Review Article Open Access
Involvement of Mitochondrial Reactive Oxygen Species in Gastric Carcinogenesis
Masato Tamura1, Michihiro Mutoh2, Gen Fujii2, and Hirofumi Matsui1*1Faculty of Medicine, University of Tsukuba 1-1-1 Ten-nohdai, Tsukuba, Ibaraki 305-8573, Japan
2Division of Cancer Prevention Research, National Cancer Center Research Institute, Japan
- *Corresponding Author:
- Hirofumi Matsui
Faculty of Medicine, University of Tsukuba 1-1-1 Ten-nohdai
Tsukuba, Ibaraki 305-8573, Japan
Tel: +81-29-853-3218
Fax: +81-29-853-3218
E-mail: hmatsui@md.tsukuba.ac.jp
Received date: July 11, 2013; Accepted date: November 06, 2013; Published date: November 14, 2013
Citation: Tamura M, Mutoh M, Fujii G, Matsui H (2013) Involvement of Mitochondrial Reactive Oxygen Species in Gastric Carcinogenesis. J Gastroint Dig Syst 3:150. doi:10.4172/2161-069X.1000150
Copyright: © 2013 Tamura M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Gastric cancer is one of the most common malignancies in many countries. Environmental gastric cancer risk is known to be associated with Helicobacter pylori infection, a high intake of salty foods, and alcohol consumption. Here, we provide evidence of linkage of mitochondrial reactive oxygen species (ROS) to several environmental gastric cancer risks. Moreover, ROS correlate with gastric cancer invasion and metastasis, the most important factor to decide a patient’s prognosis. We also show correlation between mitochondrial ROS and gastric cancer invasion by using a normal gastric mucosal cell line (RGM-1), a cancerous mutant RGM-1 subclonal (RGK-1) and a MnSOD-expressing RGK-1 cell-line, used for a scavenging mitochondrial ROS. This mini-review summarizes role of ROS in gastric carcinogenesis and aims to provide a clue in developing useful treatments against gastric cancer.
Keywords
Reactive oxygen species; Mitochondria; Gastric cells; NOX; MnSOD
Introduction
Although the incidence and mortality of gastric cancer have recently been declining, it is still a common cancer in Japan [1]. Gastric cancer remains the fourth most common cancer and the second leading cause of global cancer mortality [2]. Thus, a gastric cancer control strategy is important in Japan and around the world. Moreover, studies to clarify the etiology of gastric cancer with molecular understanding are still important work to be done. In this short review, we focus on the etiology of gastric cancer from aspects of oxidative stress.
The Environmental Risk Factors of Gastric Cancer
According to the previous report of a joint WHO/FAO Expert Consultation in 2003, a high intake of salty foods and salt, and reduced fruit and vegetable intake were evaluated as “probable” risk factors for gastric cancer [3]. An evaluation from the International Agency for Research on Cancer concludes that smoking is the “convincing” risk factor for gastric cancer [4]. Positive association is presented with chronic atrophic gastritis, drinking habits, and barbecued or grilled cooking [5]. In addition, Helicobacter pylori (H. pylori) infection is considered as an important risk factor for gastric cancer. Besides environmental risk factors, genetic factors also play crucial roles in gastric cancer development. Mutation in the E-cadherin/CDH1 gene resulted in hereditary diffuse gastric cancer [6]. People with Lynch syndrome, and familial adenomatous polyposis, are high-risk groups of gastric cancer [6].
Biology of Reactive Oxygen Species
Source of reactive oxygen species
In normal cells, energy substrates, such as ATP and NADPH are produced from the electron transport chain in the mitochondria during aerobic metabolism. However, distinct amounts of reactive oxygen species (ROS) are also produced as a by-product from the mitochondrial respiratory chain. These are so-called mitochondrial ROS. ROS include the superoxide anion radical (O2·-), hydrogen peroxide (H2O2) and the hydroxyl radical (.OH). The initial step in ROS formation is generation of O2·- and then proceeds to H2O2 production. Interaction between O2·- and H2O2 generates .OH, which causes strong damage to cells [7]. The amount of ROS can be reduced by several antioxidant enzymes, e.g. superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx) and glutathione (GSH) [8-10]. Manganese superoxide dismutase (MnSOD) is one of these antioxidant enzymes that is particularly localized at mitochondria to scavenge their O2·- [11,12]. In other words, MnSOD is the specific scavenger of superoxides produced by mitochondrial electron transport [13]. It is interesting that a hundred times number of mitochondria is observed in oocytes compared to those in somatic cells [14]. This result indicated a higher requirement of energy production in oocytes for drastic cell division, along with a high amount of generation of ROS. In the case of cancer cells, a huge amount of O2·- is often generated because acomplex I dysfunction exists in mitochondria.
On the other hand, other enzymes, such as NADPH oxidase (NOX), generate ROS. Membrane-integrated NOX family oxidases, such as NOX1 and Duox2, are known to produce O2·- or H2O2 [15]. NADPH oxidase is a multicomponent enzyme,which is formed of a complex, p22phox, gp91phox (NOX2), p40phox, p47phox, p67phox and the small GTP-binding protein Rac [16-19]. For the initial step for the activation of NADPH oxidase, it is indispensable for Rac to translocate to the membrane and interact with p67phox and p47phox [20,21]. Nox1 is highly expressed in colon epithelial cells but not in gastric epithelial cells.
Of note, it is worthwhile to mention that nitric oxide (NO) is also an important radical species that induces cellular damage. NO interacts with O2·-, and generates peroxynitrite, which damages DNA [22] and also increasesnitrotyrosine levels in exposed tissue.
Reactive oxygen species and carcinogenesis
DNA damage induced by ROS is likely to play an important role in carcinogenesis. DNA exposed to ROS results in single- or doublestrand DNA breaks, DNA-protein cross-links [23] and deoxyribose oxidation. Oxidation of guanine at the C8 position forms 8-hydroxy-2’-deoxyguanosine (8-OHdG) [24]. Such oxidized DNA bases have been shown to be mutagenic, linked to mutations commonly observed in human cancers. Lipid peroxidation generated by ROS has also been recognized to be involved in carcinogenesis, for example by modification of DNA bases and activation of transcriptional factors [25].
In addition, ROS affects cancer cell proliferation and apoptosis through activation of AP-1 and nuclear factor-kappaB (NF-κB) transcription factors [26]. Activation of AP-1 results in induction of cyclin D1 and cyclin-dependent kinase, while NF-κB induces inflammatory cytokines and growth factors. Recently, correlation between NF-E2 p45-related factor-2 (Nrf2) and Kelch-like ECHassociated protein 1 (keap1) antioxidant system has been attracting much attention because Nrf2 induces a variety of antioxidant enzymes during exposure to oxidative stress [27,28].
Relation Between Environmental Risk factors of Gastric Cancer and Reactive Oxygen Species
Helicobacter pylori-induced reactive oxygen species
H. pylori is a spiral-shaped, gram-negative rod bacteria (approximately 3.5 × 0.5 μm) that infects the stomachs of almost half of all humans [29]. H. pylori is classified as a class 1 carcinogen [30], and its infection of the stomach induces chronic gastritis and intestinal metaplasia, which are risk factors for gastric cancer [31,32]. H. pylori strains have been classified by their expression genes, such as cytotoxinassociated antigen A (Cag A), cag-pathogenicity island (cag PAI) and vacuolating cytotoxin (Vac A). It was demonstrated that H. pylori carrying Cag A is closely associated with severe gastric inflammation and the development of gastric cancer [33]. On the other hand, the cag PAI status correlates with intracellular ROS formation in the gastric epithelial cells [34].
In addition to the gastric epithelial cells, the activated inflammatory cells, including neutrophil of the infected tissues, produce ROS [35]. Current evidence suggests that neutrophil-dependent oxidative stress plays a pivotal role in the pathogenesis of gastric inflammation associated with H. pylori infection. Neutrophil infiltration of the gastric mucosa is the initial pathological abnormality in H. pylori -associated inflammation, and this induces activation of NADPH oxidase. It has been reported that H. pylori induces translocation of HSP90β from cytosol to the cell membrane with interaction with Rac1, which activates NADPH oxidase to produce ROS in gastric epithelial cells [36]. On the other hand, 15d-PGJ2 inhibits the activation of NADPH oxidase in H. pylori-infected gastric epithelial AGS cells [37].
As a brief summary, gastric oxidative stress occurs as a result of both the bacterial and host side factors in the case of H. pylori infection. It would be interesting to clarify the contribution of mitochondrial ROS to H. pylori infected gastric epithelial damage and carcinogenesis.
Salt-induced reactive oxygen species
High concentrations of salt could provide a hyperosmotic pressure environment for gastric mucosa. Consumption of high concentrations of salt has been reported to destroy the gastric mucosal barrier, evoking inflammation, diffuse erosion and degeneration of gastric mucosa [38,39]. On the other hand, plants can be damaged by salt treatment due to superoxide production [40,41]. Thus, it is speculated that salt could also induce oxidative stress in animal cells. However, there are no reports demonstrating relations between ROS and sodium chloride in animal cells.
In our previous study, we asked whether salt (NaCl) could induce oxidative stress in rat gastric mucosal cells (RGM-1) [42]. We measured living gastric epithelial cells’ ROS spectra using an electron paramagnetic resonance (EPR) apparatus, and we successfully demonstrated for the first time that hypertonic salt treatment could induce ROS production in gastric epithelial cells [43]. In this study, NaCl at a concentration of 1 M for an hour exposure resulted in all cells dying. However, exposure of NaCl less then 650 mM concentration allowed survival of several cells for a few hours with production of ROS. These results suggest that high concentrations of NaCl, such as more than 1 M, act as a necrotizing factor, while lower concentrations of NaCl act as an oxidative stress inducer. Moreover, to investigate whether the salt-induced ROS is derived from mitochondria, we treated MnSOD over expressing cells (RGM-MnSOD) [44] with NaCl, and found that MnSOD suppressed the ROS production. The results were further confirmed by measuring the amount of the cellular membrane peroxidation using DPPP as a ROS indicator. In MnSOD-expressing cells, the levels of cellular membrane peroxidation induced by NaCl were reduced compared to those of the control parent cells.
In this section, salt is not only a necrotizing factor for gastric epithelial cells, but also an oxidative stress inducer. It is also interesting to know the combined effects of salt and gastric acids on ROS induction. Strong acidic environments below pH 2 induce necrosis in gastric epithelial cells, while pH 3 and/or pH 4 environments produce ROS from gastric epithelial cell mitochondria [45].
Alcohol-induced reactive oxygen species
As reported by WHO, alcohol may cause 60 types of diseases and injuries. Moreover, around 4% of all deaths worldwide, about 2.25 million, were attributed to alcohol consumption in 2004. The gastrointestinal tract, including the stomach, is called “the first-pass metabolism of alcohol”. In the cytoplasm of gastric epithelial cells, a microsomal ethanol oxidizing system (MEOS) is activated by alcohol. For the activation of MEOS, CYP2E1 (one of the cytochrome P450 family) is required to generate oxidized NADPH [46,47]. CYP2E1 has been reported to induce the expression of cyclooxygenase-2 (COX-2) in the liver [48]. Conversely, COX-2 could be induced by ROS, partly through activation of NF-κB. It is reasonable to induce COX-2 with resultant production of prostaglandins because it protects gastric epithelial cells from an aggressive factor for the gastrointestinal tract in vivo. Taking all into consideration, we speculated that alcohol could also induce oxidative stress in an animal cell. However, there are few reports investigating the relations between ethanol-induced ROS and mitochondria.
In a previous study, we demonstrated for the first time that treatment by ethanol is involved in ROS production, especially the superoxide anion, in RGM-1 cells [49]. We measured living gastric epithelial cells’ ROS spectra using an EPR apparatus. We performed this study under conditions from 0 to 20% ethanol. Such a concentration of ethanol may represent popular alcohol beverages such as beer or wine. A concentration of ethanol of more than 15% caused immediate cell death, while a concentration of ethanol of less then 15% allowed survival of several cells for a few hours with production of ROS. Ethanol-induced cellular ROS was observed for 15 min from exposure to 1% (v/v) ethanol. Lipid peroxidation in cellular membrane was also observed with1% ethanol, examined by the intensity of DPPP fluorescence. We also tried to clarify the localization of ROS production, which may co-localize with mitochondria. To this end, we stained cells with APF and MitoRed. After treatment with 0, 1 and 5% ethanol for an hour, the MitoRed fluorescence coincided with the APF fluorescence. Moreover, we also investigated the microscopic observations with fluorescent probes JC-1 that detect mitochondrial electron potential. In this experiment, injured mitochondria were observed in 5% ethanol exposed cells. These results indicated that ethanol injured mitochondria and reduces electron potential.
In this section, ethanol is not merely a necrotizing factor for gastric epithelial cells, but also an oxidative stress inducer. Ethanol inhibits a mitochondrial electron transfer system, and results in O2·- production.
Reactive Oxygen Species and Invasion/metastasis
Cancer patients’ prognosis mainly depends on the ability of cancer cell invasion. Generally, treatment decreasing cancer cell invasive abilities could improve prognosis. However, the mechanisms that control gastric cancer invasion and metastasis have not been clarified yet. The ability of gastric cancer cell invasion is related to mutations in both oncogenes and tumor suppressor genes, and is affected by growth factors, inflammatory cytokines and angiogenesis [50-52]. In addition, cancer cellular ROS may also play an important role in their invasion and metastasis [53,54] because ROS regulate activation of actin remodeling proteins and focal adhesion proteins [55,56]. Cellular invasion needs alteration of cellular morphology, including invadopodia/invasive feet. For instance, the ability to form invadopodia in breast cancer cells is closely related to their invasive potential [57]. Regarding cancer cell invasion, ROS generated by membrane-bound NOX play a critical role. NOX is reported to accelerate invadopodia formation through O2·- production [58,59]. However, the relation between mitochondrial ROS and tumor invasion has not been well investigated.
In a previous study, we elucidated whether mitochondrial ROS was involved in tumor cell migration or not [60]. We investigated living cellular ROS spectra of EPR using RGM-1, RGK-1 and RGM-MnSOD cells [60]. Two-times higher concentration of intracellular ROS was observed by EPR measurement in RGK-1 cells compared to those in RGM-1 cells. MnSOD over expression in RGK cells significantly decreased intracellular ROS concentration. To evaluate horizontal cellular migration, cellular ruffling frequencies were measured and a wound healing assay was performed. To analyze vertical cellular migration, an invasion assay using matrigel was also performed. All cellular movement abilities were inhibited by scavenging mitochondrial ROS by over expression of MnSOD. In these cells, components forming invadopodia, such as Rac1 and cdc42 were not reduced by MnSOD over expression. These data suggest mitochondrial ROS might have a different mechanism for the tumor invasions from the NOX-ROS pathway.
In this section, we demonstrated for the first time that mitochondrial ROS are involved in cancer cellular invasion. Matrix metalloproteinase (MMP) signaling enhances tumor invasion ability by degrading collagens. It has been reported that mitochondrial ROS controls MMP signaling by inducing MMP expression or pro enzymes [61]. It will be very important to clarify the mechanism of mitochondrial ROS for tumor invasion to improve cancer patients’ prognosis.
Future Aspects
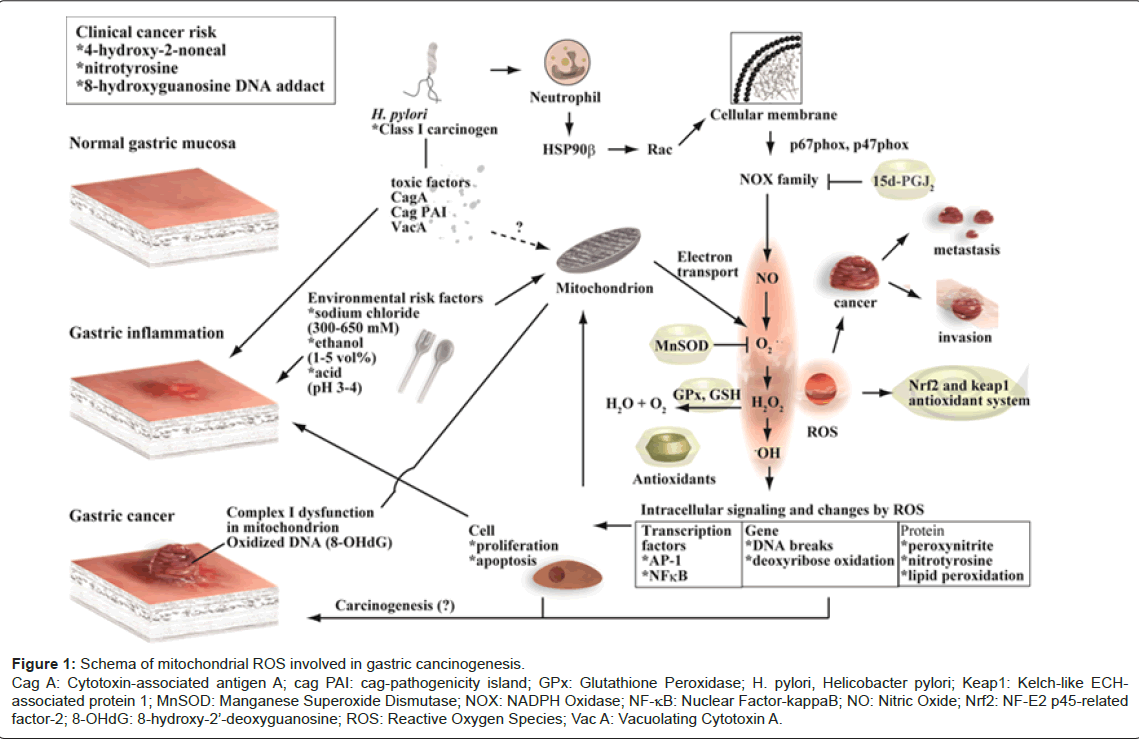
The overall five-year survival rate in gastric cancer patients is very low [62]. Thus, we should further clarify the pathogenesis of gastric cancer carefully. Subsequent studies, including experimental and clinic studies, suggest that the stomach may be susceptible to considerable oxidative stress associated with inflammation and cancer. In addition to inflammation that produce ROS in the stomach, environmental risk factors of gastric cancer also play a role in producing mitochondrial ROS, as summarized in this mini-review (Figure 1). For clinical use, monitoring the degree of 4-hydroxy-2-noneal, nitrotyrosine, 8-hydroxyguanosine DNA adducts and other lipid peroxides for free radical reaction may support identification of the degree of cancer risk. Identification or development of small molecules that scavenge superoxides, activate antioxidant enzymes and modulate activation of transcription factors, will be a useful strategy for future treatments, including chemoprevention for cancer.
Figure 1: Schema of mitochondrial ROS involved in gastric cancinogenesis. Cag A: Cytotoxin-associated antigen A; cag PAI: cag-pathogenicity island; GPx: Glutathione Peroxidase; H. pylori, Helicobacter pylori; Keap1: Kelch-like ECHassociated protein 1; MnSOD: Manganese Superoxide Dismutase; NOX: NADPH Oxidase; NF-κB: Nuclear Factor-kappaB; NO: Nitric Oxide; Nrf2: NF-E2 p45-related factor-2; 8-OHdG: 8-hydroxy-2’-deoxyguanosine; ROS: Reactive Oxygen Species; Vac A: Vacuolating Cytotoxin A.
References
- Matsuda T, Marugame T, Kamo K, Katanoda K, Ajiki W, et al. (2012) Cancer incidence and incidence rates in Japan in 2006: based on data from 15 population-based cancer registries in the monitoring of cancer incidence in Japan (MCIJ) project. Jpn J Clin Oncol 42: 139-147.
- Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55: 74-108.
- (2003) Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser 916: i-viii, 1-149, backcover.
- International Agency for Research on Cancer (2004) Tobacco smoking and tobacco smoke. IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Vol. 83. Lyon: International Agency for Research on Cancer.
- (1994) Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 61: 1-241.
- Chun N, Ford JM (2012) Genetic testing by cancer site: stomach. Cancer J 18: 355-363.
- Halliwell B (1978) Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Its role in degradation of hyaluronic acid by a superoxide-generating system. FEBS Lett 96: 238-242.
- Fridovich I (1978) The biology of oxygen radicals. Science 201: 875-880.
- MILLS GC (1957) Hemoglobin catabolism. I. Glutathione peroxidase, an erythrocyte enzyme which protects hemoglobin from oxidative breakdown. J Biol Chem 229: 189-197.
- Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59: 527-605.
- Okado-Matsumoto A, Fridovich I (2001) Subcellular distribution of superoxide dismutases (SOD) in rat liver: Cu,Zn-SOD in mitochondria. J Biol Chem 276: 38388-38393.
- Zhang HJ, Yan T, Oberley TD, Oberley LW (1999) Comparison of effects of two polymorphic variants of manganese superoxide dismutase on human breast MCF-7 cancer cell phenotype. Cancer Res 59: 6276-6283.
- Indo HP, Inanami O, Koumura T, Suenaga S, Yen HC, et al. (2012) Roles of mitochondria-generated reactive oxygen species on X-ray-induced apoptosis in a human hepatocellular carcinoma cell line, HLE. Free Radic Res 46: 1029-1043.
- Michaels GS, Hauswirth WW, Laipis PJ (1982) Mitochondrial DNA copy number in bovine oocytes and somatic cells. Dev Biol 94: 246-251.
- Sumimoto H (2008) Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J 275: 3249-3277.
- Vulcano M, Dusi S, Lissandrini D, Badolato R, Mazzi P, et al. (2004) Toll receptor-mediated regulation of NADPH oxidase in human dendritic cells. J Immunol 173: 5749-5756.
- Li JM, Shah AM (2001) Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc Res 52: 477-486.
- Muzaffar S, Jeremy JY, Sparatore A, Del Soldato P, Angelini GD, et al. (2008) H2S-donating sildenafil (ACS6) inhibits superoxide formation and gp91phox expression in arterial endothelial cells: role of protein kinases A and G. Br J Pharmacol 155: 984-994.
- Brandes RP, Schröder K (2008) Differential vascular functions of Nox family NADPH oxidases. Curr Opin Lipidol 19: 513-518.
- Dorseuil O, Quinn MT, Bokoch GM (1995) Dissociation of Rac translocation from p47phox/p67phox movements in human neutrophils by tyrosine kinase inhibitors. J Leukoc Biol 58: 108-113.
- Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, et al. (1999) Tetratricopeptide repeat (TPR) motifs of p67(phox) participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J Biol Chem 274: 25051-25060.
- Szabó C, Ohshima H (1997) DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide 1: 373-385.
- Toyokuni S, Mori T, Hiai H, Dizdaroglu M (1995) Treatment of Wistar rats with a renal carcinogen, ferric nitrilotriacetate, causes DNA-protein cross-linking between thymine and tyrosine in their renal chromatin. Int J Cancer 62: 309-313.
- Kasai H, Nishimura S (1984) Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res 12: 2137-2145.
- Niki E (2009) Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med 47: 469-484.
- Sen CK, Packer L (1996) Antioxidant and redox regulation of gene transcription. FASEB J 10: 709-720.
- Slocum SL, Kensler TW (2011) Nrf2: control of sensitivity to carcinogens. Arch Toxicol 85: 273-284.
- Yanaka A (2011) Sulforaphane enhances protection and repair of gastric mucosa against oxidative stress in vitro, and demonstrates anti-inflammatory effects on Helicobacter pylori-infected gastric mucosae in mice and human subjects. Curr Pharm Des 17: 1532-1540.
- Ding SZ, Zheng PY (2012) Helicobacter pylori infection induced gastric cancer; advance in gastric stem cell research and the remaining challenges. Gut Pathog 4: 18.
- Marshall BJ, Warren JR (1984) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1: 1311-1315.
- Correa P, Houghton J (2007) Carcinogenesis of Helicobacter pylori. Gastroenterology 133: 659-672.
- Uehara T, Ma D, Yao Y, Lynch JP, Morales K, et al. (2013) H. pylori infection is associated with DNA damage of Lgr5-positive epithelial stem cells in the stomach of patients with gastric cancer. Dig Dis Sci 58: 140-149.
- Kuipers EJ, Pérez-Pérez GI, Meuwissen SG, Blaser MJ (1995) Helicobacter pylori and atrophic gastritis: importance of the cagA status. J Natl Cancer Inst 87: 1777-1780.
- Ding SZ, Minohara Y, Fan XJ, Wang J, Reyes VE, et al. (2007) Helicobacter pylori infection induces oxidative stress and programmed cell death in human gastric epithelial cells. Infect Immun 75: 4030-4039.
- Babior BM (1984) Oxidants from phagocytes: agents of defense and destruction. Blood 64: 959-966.
- Cha B, Lim JW, Kim KH, Kim H (2010) HSP90beta interacts with Rac1 to activate NADPH oxidase in Helicobacter pylori-infected gastric epithelial cells. Int J Biochem Cell Biol 42: 1455-1461.
- Cha B, Lim JW, Kim KH, Kim H (2011) 15-deoxy-D12,14-prostaglandin J2 suppresses RANTES expression by inhibiting NADPH oxidase activation in Helicobacter pylori-infected gastric epithelial cells. J Physiol Pharmacol 62: 167-174.
- Sonnenberg A (1986) Dietary salt and gastric ulcer. Gut 27: 1138-1142.
- Tsugane S, Sasazuki S, Kobayashi M, Sasaki S (2004) Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer 90: 128-134.
- Katsuhara M, Otsuka T, Ezaki B (2005) Salt stress-induced lipid peroxidation is reduced by glutathione -transferase, but this reduction of lipid peroxides is not enough for a recovery of root growth in. Plant Sci 169: 369-373.
- Liu R, Garvin JL, Ren Y, Pagano PJ, Carretero OA (2007) Depolarization of the macula densa induces superoxide production via NAD(P)H oxidase. Am J Physiol Renal Physiol 292: F1867-1872.
- Kobayashi I, Kawano S, Tsuji S, Matsui H, Nakama A, et al. (1996) RGM1, a cell line derived from normal gastric mucosa of rat. In Vitro Cell Dev Biol Anim 32: 259-261.
- Tamura M, Matsui H, Nagano YN, Kaneko T, Indo HP, et al. (2013) Salt is an oxidative stressor for gastric epithelial cells. J Physiol Pharmacol 64: 89-94.
- Motoori S, Majima HJ, Ebara M, Kato H, Hirai F, et al. (2001) Overexpression of mitochondrial manganese superoxide dismutase protects against radiation-induced cell death in the human hepatocellular carcinoma cell line HLE. Cancer Res 61: 5382-5388.
- Rai K, Matsui H, Kaneko T, Nagano Y, Shimokawa O, et al. (2011) Lansoprazole inhibits mitochondrial superoxide production and cellular lipid peroxidation induced by indomethacin in RGM1 cells. J Clin Biochem Nutr 49: 25-30.
- Julkunen RJ, Di Padova C, Lieber CS (1985) First pass metabolism of ethanol--a gastrointestinal barrier against the systemic toxicity of ethanol. Life Sci 37: 567-573.
- Pronko P, Bardina L, Satanovskaya V, Kuzmich A, Zimatkin S (2002) Effect of chronic alcohol consumption on the ethanol- and acetaldehyde-metabolizing systems in the rat gastrointestinal tract. Alcohol Alcohol 37: 229-235.
- Uzma N, Kumar BS, Priyadarsini KI (2011) Hepatoprotective, immunomodulatory, and anti-inflammatory activities of selenocystine in experimental liver injury of rats. Biol Trace Elem Res 142: 723-734.
- Tamura M, Matsui H, Kaneko T, Hyodo I (2013) Alcohol is an oxidative stressor for gastric epithelial cells: detection of superoxide in living cells. J Clin Biochem Nutr 53: 75-80.
- Gomceli I, Demiriz B, Tez M (2012) Gastric carcinogenesis. World J Gastroenterol 18: 5164-5170.
- Ooi CH, Ivanova T, Wu J, Lee M, Tan IB, et al. (2009) Oncogenic pathway combinations predict clinical prognosis in gastric cancer. PLoS Genet 5: e1000676.
- Resende C, Thiel A, Machado JC, Ristimäki A (2011) Gastric cancer: basic aspects. Helicobacter 16 Suppl 1: 38-44.
- Nishikawa M (2008) Reactive oxygen species in tumor metastasis. Cancer Lett 266: 53-59.
- Wu WS (2006) The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev 25: 695-705.
- Schmitz AA, Govek EE, Böttner B, Van Aelst L (2000) Rho GTPases: signaling, migration, and invasion. Exp Cell Res 261: 1-12.
- Raftopoulou M, Hall A (2004) Cell migration: Rho GTPases lead the way. Dev Biol 265: 23-32.
- Yamaguchi H, Takeo Y, Yoshida S, Kouchi Z, Nakamura Y, et al. (2009) Lipid rafts and caveolin-1 are required for invadopodia formation and extracellular matrix degradation by human breast cancer cells. Cancer Res 69: 8594-8602.
- Weaver AM (2009) Regulation of cancer invasion by reactive oxygen species and Tks family scaffold proteins. Sci Signal 2: pe56.
- Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK (2008) Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68: 1777-1785.
- Tamura M, Matsui H, Tomita T, Sadakata H, Indo HP, et al. (2013) Mitochondrial reactive oxygen species accelerated gastric cancer cellular invasion. Journal of Clinical Biochemistry and Nutrition, Accepted, in press.
- Touyz RM (2006) Mitochondrial redox control of matrix metalloproteinase signaling in resistance arteries. Arterioscler Thromb Vasc Biol 26: 685-688.
- Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ (2009) Gastric cancer. Lancet 374: 477-490.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 18144
- [From(publication date):
November-2013 - Apr 07, 2025] - Breakdown by view type
- HTML page views : 13530
- PDF downloads : 4614