Investigation of Climate Changes on Metabolic Response of Plants: Interactive Effects of Drought Stress and Excess UV-B
Received: 26-Sep-2012 / Accepted Date: 12-Dec-2012 / Published Date: 15-Dec-2012 DOI: 10.4172/2157-7617.1000129
Abstract
Physiological and biochemical responses of bean (Vicia faba L.) plants to either supplementary ultraviolet (sUV-B) radiation, and/or water (WS) stress were investigated. Both stresses caused significant increases in H2O2 content and lipid peroxidation, indicating oxidative damage. Furthermore, increases in activities of stress markers indicated that sUV-B has a stronger stress effect than WS, and it caused greater membrane damage, as assessed by lipid peroxidation and osmolyte leakage.
The activities of ascorbate peroxidase (APX) and superoxide dismutase (SOD) were increased under both stresses when applied alone and in combination, while catalase (CAT) activity decreased under water stress as compared to the control. The combination of drought and UV-B, were more than additive, caused more severe damage than stress factors applied separately.
WS induced accumulation of UV-B absorbing secondary pigments (anthocyanin and flavonoids) which is likely to offer some protection from UV-B irradiation.
Keywords: sUV-B radiation; Water stress; Stress markers; Antioxidant enzymes; Proline accumulation; Secondary metabolites
8839Introduction
Anthropogenic activities have resulted in the reduction of stratospheric ozone [1,2], which led to a significant increase in ultraviolet-B (UV-B) radiation (290-320 nm) reaching the surface of the Earth [3,4]. Elevated levels of UV-B radiation can be stressful to plants [5]. Plants are exposed to a multitude of natural biotic and abiotic stressors. Most of a biotic stresses are connected to anthropogenic activities which are clearly causing major changes in atmospheric chemistry [6].
Wide inter-and intraspecific differences have been reported in response to UV-B with respect to growth and plant morphogenetic response [7-9] and physiological [10,11]. Some species show varied degrees of tolerance [12] while others are sensitive to present levels of UV-B radiation [13-15]. UV-B induced growth inhibition is usually associated with damage to the photosynthetic apparatus and reduction of photosystem II (PSII) efficiency [11,16].
Plants have evolved a variety of biochemical adjustments as mechanisms to protect and prevent damage caused by environmental stress(s) including UV-B radiation and water stress. The most widely observed mechanisms are the accumulation of UV-absorbing compounds in the epidermal cells such as flavonoids [17] and activation of antioxidant enzymes such as POD and SOD [1,15]. These enzymes scavenge free radicals from oxygen, and offer protections to lipids, proteins and nucleic [13,18].
UV-B is species specific, as other environmental stresses [1-19,20]. The different sensitivities of plants are partially explained by their abilities to respond to UV-B through the induction of defensive pathways [15-21].
Various stress factors competing with the supplemental UV radiation were shown to modify the UV radiation effects [22]. Water stress, is an important restricting factor that always affects agricultural productivity, particularly in arid and semi-arid regions. Feng et al. [23,24] showed that co-stresses of supplementary UV radiation and drought functioned synergically and one of them could alleviate the inhibitory effects of another under conditions of arid and semi-arid soils.
Although responses of crop physiology, growth, and yield to either water stress or UV-B radiation have been extensively studied in Northern Europe and the USA [24], knowledge of their interactive effects on crops, especially in developing countries, is extremely limited [10]. Moreover, there is paucity on the knowledge concerning the antioxidant response of plants to UV-B [25-26]. Furthermore, the mechanisms involved in the response of plants to both waters stress and sUV-B are yet to be identified
The aims of the present study were to understand the physiological and biochemical characteristics of broad bean (Vicia faba L.) under supplementary UV-B radiation and/or water stress, and to estimate its sensitivity and defense mechanisms under both stresses
Materials and Methods
Seeds of an Egyptian cultivar of bean (Vicia faba L.), obtained from a commercial source, were sown 20 cm apart at The Botanical Garden on 13/7/2011. Ten days after placing the plants half the plants
were subjected to progressive drought by withholding water, while well watered plants were irrigated once a week. Well-watered and waterstressed (WS) plants were divided equally between the two sections in a split-plot design. Consequently, four treatments were distributed in each plot in a randomized Latin square design: (a) control, i.e. without UV-B radiation and well-watered (b) Plots supplied with supplementary UV (sUV-B), (c) Plants subjected to drought stress (WS) without UV-B radiation and (d) plants were subjected to both sUV-B and WS. Twenty plants were used in each treatment.
No fertilisers or other fungicides were applied at either location to avoid interference with the fungicides.
Supplemental UV-B radiation was supplied by filtered Westinghouse FS-40 sunlamps oriented perpendicular to the planted rows and suspended above the plants. Lamps were filtered either with 0.13 mm thick cellulose acetate (transmission down to 290 nm) for supplemental UV-B radiation or 0.13 mm Mylar Type S plastic films (absorbs all radiation below 320 nm) as a control. The radiation filtered through the cellulose acetate supplied a weighted daily supplemental irradiance of either 3.0 or 5.1 effective kJ m-2 UV-BBE using the generalized plant response action spectrum [27] normalized to 300 nm. Plants beneath these cellulose acetate filtered lamps received supplemental doses in addition to ambient levels of UV-B radiation. These increased levels of UV-B radiation (supplemental+ambient) [28]. The weighted irradiance of Mylar filtered lamps was 0, so plants beneath these lamps received only ambient levels of UV-B (8.5 effective kJ m-2 UV-BBE on the summer solstice). Spectral irradiance beneath the lamps was measured with an Optronics Spectroradiometer (Model 742) equipped with a double monochromator with dual holographic grating and interfaced with a Hewlett Packard 85 printing calculator. The Spectroradiometer was calibrated using a National Bureau of Standards traceable 1000 W tungsten halogen lamp and wavelength alignment checked with known mercury emission lines using an Hg Arc lamp.
Non Destrctive Harvests
Net photosynthetic rate (PN) and total stomatal conductance for CO2 (gs) were measured on the youngest fully expanded leaf of the main stem. Gas exchange measurements were carried out seven times at 5 d intervals to cover all growth stages (10 days after sowing) using a LI-6200 portable IRGA (LI-COR, Lincoln, USA) between 10:00 and 14:00 h (Local time). All plants were measured on each day [29] (Table 1).
| parameter | control | UV | WS | WS +UV | LSD | d.f. |
|---|---|---|---|---|---|---|
| Fresh mass of pods [g] | 13.84+1.5 | 13.58+1.3 | 10.75+1.1 | 10.62+1.3 | 0.27 | 49 |
| No. of seeds/pod | 5.52+0.42 | 5.37+0.36 | 3.43+0.21 | 3.58+0.19 | 0.15 | 62 |
| RGR [g g-1 day-1] | 0.28+0.084 | 0.25+0.085 | 0.17+0.081 | 0.22+0.009 | 0.01 | 10 |
| PN [µmol(CO2)m-2s-1] | 18.9+2.4 | 17.8+2.2 | 14.6+2.1 | 15.3+2.5 | 0.23 | 35 |
| gs [molm-2s-1 ] | 0.33+0.02 | 0.31+ 0.02 | 0.24+0.019 | 0.25+0.02 | 0.05 | 35 |
| Fv/Fm | 0.77+0.0063 | 0.71+0.0017 | 0.53+0.0021 | 0.51+0.0019 | 0.07 | 19 |
Values are means ± SE. Least significance difference (LSD) at 5% level and d.f. are presented.
Table 1: Effects of sUV-B and drought stress, singly and in combination on yield parameters, net photosynthetic rates (PN), stomatal conductance (gs) and maximum quantum efficiency of PSII photochemistry (Fv/Fm).
Measurements of hydrogen peroxide
At the end of the drought and/or sUV-B treatment(s) (45 days after sowing), plants were harvested destructively. Fifteen leaf discs (10 mm diameter) were submerged in 750 μL reagent mixture containing 0.05% guaiacol and horseradish peroxidase (350 μL L-1, 250 Um L-1) in 25 mM sodium phosphate buffer (pH 7.0) and incubated for 2 h at 20°C in the dark [30]. Then, a volume of 250 μL was transferred into 96- well microtitre plates and the absorbance was immediately measured at 4450 nm in a plate reader photometer (SLT, Spectra, Dixons Ltd, Pure Chemicals for Laboratories, Switzerland). Commercial H2O2, which was used for standard curves, was calibrated by titration with KMnO4.
Antioxidant enzymes assays
Leaves were cut from each treatment (control, WW+sUV-B, WS?sUV-B and WS+sUV-B) and immersed in liquid nitrogen and kept in a deep freezer at ?80°C until the analyses were performed at Laboratories of Center of Excellence in Environmental Studies, King Abdulaziz University, KSA.
Samples were weighed and ground at about 0°C in 25 m Tris–HCl buffer containing 3 mM MgCl2, and then the homogenates were centrifuged at 20,000 for 15 min (Centrifuge 17 S/RS, Heraeus Sepatech). The supernatants were used for the enzyme assays and the results were expressed on protein basis [31].
All assays were performed using a final volume of 1 mL, with at least duplicate assays undertaken on each sample. Moreover, the assays were end-point determinations [29].
SOD (EC 1.15.1.1) activity was monitored [32]. The extraction mixture contained 50 mM phosphate buffer solution (pH 7.8), 13 mM L-methionine, 63 mM nitro blue tetrazolium and 2 mM riboflavin. The ability of the extract to inhibit the photochemical reduction of nitro blue tetrazolium was determined at 560 nm (Schimadzu UV-1201 spectrophotometer).
The amount of the extract resulting in 50% inhibition of nitro blue tetrazolium reaction is defined as one unit of SOD activity.
Catalase (EC, 1.11.1.6) activity was assayed in enzyme extract reaction mixture containing 50 mM phosphate buffer (pH 7.4). The reaction was started by adding 10 mM H2O2, and the reduction in absorbance was determined at 240 nm [33].
APX (EC, 1.11.1.11) activity was determined according to Maehly and Chance [33]. The reaction mixture contained 50 mM potassium phosphate, 0.5 mM ascorbate, 0.1 m Methylenedimethyl tartaric acid (EDTA) and 0.1 mM H2O2, and the absorbance was determined at 290 nm.
Protein concentrations of leaf extracts were determined as described earlier [31].
Pigment analysis
Chlorophyll was extracted in acetone from all leaves in the main stems of three plants per treatment, and determined [34].
Water-soluble pigments (flavonoids and anthocyanins) were extracted from leaves at the end of the experiment. Leaves were ground to a powder in liquid nitrogen before extraction in 10 cm3 of acidified methanol (HCl: methanol, 1: 99, v/v). Absorption spectra of the extracts were determined using a Cary 210 spectrophotometer (Varian, Palo Alto, CA, USA), and the flavonoid and anthocyanin contents were estimated from absorbances at 300 and 530 nm, respectively [35].
Measurements of free proline concentration
Leaves (0.2 g) were homogenized in 5 ml of 3% sulphosalicyclic acid solution. After centrifugation, 2 ml supernatant, 2 ml glacial acetic acid and 2 ml 2.5% acid ninhydrin solution were added in a test tube covered with Teflon cap. The absorbance of the free proline concentration was measured at 520 nm. The proline content was expressed as μg g-1 fresh weight [36].
Measurements of Lipid peroxidation
Lipid Peroxidation was measured by the amount of malondialdehyde (MDA) as end product of unsaturated fatty acid peroxidation [37].
Membrane permeability
It was measured by Electrolyte leakage [1]. Five leaves from each treatment were detached and immersed in distilled water at a room temperature and the conductivity of the solution was measured after 3 hours.
Statistical analysis
Two way ANOVA was applied to log-transformed data (Statgraphics Statistical Package 4, London, UK) to evaluate effects of WS and/or sUV-B treatments on growth and physiology of the plant. PPFD was used as a covariate in Leaf gas exchange and fluorescence data, there was no covariate used in growth measurements. The significance of difference among treatments were compared by Fisher’s least significant difference test (LSD).
Results
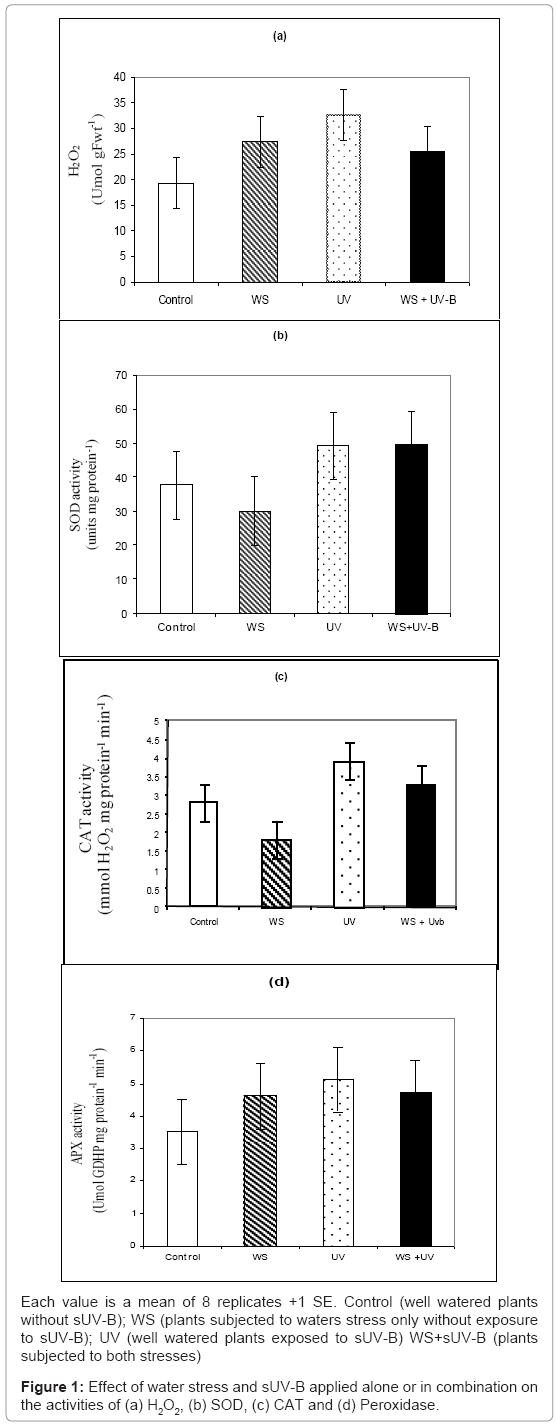
H2O2 and APX showed no significant response (P>0.05) to water stress (Figures 1a and 1d); while SOD and CAT activities were increased by 14 and 20%, respectively (Figures 1b and 1c). Exposure to sUV-B caused increases in these parameters by 18, 21, 47 and 56%, respectively (Figures 1a-1d).
Exposure to both stresses was more than additive as it caused an increase by 33% in APX and H2O2, while it was less than additive in case of CAT and SOD, as they were decreased by 20 and 26%, respectively, (Figure 1). Furthermore, there was negative correlation between shoot fresh weight and H2O2 content (Data not shown).
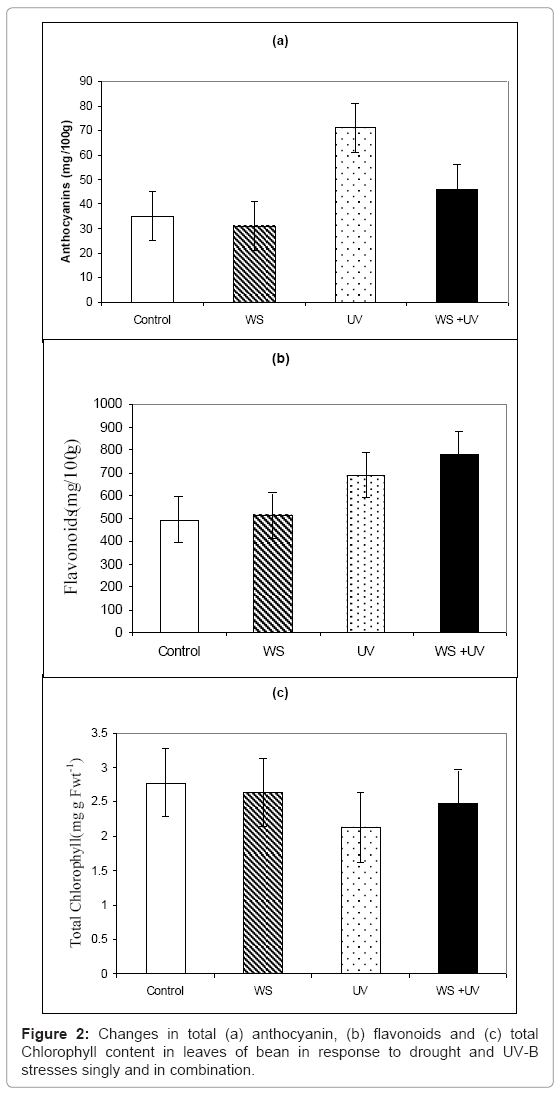
Exposure to sUV-B caused reduction in anthocyanine by 24% (Figure 2a), while it had no significant (P>0.05) effect on total flavonoids (Figure 2b). On the other hand, WS caused increases by 75 and 46% in both pigments, respectively (Figures 2a and 2b). Moreover, plants exposed to both stresses simultaneously shoed increases in these pigments by 35 and 59%, respectively (Figures 2a and 2b).
However, chlorophyll content showed the same response as growth parameters, as it was decreased by 22, 16 and 20%, due to exposure to WS, UV and both stress together, respectively (Figure 2c).
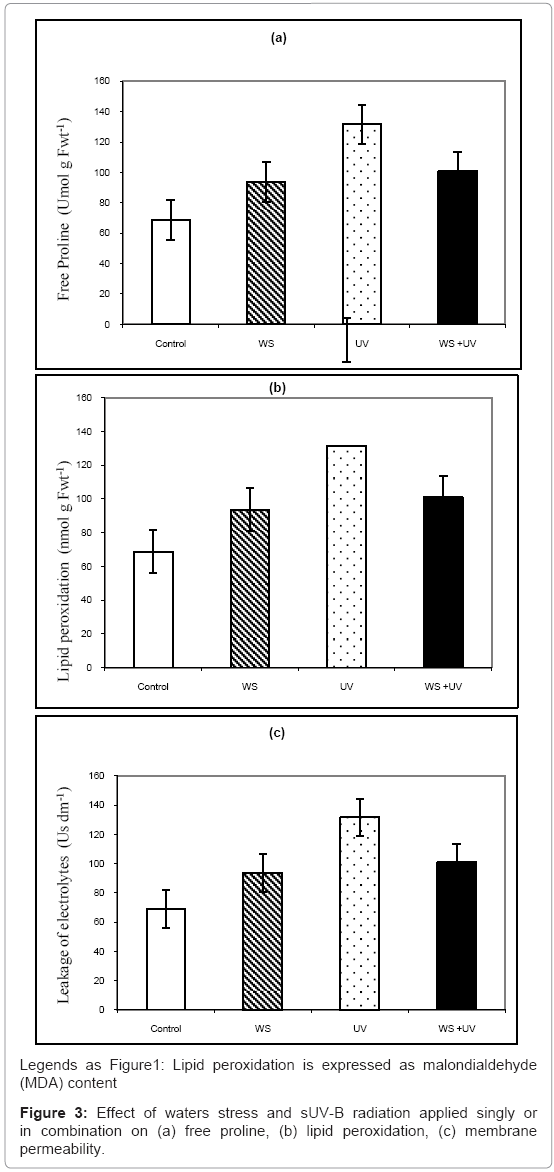
Figure 3 showed effect of waters stress and UV-B radiation applied singly or in combination on free proline, lipid peroxidation and membrane permeability. Proline was increased by 76 and 34% after exposure to WS and sUV-B, respectively (Figure 3a), while their interaction was less than additive (19%). On the other hand, Lipid peroxidation and membrane permeability were increased by 40 and 43%, respectively (Figures 3b and 3c) due to exposure to UV irradiation while water tress had no significant effect. Interaction between both stresses caused increases in these parameters by 31 and 47%, respectively (Figures 3b and 3c).
Discussion
Alexieva et al. [1] reported that there is an inter-relationship between drought and ultraviolet-B (UV-B) radiation in plant responses, in that both stresses provoke an oxidative burst. However, the mechanisms involved in the response of plants to both stresses are yet to be identified. Thus, elucidation of their interaction would help plants cope with changing environmental conditions [38].
The significant effect of sUV-B on the concentrations of H2O2 is in agreement with results of other researchers [39-41]. Increase in lipid production caused by stress may have occurred because of the accelerated formation of reactive oxygen species (ROS) [i.e., singlet oxygen (1O2) and ·OH]; ROS attack lipids, particularly unsaturated fatty acids, and the accelerated formation of ROS results in the formation of peroxidation products, the main one of which is MDA [15].. The reaction of such radicals with macromolecules, particularly lipoproteins, can cause faster peroxidative damages as observed from the destruction of membrane lipids [1].
On the other hand, anthocyanins and flavonoids are affected differently by UV radiation. These pigments play an important role against UV damage in higher plants [22]. We found the highest levels of anthocyanin and flavonoids were obtained in UV-B radiation while the lowest content were observed in plants exposed to water stress (Figure 2). An increase of UV absorbing compounds caused by UV was well documented in previous studies [1,22,42]. Flavonoids compounds have effective radical scavenging capabilities and can directly contribute to enhanced photo protection against UV-B radiation. The increases in UV-B absorbing compounds, mainly in flavonoids, are recognized as a general response to UV-B stress [43].These results suggest that the UV-B absorbing compounds are mainly synthesized in leaves and they are used to protect leaf tissue under exposure to UV. However, it seems to be produced through similar mechanisms as in the case of UV induction. Flavonoids and related compounds absorb strongly in the UV-region but not in the photosynthetically active regions of the spectrum [44], allowing photosynthesis to continue while UV wavelengths are attenuated at the epidermis.
Water stress and UV radiation lead to the increase of the contents of proline in leaves of bean in this experiment, which indicates that some wilting-induced proline accumulation occurred (Balouhci et al. [22]). It was reported that plants exposed to UV radiation accumulate proline that could protect plant cells against UV radiation-induced peroxidative processes [45]. A marked increase in proline accumulation under UV-B in the present study is in agreement with the results of Balouchi et al. [22] and this could represent adaptive responses to oxidative damage induced by UV radiation. Proline is known to be involved in alleviating cytosolic acidic associated with several stresses [46]. The removal of excess H+ occurring as a result of proline synthesis may have a positive effect on reduction of the UV-B induced damage. It suggests that UV radiation-induced proline accumulation protects plants against UV radiation promoted peroxidation processes.
CAT, APX, and SOD are key enzymes of the antioxidant defense system. The SOD, POD, APX, and CAT activities are also associated with UV-B exposure and other stresses such as water stress, as these enzymes act as antioxidant compounds to help reduce photooxidative damage in plant leaves. SOD accelerates the conversion of superoxide to H2O2, whereas CAT and APX catalyze H2O2 breakdown [47,48]. The results of the present study indicate that the CAT, APX, and SOD activities were positively affected by supplementary UV-B radiation. Therefore, a preferential synthesis activation of this enzyme by bean leaves in the present study counteracts oxidative stress. The increase in the CAT, APX, and SOD activities are frequently observed under stressful conditions [49-55].
Conclusion
In conclusion, we found that UV-B radiation and water stress increased UV screen pigments, MDA and antioxidant enzymes although water stress decreased pigment production. There was an interaction between sUV-B and WS, where the first delayed and reduced the severity of the latter. Our understanding of the relationships between crop growth and the atmospheric environment was developed substantially in the past few decades. Still, the factor of climate change and its impact on crops and food production will be further explored in future studies because global change climate might be critical event in future centuries; available data may not adequately characterize the potential effect of future, such as simultaneous changes in climate change and UV-B radiation.
Acknowledgements
This work was funded by a grant from cees (2/H/1433).
References
- Alexieva V, Sergiev I, Mapelli S, karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant, Cell & Environment 24: 1337.
- Molina MJ, Rowlands FS (1974) Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature 249: 810-812
- Blumthaler M, Amback M (1990) Indication of increasing solar ultraviolet-B radiation flux in alpine regions. Science 248: 206-208.
- Garty J, Tamir O, Levin T, Lehr H (2007) The impact of UV-B and sulphur- or copper-containing solutions in acidic conditions on chlorophyll fluorescence in selected Ramalina species. Environ Pollut 145: 266-273.
- Paul ND, Gwynn-Jones D (2003) Ecological roles of solar UV radiation: towards an integrated approach Trends Ecol Evol 18: 48-55.
- Kakani Vg, Reddy K, Zhao R, Salaja K (2003) Field crop responses to ultraviolet-B radiation: a review. Agricult. Forest Met 120: 191-218.
- Kramer GF, Norman HA, Krizek DT, Mirecki RM (1991) Influence of UV-B radiation on polyamines, lipid peroxidation and membrane lipids in cucumber. Phytochemistry 30: 2101-2108.
- Rozema J, Staaij JVD, Bjorn LO, Caldwell M (1997) UV-B as an environmental factor in plant life: stress and regulation. Trends Ecol Evol, 12: 22-28.
- Milchunas DG, King JY, Mosier AR, Moore JC, Morgan JA, et al. (2004) UV Radiation Effects on Plant Growth and Forage Quality in a Shortgrass Steppe Ecosystem, Photochemistry and photobiology, 79: 404-410.
- Agrawal S, Rathore D (2007) Changes in oxidative stress defense system in wheat (Triticum aestivum L.) and mung bean (Vigna radiate L.) cultivars grown with and without mineral nutrition and irradiant by supplement ultraviolet-B. Environ Exper Bot 39: 21-33.
- Tsormpatsidis E, Henbest RGC, Battey NH, Hadely P (2010) The influence of ultraviolet radiation on growth, photosynthesis and phenolic levels of green and red lettuce: potential for exploiting effects of ultraviolet radiation in a production system. Annals of Applied Biol 156: 357-366.
- Kolb CA, Kaser M A, Kopecky J, Zotz G, Riedere M, et al. (2001) Effects of Natural Intensities of Visible and Ultraviolet Radiation on Epidermal Ultraviolet Screening and Photosynthesis in Grape Leaves. Plant Physiology 127: 863-875.
- Teramura AH, Sullivan JH (1991) Potential impacts of increased solar UV-B on global plant productivity In: Photobiology (Ed. E. Riklis). New York, Plenum press 645-634.
- Moussa HR, Khodary SEA (2008) Changes in growth and 14CO2 fixation of Hordeum vulgare and Phaseolus vulgaris induced by UV-B radiation. J Agric Soc Sci 4: 59-64.
- Zu YG, Wel XX, Yu JH, Li DW, Pang HH, et al. (2011) Responses in the physiology and biochemistry of Korean pine (Pinus koraiensis) under supplementary UV-B radiation. Photosynthetica, 49: 448-458.
- Gartia S, Pradhan MK, Joshi PN, Biswal UC, Biswal B (2003) UV-A Irradiation Guards the Photosynthetic Apparatus Against UV-B-Induced Damage. Photosynthetica 41: 545-549.
- Treutter D (2005) Significance of flavonoids in plant resistance and enhancement of their biosynthesis (Special issue: mechanism in resource allocatopn). Plant Biology 7: 581-591.
- Jain K, Kataria S, Guruprasad KN (2004) Effect of UV-B radiation on antioxidant enzymes and its modulation by benzoquinone and a-tocopherol in cucumber cotyledons. Curr Sci India 87: 87-90.
- Smith JL, Burrritt DJ, Bannister P (2000) Shoot Dry Weight, Chlorophyll and UV-B-absorbing Compounds as Indicators of a Plant’s Sensitivity to UV-B Radiation. Ann Bot 1057-1063.
- Zu Y, Li Y, Chen J, Chen H (2004) Intraspecific responses in grain quality of 10 wheat cultivars to enhanced UV-B radiation under field conditions. J Photochem Photobiol B Biol 74: 95-100.
- Creelman RA, Mulletl JE (1997) Oligosaccharins, brassinolides, and jasmonates: nontraditional regulators of plant growth, development, and gene expression. Plant Cell 9: 1211-1223.
- Balouchi HR, Sanavy SAM, Emam Y, Dolatabadian A (2009) UV radiation, elevated CO2 and water stress effect on growth and photosynthetic characteristics in durum wheat. Plant Soil Environ 55: 443–453.
- Fengh LIS, Xue L, An L, Wang X (2007) The interactive effects of enhanced UV-B radiation and soil drought on spring wheat. S Afr J Bot 73: 429–434.
- Zhao D, Reddy KR, Kakani VG, Koti S, Gao W (2005) Physiological causes of cotton fruit abscission under conditions of high temperature and enhanced UV-B radiation. Physiol Plant. 124: 189-199.
- Costa H, Gallego SM, Tomaro ML (2002) Effect of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci 162: 939-945.
- Hassan IA, Abou Zeid HM, Basahi J (2011) Photosynthetic response of Egyptian cultivar of broad bean (Vicia faba L.) to UV-B and drought, singly and in combination. Int J Agri Sci Soil Sci 1: 455-461.
- Caldwell MM, Robberecht R, Nowak RS, Billings WD (1982) Differential photosynthetic inhibition by ultraviolet radiation in species from the arctic-alpine life zone. Arctic and Alpine Res 14: 195-202.
- Green AES, Cross KR, Smith LA (1980) Improved analytic characterization of Ultraviolet skylight. Photochem. Photobiol 31: 59-65.
- Hassan IA (2006) Physiological and biochemical response of potato (Solanum tuberosum L. Cv. Kara) to O3 and antioxidant chemicals: possible roles of antioxidant enzymes. Annals of Applied Biology, 148: 197-206.
- Wu Y, Tiedemann AV (2002) Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut 116: 37-47.
- Bradford MM (1976) A Rapid and Sensitive Method for the Quantitation of Microgram Quantitives of Protein Utilizing the Principle of Protein-Dye Binding. Analytical Biochemistry 72: 248-254.
- Lee EH, Upadhyaya A, Agrawal M, Rowland RA (1997) Mechanisms of ethylenediurea (EDU) induced ozone protection: Reexamination of free radical scavenger systems in snap bean exposed to O3. Environmental and Experimental Botany 38: 199-209
- Maehly AC, Chance B (1954) The assay of catalase and peroxidase. In: Methods of Biochemical analysis. Glick D (ed). New York: Interscience: 357-424.
- Khan MR, Khan MW (1994) Single and interactive effects of O3 and SO2 on tomato. Environ Exp Bot 34: 461-469.
- Nogués S, Baker NR (2000) Effects of drought on photosynthesis in Mediterranean plants grown under enhanced UV-B radiation. J Exp Bot 5: 1309-1317.
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant and Soil 39: 205-207.
- Hassan IA, Twefik I (2006) CO2 photoassimilation, chlorophyll fluorescence, lipid peroxidation and yield in cotton (Gossypium hirsutum L. cv Giza 65) in response to O3. World Review of Science, Technology and Sustainable Development 3: 70-79
- Tian XR, Lei YP (2007) Physiological responses of wheat seedlings to drought and UV-B radiation. Effect of exogenous sodium nitroprusside application. Russian J Plant Physiolgy 54: 676-682.
- Rao MV, Ormrod DP (1995) Impact of UVB and O3 On the Oxygen Free Radical Scavenging System in Arabidopsis thaliana Genotypes Differing in Flavonoid Biosynthesis. Photochem Photobiol 62: 719-726.
- Prasad SM, Kumar D, Zeeshan M (2005) Growth, photosynthesis, active oxygen species and antioxidants responses of paddy field cyanobacterium Plectonema boryanum to endosulfan stress. J Gen Appl Microbiol 51: 115-123.
- Hideg E, Vass I (1996) UV-B induced free radical production in plant leaves and isolated thylakoid membranes. Plant Sci 115: 251-260.
- Rozema J, Björn LO, Bornman JF, Gaberšcik A, Häder DP, et al. (2002) The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. J Photochem Photobiol B 66: 2-12.
- Hidema J, Kumagai T (2006) Sensitivity of Rice to Ultraviolet-B Radiation. Ann Bot 97: 933-942.
- Ceny P, Bornman JF (1993) The effect of exposure to enhanced UV-B radiation on the penetration of monochromatic and polychro- matic UV-B radiation in leaves of Brassica napus. Plant Physiology 87: 249-255.
- Saradhi PP, Arora AS, Prasad SK (1995) Proline accumulates in plants exposed to UV radiation and protects them against UV-induced peroxidation. Biochem Biophys Res Commun 209: 1-5.
- kurkdjian A, Guern J (1989) Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol 40: 271-303.
- Ziska LH, Teramura AH (1992) CO2 Enhancement of Growth and Photosynthesis in Rice (Oryza sativa). Plant Physiol 99: 473-481.
- Wang SW, Xie BT, Yin LN, Duan LS, Li ZH, et al. (2010) Increased UV-B radiation affects the viability, reactive oxygen species accumulation and antioxidant enzyme activities in Maize (Zea mays L.) pollen Photochem. Photobiol 86: 110-116.
- Yazici I, Türkan I, Sekmen AH, Demiral T (2007) Salinity tolerance of purslane (Portulaca oleracea L.) is achieved by enhanced antioxidative system, lower level of lipid peroxidation and proline accumulation Environ. Exp Bot 61: 49-57.
- Mishra V, Srivastava G, Prasad SM (2009) Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic 120: 373-378.
- Pal M, Sengupta UK, Srivastava AC, Jain V, Meeena RC (1999) Changes in growth and photosynthesis of mungbean induced by UV-B radiation. Indian J Plant Physiol 4: 79-84
- Hojati M, Sanavy S, kareimi M, Ghannat F (2011) Responses of growth and antioxidant systems in Carthamus tinctorius L. under water deficit stress. Acta Phsiol Plant 33: 105-112.
- Feng H, An L, Tan L, Hou Z, Wang X (2000) Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 taxa in vitro. Environmental and Experimental Botany 43: 45-53.
- Lapos R, Veras S, Meszaros I (2002) Photosynthesis-ecophysiological properties of beech (Fagus sylvatica L.) under the exclusion of ambient UV-B radiation. Acta Biol Szegediensis 46: 243- 245.
- Zancan S, Suglia I, Rocca NL, Ghisi R (2008) Effects of UV-B radiation on antioxidant parameters of iron-deficient barley plants. Environ Exp Bot 63: 71-79.
Citation: Hassan IA, Basahi JM, Haiba NS, Kadi MW (2013) Investigation of Climate Changes on Metabolic Response of Plants; Interactive Effects of Drought Stress and Excess UV-B. J Earth Sci Climate Change 4: 129. DOI: 10.4172/2157-7617.1000129
Copyright: ©2013 Hassan IA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 16073
- [From(publication date): 3-2013 - Nov 13, 2025]
- Breakdown by view type
- HTML page views: 11212
- PDF downloads: 4861