Research Article Open Access
Interlaboratory Development and Cross Validation of a Chromatographic Method for Determination of Lumefantrine in Human Plasma-A Proficient Capacity Assessment of Bioanalytical Laboratories in East Africa
Minzi O1*, Ngaimisi E1, Shewiyo DH2, Sasi P3 and Ignace AM1
1Unit of Pharmacology and Therapeutics, School of Pharmacy, Muhimbili University of Health and Allied Sciences, PO BOX 65013, Dar Es Salaam, Tanzania
2Directorate of laboratory Services, Tanzania Food and Drugs Authority, P. O. Box 77150, Dar Es Salam, Tanzania
3Department of Clinical Pharmacology, School of Medicine, Muhimbili University of Health and Allied Sciences, P.O. BOX 65013, Dar Es Salaam, Tanzania
- *Corresponding Author:
- Minzi O
Unit of Pharmacology and Therapeutics, School of Pharmacy
Muhimbili University of Health and Allied Sciences
P.O. BOX 65013, Dar Es Salaam, Tanzania
E-mail: Minziobejayesu@gmail.com, mashiku2005@yahoo. com, ominzi@muhas.ac.tz
Received date: March 28, 2012; Accepted date: April 24, 2012; Published date: April 26, 2012
Citation: Minzi O, Ngaimisi E, Shewiyo DH, Sasi P, Ignace AM (2012) Interlaboratory Development and Cross Validation of a Chromatographic Method for Determination of Lumefantrine in Human Plasma-A Proficient Capacity Assessment of Bioanalytical Laboratories in East Africa. J Anal Bioanal Tech 3:131. doi: 10.4172/2155-9872.1000131
Copyright: © 2012 Minzi O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Background: Bioanalytical laboratories in developing countries face many challenges. The objective of this work was to assess the capacity of bioanalytical laboratories in emerging countries in setting and validating analytical methods. An HPLC method for determination of lumefantrine in human plasma was used to assess three medical university laboratories of Tanzania, Uganda and Kenya.
Methodology: Bioanalytical experts from Analytical Clinical Concept, Leidersbach, in Germany (ACC GmbH) developed the HPLC method and assigned the 3 laboratories to set up and validate the method. The laboratories were tasked to determine the concentrations of blinded plasma samples spiked with lumefantrine and had to submit their analysis reports to ACC for evaluation within 6 weeks. Each laboratory was provided with reference standard, internal standard columns and precolumns. Spiked plasma samples were shipped under dry ice from Germany to “Gesellschaft fuer Internationale Zusammenarbeit” (GIZ) local office of each participating country. All other requirements were procured by individual laboratories.
Results: The results from MUHAS Bioanalytical laboratory (Tanzania) Laboratory met the criteria set by ACC laboratory. The results were within the range set by ACC laboratory and most calibration curves had good linearity with coefficient of correlation always > 0.990. The inter-day precision and accuracy (Relative standard deviation=RD of recovery) were always < 15%. The relative deviation of the results obtained compared to assigned concentrations for blinded plasma samples were between -15% and -25%. The Laboratory met the stipulated time line and the obtained validation results were within the range set by the ACC experts. The results from other laboratories were also satisfactory.
Conclusion: The results indicate that with further little infrastructural and technical assistance the capacity of these bioanalytical laboratories in conducting bio-analyses will be more strengthened and can serve as centers for training bioanalytics and running bioequivalence studies in the region.
Keywords
Bioanalytical laboratories; Bioanalytical method; Bioequivalence
Introduction
Bio-analytical laboratories are increasingly gaining importance in developing countries as they are becoming instrumental in fighting substandard medicines and supporting efficacy, medication compliance and drug utilization studies for anti-malarial, anti-tuberculosis and an- tiretroviral drugs in developing countries [1-3]. With limited capacity to procure branded innovators’ medicines, developing countries rely on generic medicines despite reports of poor quality generics marketed in the countries [4,5]. The use of substandard medicines may lead to drug resistance, delayed cure and increased total costs of treatments [6,7]. Due to lack of well-functioning analytical laboratories, the National Drug Regulatory Authorities (NDRAs) in most African countries currently register drugs based on the product dossier evaluation, inspection reports for good manufacturing practices and comparative in vitro data [communication with drug regulators]. There is therefore a need for more well-functioning analytical laboratories with capacity to set and validate analytical methods in these countries. Such laboratories will sustain efforts to monitor drug quality, efficacy and rational drug use in order to ensure safety and good treatment outcomes.
Establishing fully functioning bioanalytical laboratories, which imple- ment international Good Laboratory Practice (GLP), requires human and infrastructure capacity building through training, financial and technical support [8]. Due to resource constrains, most analytical laboratories in developing countries lack capacity to develop analytical methods, but can set HPLC methods developed elsewhere. For sustainable moni- toring of drug quality and efficacy, availability of well-structured ana- lytical laboratories equipped with trained human resource is a matter of urgency. Emerging bioanalytical laboratories in developing countries have an exclusive role to see that this is achieved despite many challeng- es facing them. These laboratories are currently equipped with some analytical instruments mostly HPLC systems, but majority has limited capacity to analyze biological samples let alone developing analytical methods.
A pilot study recently conducted by Dr. Said Laik Ali the GIZ Consultant; [unpublished data] revealed that, some few centers in the East African region could run Bioequivalence (BE) studies but not satisfactorily. This is contributed by many factors including lack of capacity to promptly repair and service their laboratory equipment; as a result a number of many laboratory instruments are beyond repair and are idly piled up in corridors.
At present, Medical Universities in Kenya, Tanzania and Uganda, have few bio analytical laboratories which they are trying to strengthen up in order to meet WHO guidelines for Good laboratory practice and hence prequalification to conduct bioequivalence and Therapeutic Drug Monitoring (TDM) activities in the region. Therefore, the objective of this work was to assess the capacity of bio analytical laboratories in Kenya, Tanzania and Uganda in setting and validating an HPLC method for determination of lumefantrine in human plasma. The bio analytical method was developed by ACC laboratory in Germany (GmbH). We hereby report the results obtained by the Tanzania bioanalytical laboratory after receiving feedback from ACC.
Methods
The Germany consultant, Dr. Lak Ali - Consultant for German Gesellschaft fuer Internationale Zusammenarbeit” (GIZ), a Germany agency for international cooperation and development” wrote a concept note in 2009 proposing an initiative to address the lack of bio analytical services in the EAC region. A collaborative trial was proposed to assess the performance among the target laboratories. This idea was presented at a consultative meeting (October 2009 in Addis Ababa, Ethiopia) in which the laboratory representatives from Tanzania, Kenya and Uganda were involved. The proposed capacity building initiative beginning with a collaborative trial was accepted by all the representatives and a common drug molecule, Lumefantrine, for inclusion in the trial was agreed. The World health Organization (WHO), Geneva and GIZ, Eschborn, Germany agreed to fund this project.
The study involved a 2-day pre-trial training in Kenya, Tanzania and Uganda and the trial itself. For the bioanalytical team in Tanzania, the training was conducted in April, 2010 in Dar es Salaam, and the team was trained on various aspects of Good Laboratory Practice (GLP) bioanalysis including: development and implementation of Standard Operating Procedures according (SOP’s) to GLP, Good Clinical Practice (GCP), and WHO guidelines; methods and procedures of sample preparation and measurement; Considerations when establishing an Internal Quality Assurance Unit (QAU); method development, validation and sample measurement: requirements, procedures, and acceptance criteria according to WHO, European Medicines Agency (EMA), and the US Food and Drug administration (FDA) guidelines; and collaborative trial procedures.
Collaborative trial procedures
The ACC laboratory in Germany (GmbH) developed and validated the lumefantrine method in their laboratory after which, they prepared the trial samples (plasma spiked with lumefantrine in concentrations, which were concealed to the teams in Kenya, Tanzania and Uganda. At this point, ACC GmbH laboratory sent the method SOP and a list of needed materials, reagents to our team and finally, they shipped the plasma samples (unknown test samples) in dry ice to Dar es Salaam, Kampala and Nairobi. ACC GmbH also shipped the pure compounds: lumefantrine (analyte), halofantrine (internal standard), and HPLC columns and guard columns to the laboratories. The plasma samples were received in excellent condition and were stored at -80°C before analysis. The teams in Tanzania, Kenya and Uganda, had to establish an external quality assurance system monitor; procured the needed materials, reagents, and prepared the equipment and apparatus, including performing a system suitability test for the laboratory’s HPLC system, before re-validating the method and analyzing the plasma samples for the collaborative trial.
Reference standards were weighed and dissolved in solvents; the method was cross-validated for selectivity, linearity and precision/accuracy. A standard curve was prepared and measurement of QC’s as well as plasma samples was performed. All these activities followed the SOP’s prepared by ACC GmbH and were overseen by an external quality assurance monitor/officer from Tanzania Food and Drug Authority(TFDA) in the case of Tanzania. Cross-validation data, the standard curve, results of QCs and collaborative trial plasma samples; and the calculated concentrations and the chromatograms were submitted to ACC GmbH for evaluation.
Bioanalytics
Instrumentation: The specifications hereby presented are based on MUHAS Bioanalytical Laboratory in Tanzania. The HPLC system consisted of a pump (LC-20AT, Shimadzu®, Kyoto, Japan), auto sampler (SIL-20A, Shimadzu®, Kyoto, Japan), and UV/VIS detector (SPD-20AV, Shimadzu®, Kyoto, Japan).
Reagents: All chemicals used were of analytical grade. Lumefantrine and halofantrine reference standards were supplied by ACC, Leidersbach, Germany. Acetonitrile, methanol, potassium dihydrogen phosphate were from Romil LTD, waterbeach-Cambridge, UK. Acetic acid, hydrochloric acid and ortho-phosphoric acid were from Fluka Chemie, GmbH, Switzerland. Distilled water was prepared locally at Muhimbili University of Health and Allied Sciences (MUHAS).
Preparation of standard solutions, calibration and quality control samples: The SOP’s for preparation of stock and reference standard solutions as well as sample extraction were provided by ACC, which also provided all the SOP’s for preparation of calibration curves and determination of lumefantrine concentrations from spiked plasma samples.
Lumefantrine stock solution (double weighing) was prepared by dissolving 10 mg of Lumefantrine in a mixture of methanol: acetic acid (99.8:0.2, v/v) up to 20.0 mL. For standard solutions preparations, different volumes of the stock solution were diluted using 0.1% acetic acid solution in methanol: water (1:1, v/v) up to 20 mL. For preparation of the standard curves, 50.0 μl of the respective standard solution were added to 500.0 μl of blank plasma. The calibration curves prepared were in a concentration range of 0.05-10.0 μg/ml.
Lumefantrine quality control solutions were obtained by dilution of the stock solution to achieve 80.0 μg/ml, 10.0 μg/ml, 1.0 μg/ml and 0.50 μg/ml as high, middle and low level quality control samples respectively. Final QC samples were prepared by adding 50.0 μl of each QC solution to 500.0 μl of plasma. Halofantrine (internal standard) stock solution was prepared by dissolving 10.0 mg into 20.0 mL of methanol which was then diluted 4 times in methanol to obtain working internal standard solution.
Preparation of samples for HPLC analysis: Blank plasma (500.0 μl) was mixed with 50.0 μl of lumefantrine standard solutions (for calibration/ standard curve); 50.0 μl of the internal standard (halofantrine: 100.0 μg/ml) ; and 50.0 μl of hydrochloric acid (0.1 M). For test samples (plasma with unknown concentrations), 50.0 μl of the internal standard and 50.0 μl of hydrochloric acid were added. In each unknown sample 50 μl of methanol was added to make its volume similar to that of STD and QC. The mixtures were vortexed for 5 s at 2000 U/min, then 2 ml of diethyl ether: ethyl acetate (2:1 v:v) was added and the mixture was shaken for 15 min at 300 U/min and then centrifuged for 10 min at 2750 g. The organic layer (1200.0 μl) was transferred into a tube and evaporated to dryness under a gentle stream of nitrogen at 40°C. The nitrogen gas was purchased from Tanzania Oxygen Limited, Dar es salaam, Tanzania. The nitrogen gas evaporation system was designed by the MUHAS Bioanalytical laboratory team and assembled locally by a blacksmith. The residue was reconstituted in 300.0 μl of mobile phase and shaken for 2 s at 2000 U/min. The solutions were transferred into auto sampler vials and 20.0 μl was injected into the chromatograph.
Method re-validation: Inter-day method linearity, precision and accuracy were assessed by processing one batch each day for three different days. Validation batches consisted of extracts of blank plasma spiked with internal standard, 8 calibration samples (0.05, 0.1, 0.2, 0.5, 1.0, 2.0, 5.0 and 10.0 μg/ml) and explicates for each of the 4 quality control samples (0.05, 0.1, 1.0 and 8.0 μg/ml). Unknown concentrations were run in a batch that had similar components except that quality control samples were run in triplicates.
Chromatographic conditions: The mobile phase was prepared by dissolving 9.52 g of potassium dihydrogen phosphate in 700 ml distilled water. The obtained solution was mixed with acetonitrile at a ratio of (7:13) and the mixture was adjusted to a pH of 3.1 with ortho-phosphoric acid. The pre column (LiChrospher 100) RP 18, 5 μm; 5×4 mm and the column (LiChrospher 100) RP18, 5 μm; 125 × 4 mm were used. The flow rate was 1.2 ml/min, detection was achieved at 335 nm and the total run time was 20 min.
Results
All the 3 laboratories finished the assignment and send their results to ACC for evaluation. However, due to reasons beyond the scope of the authors of this paper, it was not possible to access and include the analysis results from Kenya and Uganda. However, according to ACC, the results which were later on reported by these laboratories were also satisfactory. The results hereby presented, are based on MUHAS Bioanalytical Laboratory in Tanzania.
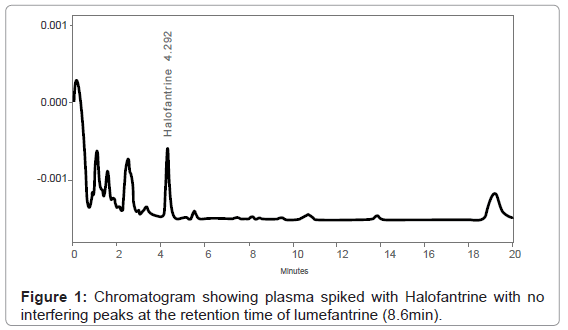
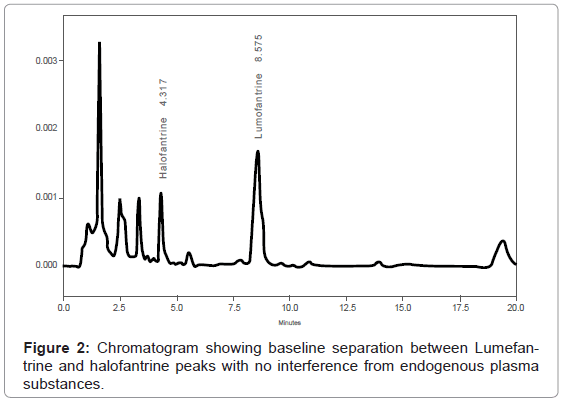
The method set up by laboratory was successful and analyte detection and better resolution was achieved without any adjustments. (Figure 1) demonstrates a chromatogram obtained from extracted blank plasma spiked with only internal standard. The peaks of halofantrine (internal standard) and lumefantrine eluted at 4.3 min and 8.6 minutes, respectively (Figure 2), and there were no interferences by endogenous plasma substances (Figure 1 and Figure 2) indicating good selectivity of method in analyzing biological samples. The retention times of the peaks obtained were always consistent.
Cross-validation of the method
Method validation was performed with respect to linearity, precision and accuracy in order to evaluate the reliability of the results. The obtained validation results are summarized in Table 1.
| Linearity | Precision (CV %) | Accuracy (RD %) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | correlation coefficient | High QC | Middle QC | Low QC | High QC | Middle QC | Low QC | |
| Day 1 | 0.003091 | 0.032631 | 0.9968 | 2.4 | 2.5 | 4.9 | 4.2 | 2.2 | 1.8 |
| Day 2 | 0.003115 | 0.025557 | 0.999 | 9 | 3.7 | 7.4 | -1.1 | -4.6 | -4.7 |
| Day 3 | 0.002956 | -0.01273 | 0.997 | 3.1 | 4 | 10.1 | 0.9 | -0.1 | -7 |
Table 1: The interday results for calibration curve, precision and accuracy of the method.
The precision of the method, expressed as percentage coefficient of variation (%CV) was evaluated with measurement of the high QC, middle QC and low QC samples in which the results ranged from 2.4 – 9, 2.5 – 4 and 4.9 - 10.1, respectively (Table 1). These values complied with requirements described in the ACC document of %CV < 15%.
Evaluation of the accuracy of the method was performed using the quality control samples mentioned above, where the difference between the observed and the assigned concentration values for each level were calculated and expressed as percentage values with RD (Table 1). All QC samples complied well with the 15% RD limit. The results varied from -7.0 to 4.2.
Determination of unknown concentrations
The result of the determination of the concentration of lumefantrine in the plasma samples that contained unknown levels are indicated in (Table 2). Sample 3 complied with the zero deviation limits for this concentration level. Sample 1 and 5 were within the deviation limit of 15% RD, however, samples 2 and 4 had values (-16.3% and -25.3%) which did not comply with the limit. The validation work and the concentration results of lumefantrine obtained from the plasma samples with unknown levels were in line with the acceptance criteria and assigned values. In general, we were able to accomplish the assigned task within the given time line (6 weeks) and the results obtained were in line with ACC requirements.
| Sample No. | Assigned Conc. (ng/ml) | Found conc. (ng/ml) | Deviation (%) |
|---|---|---|---|
| 1 | 600.0 | 510.1 | -15.0 |
| 2 | 7000.0 | 5857.7 | -16.3 |
| 3 | <50.0 | <50.0 | 0.0 |
| 4 | 180 | 134.4 | -25.3 |
| 5 | 2800.0 | 2398.7 | -14.3 |
Table 2: Results of determined lumefantrine levels from plasma samples containing unknown amounts compared to assigned levels.
The other two laboratories also had satisfactory results as per feedback from ACC Laboratory.
Discussion
This study involved inter-laboratory cross-validation of an HPLC method for determination of lumefantrine in plasma using Ultra Violet light (UV) detection. The method was developed by ACC laboratory in Leidersbach, Germany. In this study, the MUHAS bio analytical Laboratory completed analysis of the collaborative trial within the required time (6 weeks) and the cross validation results were reliable (Table 1 and Table 2). As illustrated by Figure 1 and Figure 2, the retention times of the obtained peaks were always consistent, indicating the capacity of our laboratory in setting up and cross-validating analytical methods developed elsewhere.
Transferring a method to another laboratory with different level of advancement and expertise and instrument specifications always faces challenges due to strict method validation requirements especially when dealing with bio analytical methods [9,10]. As opposed to drug formulations, analysis of biological samples deals with very low concentrations and is affected by many factors, including the variation in the biological matrix and presence of endogenous substances some of which may have some characteristics resembling the compounds of interest. Therefore, a bio-assay requires a sensitive and selective method enough to detect low concentrations of the analytes and overcome such interferences [11,12].
Based on our long term experience in developing and running bio analytical assays, it has shown that, adapting a method developed by others is not a straight forward task and sometimes it may require more than a month to achieve consistency and method cross-validation [9,10].
Bioanalytical Laboratories are currently gaining importance even in developing world where major infectious diseases (AIDS, Malaria and Tuberculosis) are predominant, affecting mainly poor people. Currently, Phase II, III and IV clinical trials have gained momentum in developing countries necessitating availability of locally well-functioning bioanalytical laboratories. This in turn will cut down the number of biological samples transported abroad.
Until the participation in the study hereby reported, the performance of the MUHAS bioanalytical laboratory was optimal but required improvement to international standards in terms of good laboratory practice.
This study has shown the importance of collaboration between North and South and has opened doors for building capacity of laboratories in the developing world through forming partnerships with laboratories in developed countries. Through this study the bioanalytical laboratory teams in Tanzania, Kenya and Uganda have gained considerable experience in performing bioanalytical procedures according to internationally acceptable standards. This suggests that, with further little infrastructural support and technological transfer from our partners, these laboratories will have adequate capacity to provide quality bioanalytical services to satisfy drug registration requirements; to support GCP based clinical trials and conduct therapeutic drug monitoring.
The MUHAS bioanalytical laboratory was established in collaboration with Karolisnka Institute (KI) scientists (Professor Lars Gustafsson) with support from Swedish development Agency (Sida) in 2003 and currently is equipped with 2 HPLC systems. So far the laboratory has supported 7 PhD theses and 4 master degree dissertations. There is now a strong will and commitment by both, the collaborating laboratory (ACC) and other development partners to transform this laboratory into a WHO qualified bioanalytical laboratory so that it can acquire adequate capacity to conduct BE studies in the region.
This work has set a benchmark for a need of continued collaborations with partner countries particularly, those with advanced technology and experience in conducting bio-analysis. This is crucial particularly in ensuring quality performance and sustainability of the emerging bio-analytical laboratories in developing world. With current emerging international support and other initiatives such as the EDCTP, the development of bio-analytical capacity in developing countries can be accelerated through forming partnerships with such laboratories in the developed world. Through such networks, bio-analysts in resource-poor countries will accumulate experience in bio-analytical science and technology especially through technological transfer from developed world, which is critical for sustainable capacity building [8].
As the East African countries move towards establishing a common market, the issue of ensuring availability of medicines with safety and acceptable quality is of paramount importance. Already, there are reports which indicated existence of some drugs with low bioavailability in the region [4,13,14]. It is our hope that the East African countries will make use of the emerging collaboration with ACC laboratories support in seeking advancement to meeting GLP requirements and international recognition of the existing bioanalytical laboratories in the region.
Conclusion
Based on the potential to improvement demonstrated by these laboratories, the results obtained by this collaborative trial indicate that, with further little infrastructural and technical assistance the capacity of these laboratories in conducting bio-analyses will be more strengthened and can serve as centers for training bioanalytics and running bioequivalence studies in the region.
Acknowledgements
This study was partly financed by WHO, Geneva for carrying out 2 days pretrial training in Dar-es-Salam by GIZ consultants Dr. Syed Laik Ali and Dr. Bernhard Scheidel, Managing Director of ACC in Leidersbach, Germany. Mrs. Dr. Kerstin Steigerwald of ACC supervised all the analytical work for this collaborative trial and helped also in the evaluation of the data submitted. GIZ in Eschborn, Germany covered all other costs and expenses for preparing the trial plasma samples, purchase of materials including the price of sending the deep frozen plasma samples in dry ice through World Courier to Tanzania. The MUHAS bioanalytical laboratory used some Funds from the Sida supported Malaria Project at MUHAS. We thank GIZ office in Tanzania for facilitating the handling and clearance of the samples sent from Germany. We also thank Ms Doris Nanage for participating in the bioanalytical part of this work.
References
- Minzi O, Mugoyela V, Gustafsson L (2011) Correlation between lamivudine plasma concentrations and patient self-reported adherence to antiretroviral treatment in experienced HIV patients. Ther Clin Risk Manag 7: 441-446.
- Minzi OM, Gupta A, Haule AF, Kagashe GA, Massele AY, et al. (2007) Lack of impact of artesunate on the disposition kinetics of sulfadoxine/pyrimethamine when the two drugs are concomitantly administered. Eur J Clin Pharmacol 63: 457-462.
- Heysell SK, Mtabho C, Mpagama S, Mwaigwisya S, Pholwat S, et al. (2011) Plasma drug activity assay for treatment optimization in tuberculosis patients. Antimicrob Agents Chemother 55: 5819-5825.
- Minzi OM, Massele A, Justin-Temu M, Ericsson O, Gustafsson LL (2006) Existence of antimalarial formulations with low bioavailability in Tanzania. Trop Doct 36: 93-97.
- Minzi OM, Moshi MJ, Hipolite D, Massele AY, Tomson G, et al. (2003) Evaluation of the quality of amodiaquine and sulphadoxine/pyrimethamine tablets sold by private wholesale pharmacies in Dar Es Salaam Tanzania. J Clini Pharm Ther 28: 117-122.
- Nsimba SE (2008) Problems associated with substandard and counterfeit drugs in developing countries: a review article on global implications of counterfeit drugs in the era of antiretroviral (ARVs) drugs in a free market economy. East Afr J Public Health 5: 205-210.
- Penzak SR, Acosta EP, Turner M, Tavel JA, Masur H (2004) Antiretroviral drug content in products from developing countries. Clin Infect Dis 38:1317-1319.
- Brockman A, Wu JT (2011) Basic Guidelines for Building a New GLP Bioanalytical Laboratory Part I &II: Qualifying and Staffing the facilities. American Pharmaceutical Review.
- Rozet E, Dewe W, Ziemons E, Bouklouze A, Boulanger B, et al. (2009) Methodologies for the transfer of analytical methods: a review. J Chromatogr B Analyt Technol Biomed Life Sci 877: 2214-2223.
- Lin ZJ, Li W, Weng N (2011) Capsule review on bioanalytical method transfer: opportunities and challenges for chromatographic methods. Bioanalysis 3: 57-66.
- Hartmann C, Smeyers-Verbeke J, Massart DL, McDowall RD (1998) Validation of bioanalytical chromatographic methods. J Pharm Biomed Anal 17: 193-218.
- Dadgar D, Burnett PE (1995) Issues in evaluation of bioanalytical method selectivity and drug stability. J Pharm Biomed Anal 14: 23-31.
- Kayumba PC, Risha PG, Shewiyo D, Msami A, Masuki G, et al. (2004) The quality of essential antimicrobial and antimalarial drugs marketed in Rwanda and Tanzania: influence of tropical storage conditions on in vitro dissolution. J Clin Pharm Ther 29: 331-338.
- Risha PG, Shewiyo D, Msami A, Masuki G, Vergote G, et al. (2002) In vitro evaluation of the quality of essential drugs on the Tanzanian market. Trop Med Int Health 7: 701-707.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14185
- [From(publication date):
May-2012 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 9595
- PDF downloads : 4590