt concentration; thus, minimizing protein denaturation. It was reasoned that the gradual increasing of salt concentration might release protein- and cell-bound IPX-750. Derivatization with TMS is designed to reduce the exposed charge on compounds, theoretically reducing protein and cell binding. Thus,in an effort to capture released IPX-750 in the lyophilized product, derivatization reagent (TMS) was added directly to the lyophilized plasma, blood cell or tissue powder. As of the present date, no experiments have been conducted to determine the recovery and will be the subject of future studies.
Blood levels of IPX-750 following intraperitoneal or gavage administration
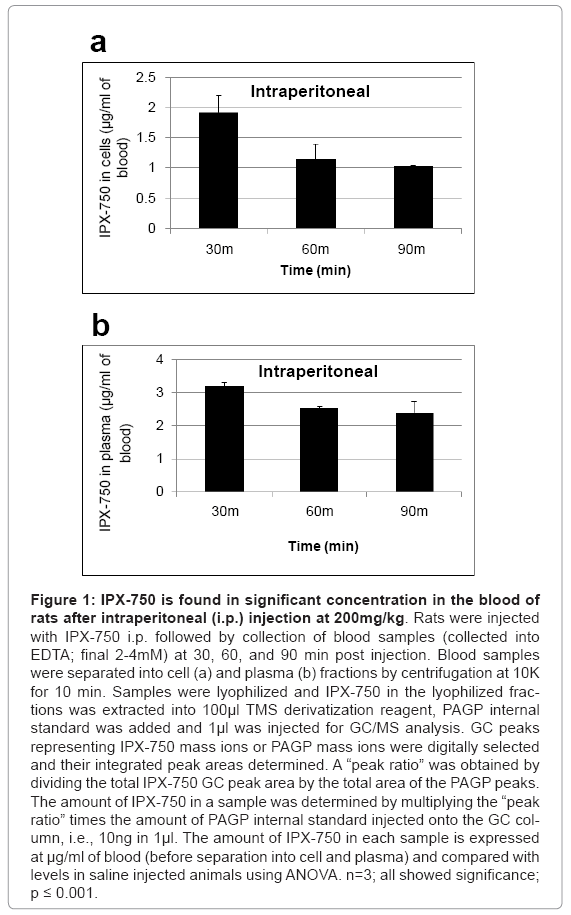
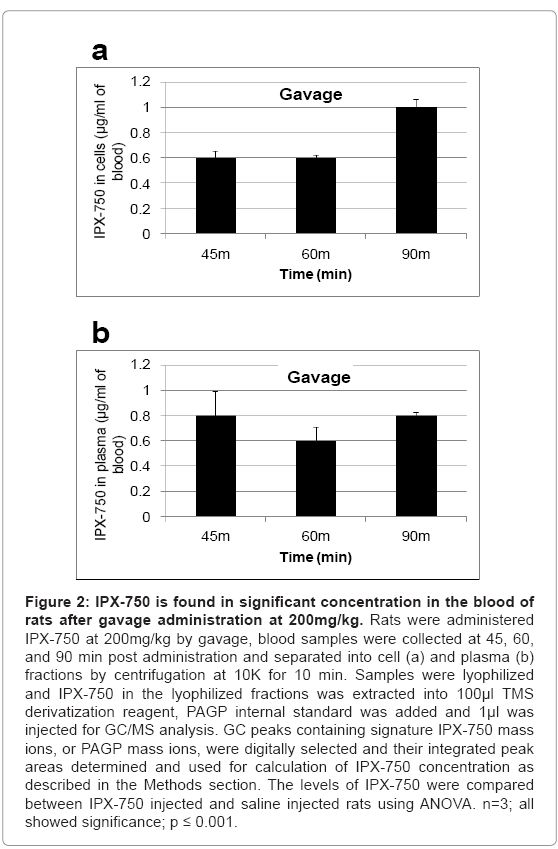
Blood samples (collected into EDTA; final 2-4 mM) were separated into plasma and cell fractions by centrifugation (microfuge; 10K; 10 min). IPX-750 in the lyophilized fractions was extracted into 100 μl TMS derivatization reagent (as described above), PAGP internal standard was added (as described above) and 1μl was injected for GC/ MS analysis. GC peaks containing signature IPX-750 mass ions, or PAGP mass ions, were digitally selected and their integrated peak areas determined. A “peak ratio” was obtained by dividing the total IPX-750 GC peak area by the total area of the PAGP peaks. The amount of IPX- 750 in a sample was determined by multiplying the “peak ratio” times the amount of PAGP internal standard injected onto the GC column, i.e., 10 ng in 1μl. For blood samples, the peak areas, peak ratios and calculations are summarized and presented in Figures 1 and 2. Not surprisingly, blood levels were about 1.9 to 3-fold higher at 30-90 min. post-IP administration relative to gavage administration. Interestingly, the calculated blood cell levels of IPX-750 at 90 min post-gavage were 96% of those achieved by IP administration. No attempts were made in the present studies to determine the rates of intestinal absorption of IPX-750 or of possible red blood cell loading with IPX-750 in the intestinal microvasculature.
Figure 1: IPX-750 is found in significant concentration in the blood of rats after intraperitoneal (i.p.) injection at 200mg/kg. Rats were injected with IPX-750 i.p. followed by collection of blood samples (collected into EDTA; final 2-4mM) at 30, 60, and 90 min post injection. Blood samples were separated into cell (a) and plasma (b) fractions by centrifugation at 10K for 10 min. Samples were lyophilized and IPX-750 in the lyophilized fractions was extracted into 100μl TMS derivatization reagent, PAGP internal standard was added and 1μl was injected for GC/MS analysis. GC peaks representing IPX-750 mass ions or PAGP mass ions were digitally selected and their integrated peak areas determined. A “peak ratio” was obtained by dividing the total IPX-750 GC peak area by the total area of the PAGP peaks. The amount of IPX-750 in a sample was determined by multiplying the “peak ratio” times the amount of PAGP internal standard injected onto the GC column, i.e., 10ng in 1μl. The amount of IPX-750 in each sample is expressed at μg/ml of blood (before separation into cell and plasma) and compared with levels in saline injected animals using ANOVA. n=3; all showed significance; p ≤ 0.001.
Figure 2: IPX-750 is found in significant concentration in the blood of rats after gavage administration at 200mg/kg. Rats were administered IPX-750 at 200mg/kg by gavage, blood samples were collected at 45, 60, and 90 min post administration and separated into cell (a) and plasma (b) fractions by centrifugation at 10K for 10 min. Samples were lyophilized and IPX-750 in the lyophilized fractions was extracted into 100μl TMS derivatization reagent, PAGP internal standard was added and 1μl was injected for GC/MS analysis. GC peaks containing signature IPX-750 mass ions, or PAGP mass ions, were digitally selected and their integrated peak areas determined and used for calculation of IPX-750 concentration as described in the Methods section. The levels of IPX-750 were compared between IPX-750 injected and saline injected rats using ANOVA. n=3; all showed significance; p ≤ 0.001.
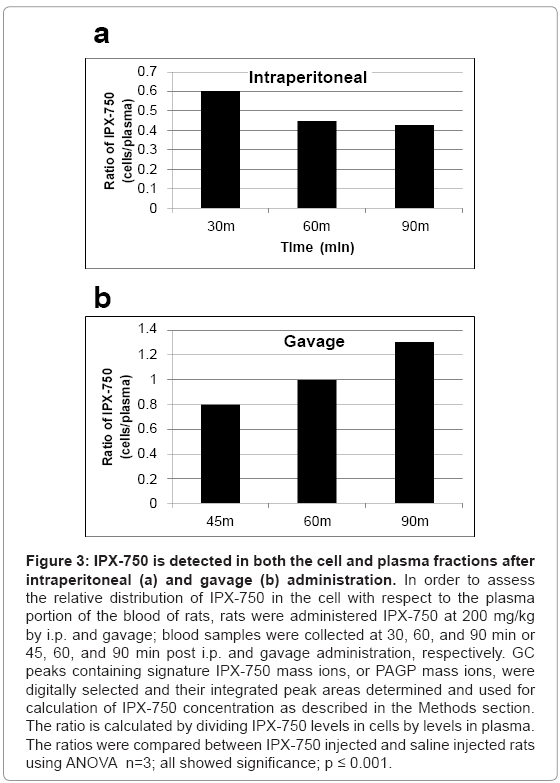
Blood cell levels of IPX-750 relative to plasma levels
Figure 3 represents the relative levels of IPX-750 in the plasma and blood cell fractions at different times after intraperitoneal or gavage administration. The levels of IPX-750 associated with blood cells were 43-130% of the levels in plasma.
Figure 3: IPX-750 is detected in both the cell and plasma fractions after intraperitoneal (a) and gavage (b) administration. In order to assess the relative distribution of IPX-750 in the cell with respect to the plasma portion of the blood of rats, rats were administered IPX-750 at 200 mg/kg by i.p. and gavage; blood samples were collected at 30, 60, and 90 min or 45, 60, and 90 min post i.p. and gavage administration, respectively. GC peaks containing signature IPX-750 mass ions, or PAGP mass ions, were digitally selected and their integrated peak areas determined and used for calculation of IPX-750 concentration as described in the Methods section. The ratio is calculated by dividing IPX-750 levels in cells by levels in plasma. The ratios were compared between IPX-750 injected and saline injected rats using ANOVA n=3; all showed significance; p ≤ 0.001.
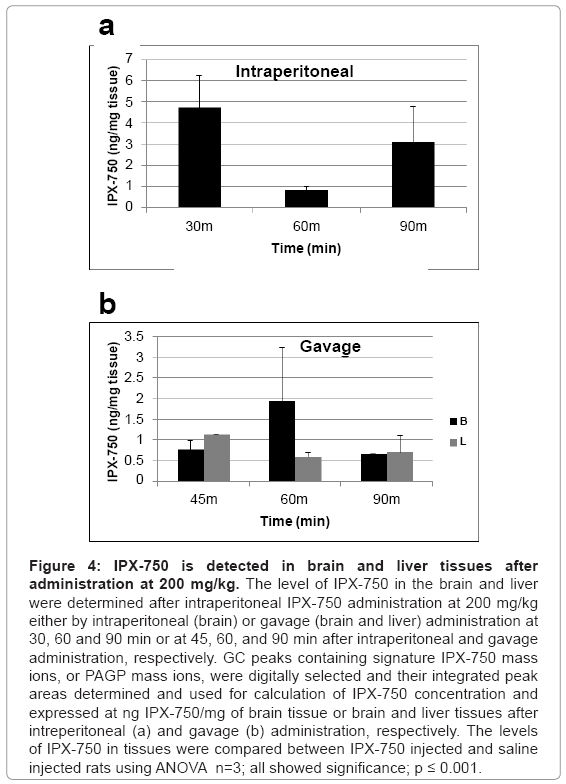
Brain and liver levels of IPX-750 following intraperitoneal or gavage administration
Figure 4 shows the levels of IPX-750 brain tissues or brain and liver tissues at times following IP or gavage administration, respectively. The data shows that detectable levels of IPX-750 were present in the brain and liver at 30-90 min after administration of IPX-750. Brain levels in animals administered IPX-750 by gavage were 55-331% of the extracted liver levels, i.e., brain penetration indices of 0.55, 3.31 and 0.90 (Figure 5).
Figure 4: IPX-750 is detected in brain and liver tissues after administration at 200 mg/kg. The level of IPX-750 in the brain and liver were determined after intraperitoneal IPX-750 administration at 200 mg/kg either by intraperitoneal (brain) or gavage (brain and liver) administration at 30, 60 and 90 min or at 45, 60, and 90 min after intraperitoneal and gavage administration, respectively. GC peaks containing signature IPX-750 mass ions, or PAGP mass ions, were digitally selected and their integrated peak areas determined and used for calculation of IPX-750 concentration and expressed at ng IPX-750/mg of brain tissue or brain and liver tissues after intreperitoneal (a) and gavage (b) administration, respectively. The levels of IPX-750 in tissues were compared between IPX-750 injected and saline injected rats using ANOVA n=3; all showed significance; p ≤ 0.001.
Figure 5: The brain penetration index for IPX-750 is highest at 60 min after administration by gavage. To determine the relative distribution of IPX-750 in the brain and liver tissues, the level of IPX-750 in the brain in ng/ mg of tissue is divided by the amount of IPX-750 in the liver at the same time point. This number is the brain penetration index.
Conclusion
The present study was the first designed to detect IPX-750 in tissue and blood samples and, if possible, estimate the levels of compound achieved in tissues. The results presented here show: 1) the feasibility of GC/MS for monitoring tissue and blood levels and confirm the presence of IPX-750 in blood and tissues, including brain, following intraperitoneal or gavage dosing; 2) the presence of IPX-750 in the blood, liver and brain tissues after intraperitoneal and gavage administration, and 3) the ability of IPX-750 to cross into the brain after intraperitoneal and gavage administrations. Follow-up studies need to be conducted in order to determine the exact mechanism by which IPX-750 crosses the blood brain barrier. Because of the intricate and bulky natures of the raw tables, they are not presented here but instead figures representing the results are. We feel that the readers would benefit more from the figures than the raw tables. However, we will provide the raw data for each figure upon request.
Acknowledgements
We would like to thank ASFA and the UAB CORD Program for their support of Karan Jani and Vestavia High School for their support of Wendy Feng. The authors would also like to thank the LSU Department of Biological Sciences for the use of their equipment and space.
References
- Pahwa R, Koller WC (1996) Treatment of Parkinson's disease with controlled-release Carbidopa/L-DOPA. Advances in neurology 69: 487-491.
- Tyce GM, Dousa MK, Muenter MD (1990) MAO and L-dopa treatment of Parkinson's disease. Journal of neural transmission. Supplementum 29: 233-239.
- Admani AK, Verma S, Cordingley GJ, Harris RI (1985) Patient benefits of l-dopa and a decarboxylase inhibitor in the treatment of Parkinson's disease in elderly patients. Pharmatherapeutica 4: 132-40.
- Lopez Lozano JJ, Moreno Cano R (1995) [Preparation of a levodopa/carbidopa solution in ascorbic acid (citridopa) and chromatographic and electrochemical assessment of its stability over 24 hours]. Neurologia 10:155-158.
- Markham CH (1974) The "on-off" side effect of L-DOPA. Adv Neurol 5: 387-96.
- Fatehi MI, Gerhart DZ, Myers TG, Drewes LR (1987) Characterization of the blood-brain barrier: glycoconjugate receptors of 14 lectins in canine brain, cultured endothelial cells, and blotted membrane proteins. Brain Res 415: 30-39.
- Walters A, Jackson-Lewis V, Fahn S (1984) Little effect of dimethyl sulfoxide on blood-brain barrier to dopamine. Experientia 40: 859-861.
- Harris AP, Robinson R, Koehler RC, Traystman RJ, Gleason CA (2001) Blood-brain barrier permeability during dopamine-induced hypertension in fetal sheep. J Appl Physiol 91: 123-129.
- Martel CL, Mackic JB, Adams JD Jr, McComb JG, Weiss MH, et al. (1996) Transport of dopamine at the blood-brain barrier of the guinea pig: inhibition by psychotropic drugs and nicotine. Pharm Res 13: 290-295.
- Hardebo JE, Edvinsson L, Owman C, Rosengren E (1977) Quantitative evaluation of the blood-brain barrier capacity to form dopamine from circulating L-DOPA. Acta Physiol Scand 99: 377-384.
- Jankovic J (2002) Levodopa strengths and weaknesses. Neurology 58: S19-32.
- Bonina F, Puglia C, Rimoli MG, Melisi D, Boatto G, et al. (2003) Glycosyl derivatives of dopamine and L-dopa as anti-Parkinson prodrugs: synthesis, pharmacological activity and in vitro stability studies. J Drug Target 11: 25-36.
- Fernandez C, Nieto O, Fontenla JA, Rivas E, de Ceballos ML, et al. (2003) Synthesis of glycosyl derivatives as dopamine prodrugs: interaction with glucose carrier GLUT-1. Org Biomol Chem 1: 767-771.
- Fernandez C, Nieto O, Rivas E, Montenegro G, Fontenla JA, et al. (2000) Synthesis and biological studies of glycosyl dopamine derivatives as potential antiparkinsonian agents. Carbohydr Res 327: 353-365.
- Jiang C, Wan X, Jankovic J, Christian ST, Pristupa ZB, et al. (2004) Dopaminergic properties and experimental anti-parkinsonian effects of IPX750 in rodent models of Parkinson disease. Clin Neuropharmacol 27: 63-73.
- Gorton LM, Vuckovic MG, Vertelkina N, Petzinger GM, Jakowec MW, et al. (2010) Exercise effects on motor and affective behavior and catecholamine neurochemistry in the MPTP-lesioned mouse. Behav Brain Res 213: 253-262.
- Boulamery A, Simon N, Vidal J, Bruguerolle B (2010) Effects of L-Dopa on circadian rhythms of 6-OHDA striatal lesioned rats: a radiotelemetric study. Chronobiol Int 27: 251-64.
- Cai J, Yang M, Poremsky E, Kidd S, Schneider JS, et al. (2010) Dopaminergic neurons derived from human induced pluripotent stem cells survive and integrate into 6-OHDA-lesioned rats. Stem cells Dev 19: 1017-1023.
- Jankovic J, Chen S, Le WD (2005) The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Prog Neurobiol 77: 128-38.
- Eells JB, Misler JA, Nikodem VM (2006) Reduced tyrosine hydroxylase and GTP cyclohydrolase mRNA expression, tyrosine hydroxylase activity, and associated neurochemical alterations in Nurr1-null heterozygous mice. Brain Res Bull 70: 186-195.
- Jiang C, Wan X, He Y, Pan T, Jankovic J, et al. (2005) Age-dependent dopaminergic dysfunction in Nurr1 knockout mice. Exp Neurol 191: 154-162.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 25401
- [From(publication date):
August-2012 - Apr 26, 2025] - Breakdown by view type
- HTML page views : 20661
- PDF downloads : 4740