Review Article Open Access
Insights for Developing Pharmacological Treatments for Psychostimulant Relapse Targeting Hypothalamic Peptide System
Morgan H. James, Jiann W. Yeoh, Brett A. Graham and Christopher V. Dayas*Neurobiology of Addiction Laboratory, School of Biomedical Sciences and Pharmacy and the Centre for Translational Neuroscience and Mental Health Research, University of Newcastle and the Hunter Medical Research Institute, Newcastle, NSW 2038, Australia
- *Corresponding Author:
- Christopher V. Dayas
School of Biomedical Sciences & Pharmacy
University of Newcastle and the Hunter Medical Research Institute
Newcastle, NSW 2308, Australia
Email: Christopher.Dayas@newcastle.edu.au
Received December 28, 2012; Accepted February 17, 2012; Published February 21, 2012
Citation: James MH, Yeoh JW, Graham BA, Dayas CV (2012) Insights for Developing Pharmacological Treatments for Psychostimulant Relapse Targeting Hypothalamic Peptide Systems. J Addict Res Ther S4:008. doi: 10.4172/2155-6105.S4-008
Copyright: © 2012 James MH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Effective pharmacotherapeutic treatment options for psychostimulant addiction are lacking, in part due to an incomplete understanding of the complex neural circuitry involved in renewed drug-seeking and relapse. The lateral hypothalamus (LH) has received renewed interest with respect to its role in addiction-related behaviours, prompted largely by the identification of a number of hypothalamic neuropeptides shown to be important mediators of reward-seeking. In particular, orexin (hypocretin) and cocaine- and amphetamine-regulated transcript (CART) have been shown to play largely opposing roles in feeding behaviour and these roles have recently been shown to extend to drug-seeking behaviour. We have previously proposed that these two peptide systems may interact with the ‘classical’ reward circuitry via the paraventricular thalamus (PVT), as this region is densely innervated by both orexin and CART –positive fibres and projects to a number of regions critical to drug-seeking [1]. The present review provides a comprehensive overview of the current literature implicating both orexin and CART in drug-seeking and relapse behaviour and presents a revised summary of the role of the PVT in mediating the actions of these two peptides. In addition, we provide novel data demonstrating that blockade of orexin receptor 1 (OXR1) signaling within the ventral tegmental area (VTA) alters Fos-protein expression in relapse-relevant regions, including the PVT and the nucleus accumbens shell (NacS). Further, whilst previous findings have shown that blockade of OXR1 in the VTA prevents reinstatement of cocaineseeking, we show here that this treatment does not affect natural reward seeking for sweetened condensed milk. In light of the reviewed body of literature, as well as the novel data presented, we discuss the considerations for future pharmacotherapies targeting the orexin and CART systems for relapse prevention.
Keywords
Hypothalamus; Orexin; Hypocretin; CART; Cocaine; Reinstatement; Relapse; Fos; Reward seeking; Drug seeking
Introduction
The last two decades have seen significant advances in our understanding of the brain circuitry and molecular changes that drive the addiction process. However, relapse to drug taking continues to represent a significant impediment to the successful treatment of addiction. Indeed, treatment options such as effective pharmacotherapies are lacking, particularly in the case of psychostimulant addiction [2]. Despite significant recent progress, it is likely that the lack of pharmaceutical options to treat this phase of the addiction cycle is in part due to the inadequate understanding of the complex interactions within the neural circuitry that triggers renewed drug-seeking and relapse. Accordingly, in the present review, we aim to highlight some of the recent progress in our understanding of the brain mechanisms underpinning psychostimulant relapse. In particular, we will focus upon the emerging evidence of an important role for neuropeptide systems expressed within the lateral hypothalamus (LH) in modulating drug-seeking and relapse to psychostimulant use. Of the multiple peptides expressed in the LH that appear to regulate reward-seeking, we will focus on two, orexin and cocaine- and amphetamine- regulated transcript (CART), which appear to have opposing effects on food-seeking behaviour. We outline current research that is investigating how these peptide systems might interact with the ‘classic’ neural circuitry implicated in the addiction and relapse process, as well as other regions that have ‘re-emerged’ as potential mediators of addiction-related behaviours [3].
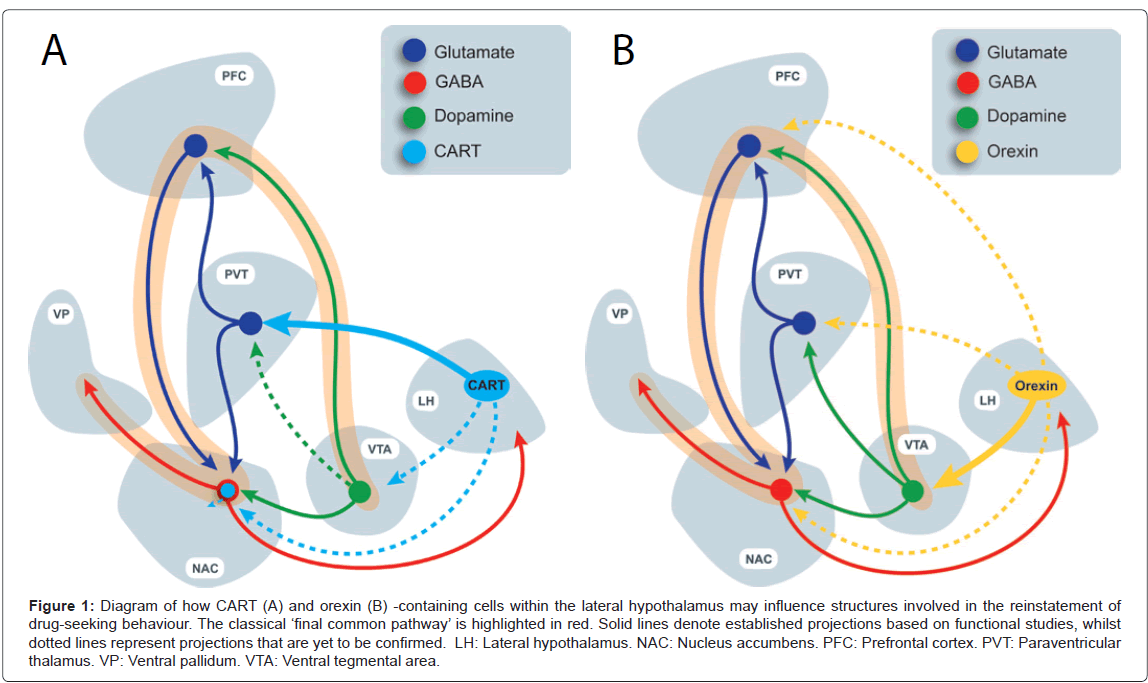
Although imaging techniques for human research have dramatically improved, much of the progress in our understanding of the brain mechanisms involved in addiction and relapse continues to come from preclinical studies. Using experimental animals, researchers have attempted to model human relapse through the use of the experimental procedure known as REINSTATEMENT. Progress from such studies has led to the formulation of a putative ‘final common pathway’ comprised of a series of interconnected brain regions shown to be critical to reinstatement or relapse-like behaviour [4,5]. Briefly, this pathway is thought to involve glutamatergic projections from the prefrontal cortex (PFC) to the nucleus accumbens (NAC), which in turn results in the disinhibition of the ventral pallidum [4,5] (Figure 1). Importantly, dopamine released in the PFC, amygdala and NAC is thought to be important for drug-motivated behaviours including relapse-like behavior [6-8]. Dopamine input to these regions arises primarily from the ventral tegmental area (VTA) A10 dopamine neurons [9-14]. Although activation of this pathway is clearly central to the drug-seeking response, other brain regions have recently been found to, or have ‘re-emerged’ as, important modulators of this ‘brain relapse network’ [3]. As such, there is a significant current focus on understanding how these regions of the brain may interface with the ‘classic relapse circuitry’ described above. In particular, significant recent attention has been given to the hypothalamus, as well as the paraventricular thalamus (PVT), the latter being a proposed integrator and relay of hypothalamic neuropeptide signals to higher centres.
Figure 1: Diagram of how CART (A) and orexin (B) -containing cells within the lateral hypothalamus may influence structures involved in the reinstatement of drug-seeking behaviour. The classical ��?final common pathway��? is highlighted in red. Solid lines denote established projections based on functional studies, whilst dotted lines represent projections that are yet to be confirmed. LH: Lateral hypothalamus. NAC: Nucleus accumbens. PFC: Prefrontal cortex. PVT: Paraventricular thalamus. VP: Ventral pallidum. VTA: Ventral tegmental area.
Renewed Interest in Hypothalamic and Dorsal Thalamic Circuitry in Mediating Drug ‘Relapse’
Hypothalamus
The hypothalamus has long been recognized as an important regulator of reward-seeking behavior [15,16]. For example, early studies implicated the LH as the brain’s ‘feeding centre’ following demonstrations that stimulation of the LH resulted in an increase in feeding behaviour and lesions of the LH promoted aphagia and weight loss [15]. In contrast, the ventromedial hypothalamus (VMH) was thought to function as a ‘satiety centre’, with lesions of this region reported to produce hyperphagia and obesity [16]. Although this ‘dualcentre’ hypothesis is undoubtedly oversimplified, this body of work has been influential in establishing that the LH is a key component of a ‘brain reward-seeking system’ [17,18]. Additional support for this proposal can be found in the work of Olds and Milner [19] showing that the lateral portion of the hypothalamus supports INTRACRANIAL SELF-STIMULATION (Table 1) as well as anatomical studies demonstrating that the LH is reciprocally connected with key nodes of the brain circuitry that control motivation and reward, including the PFC, NAC and VTA [20]. Surprisingly, despite this well-established role in reward seeking, only recently has significant attention returned to the LH in terms of its involvement in pathological rewarding-seeking behavior [21]. An important catalyst for this renewed interest in LH control of reward-seeking was the discovery of the hypothalamic neuropeptide transmitters cocaine- and amphetamine- regulated transcript (CART) and orexin (hypocretin). Interestingly, following their identification in the late nineties, studies have generally showed opposing roles for orexin and CART in feeding behaviour. Indeed, subsequent work has shown that a similar relationship may exist for drug-seeking behaviour. Thus, a role for orexin in promoting relapselike behaviour is now well established and recent reports suggest that CART may negatively modulate relapse-like behaviour. These findings have driven speculation that modulators of LH neuropeptide systems may lead to new treatments for psychostimulant relapse and other neurological disorders [22].
Paraventricular thalamus
The PVT is another structure that has undergone a ‘renaissance’ of sorts in relation to its role in motivated behaviors. The PVT is a component of the dorsal midline thalamic group [23] and has been implicated in stress reactivity [24], reward [25] and general arousal [25]. Our interest in the PVT was stimulated by studies showing that the PVT is densely innervated by peptide expressing neurons originating from the hypothalamus [26-28], as well as midbrain dopamine and brainstem catecholamine neurons [29]. Compellingly, the PVT receives perhaps the strongest orexin and CART peptide input in the forebrain and anatomical evidence indicates that this region may relay hypothalamic neuropeptide activity to the striatal axis and other reward-related regions. For example, Parsons et al. [28] showed that NAC shell-projecting neurons in the PVT receive both orexin and CART terminals. These findings provide an anatomical basis for the influential proposal by Kelley and colleagues [25] that the PVT is as a key relay in a hypothalamic-thalamic-striatal axis that regulates motivation and reward-seeking [25]. Interestingly, the PVT also has strong projections to other important reward-related brain regions, including the PFC, NAC and basolateral amygdala [30-37]. Indeed, anatomical studies have shown that single PVT neurons send branched projections to both the NAC and PFC [30,33]. It is also noteworthy that early studies suggested an important role for the PVT in drug-related behaviours, with lesions of the PVT shown to block BEHAVIOURAL SENSITISATION to cocaine [38]. Furthermore, Fos-protein expression has been shown to increase in the PVT following re-exposure to cocaine-paired environments after experimenter administered drug injections [39,40].
| Behavioural sensitisation: A progressive increase in the locomotor-activating effects of drug following repeated exposure to a set drug dose (typically 5-10 days). The expression of behavioural sensitisation is typically assessed during withdrawal following an acute drug challenge at a dose equivalent to that administered during the sensitisation exposure. |
| Conditioned place preference (CPP): A classical (Pavlovian) conditioning paradigm whereby animals are administered either drug or saline (delivered intraperitoneally). After injection animals are immediately placed into one of two distinct contexts that differ in terms of their contextual cues (e.g. wall colour/pattern, floor texture). Following conditioning, the animals are tested for their preference of the drug- vs. saline- paired chamber, as measured by time spent in each chamber. An increase in preference for the drug-paired context is thought to indicate a positive reinforcing effect of the drug. |
| Conditioned place aversion (CPA): Similar to CPP, however, one context is associated with an aversive stimulus, whilst the other is associated with a neutral stimulus. Following conditioning, animals are tested for their preference for the contexts. An increase in preference for the neutral context indicates an aversive effect of the test stimulus. |
| Operant drug self-administration model: A procedure whereby animals are trained to respond (typically lever press or nose poke) for a drug reward. Psychostimulants such as cocaine and amphetamine are typically delivered to the animal via a chronic indwelling jugular catheter, whereas drugs such as alcohol are delivered in small volumes for oral consumption. This model can also be used to train animals to respond for natural rewards, such as a sucrose solution or sucrose/food pellets. |
| Extinction learning: A reduction in drug-seeking produced by breaking the contingency between drug-seeking behaviour or drug-predictive stimuli and delivery of drug reward. Importantly, extinction learning involves new learning, and is not a ‘forgetting’ of the previous association. |
| Intracranial self-stimulation (ICSS): A procedure whereby animals learn to respond for direct electrical stimulation via an electrode implanted into a specific region of the brain. Higher rates of responding for ICSS are thought to be caused by decreased ‘reward’ pathway sensitivity whereas lower rates of responding for ICSS are thought to result from increased ‘reward’ pathway sensitivity. |
| Progressive ratio schedule: A reinforcement schedule applied to the drug self-administration procedure to assess an animals’ motivation to obtain a natural or drug reward. Under this schedule, the number of responses required to obtain a drug reward is increased following every reward. The point at which the animal ceases to press is referred to as the animals’ ‘break point’. |
| Operant reinstatement models of relapse-like behaviour: In the drug self-administration procedure, animals are trained to self-administer drug before their responding is extinguished to a set criterion e.g. numbers of days of extinction training or number of responses/session. After reaching the extinction criterion, ‘reinstatement’ of responding can be elicited by exposure to drug-associated stimuli, psychological stress or re-exposure to the drug itself (also known as a drug-prime) – analogous stimuli are thought to elicit drug-seeking and relapse in humans. |
| Reinstatement of a CPP: CPP is induced by drug administration as described above. The CPP is then extinguished by repeatedly placing the animal into the CPP apparatus without drug/saline injections. Reinstatement of a CPP can be induced by drug-priming injections or exposure to stress. |
| Renewal: Animals are trained to self-administer drug in Context A, before drug-seeking is extinguished in Context B. Context A and B differ from one another in terms of various contextual cues, including wall colour/pattern, floor texture. ‘Renewal’ of drug seeking can then be elicited by placing the animal back into Context A. |
Table 1: Glossary of terms.
Based on these observations, we recently proposed that the PVT is a site central to the integration of CART and orexin signaling in controlling drug-seeking [1]. Consistent with our hypothesis, we recently identified an interesting anatomical relationship involving CART and orexin terminals closely apposed to PVT neurons recruited by exposure to ethanol-linked discriminative cues in an operant reinstatement paradigm to model alcohol relapse [1]. Important studies have shown that a role for the PVT extends to renewal of alcohol-seeking [37,41], reinstatement of cocaine-seeking [42,43], as well as the expression of EXTINCTION behaviour [44,45]. Below, we review the evidence implicating both the CART and orexin peptides in psychostimulant relapse and discuss recent data demonstrating that CART signaling within the PVT appears to negatively modulate drug seeking behavior. We also review recent findings from our laboratory demonstrating that, in contrast to our original hypothesis, orexin signaling in the PVT does not appear to be critical to drug-seeking behavior. We therefore discuss evidence that the VTA is a more likely target of relapse-relevant orexin signaling. In addition, we present new, unpublished, data showing that orexin receptor antagonism in the VTA is associated with changes in Fos-protein expression patterns in relapse-relevant regions. We also show that whilst VTA orexin receptor antagonism prevents cue-induced cocaine-seeking behavior, similar doses of this antagonist does not affect natural reward-seeking. These preliminary data raise the possibility that orexin antagonisms for relapse prevention may be possible without producing significant off-target effects. Firstly, however, we review the historical evidence linking both CART and orexin with rewarding-seeking in general and subsequently their role in drug-motivated behaviours.
The CART Neuropeptide System
In 1996, Douglass and colleagues [46] identified an mRNA that was upregulated in the striatum of rats following acute cocaine and amphetamine administration. Interestingly, this mRNA was found to encode a gene product that had been identified by Spiess et al. [47] in the ovine hypothalamus close to 15 years earlier. This peptide was subsequently named cocaine- and amphetamine- regulated transcript (CART). Human and rat CART mRNA share 91% sequence homology but despite this similarity the rat has long and short splice variants of the CART peptide whereas only the short form is present in humans [48]. Two active fragments of the rat long form (CART 55-102 and CART 61-102) have been identified, however, a CART receptor has yet to be fully characterized, meaning that a specific CART receptor antagonist is yet to be developed. CART cell bodies are expressed in a number of hypothalamic regions, including the LH, the paraventricular nucleus (PVN), supraoptic nucleus and the arcuate nucleus (ARC) [46-50]. In addition to this distribution, CART is co-expressed with a number of other neuropeptides including thyrotropin-releasing hormone (TRH) in the PVN, melanin concentrating hormone (MCH) in the DMH/LH and proopiomelanocortin (POMC) in the ARC nucleus and retrochiasmatic area (RCA). It is interesting and somewhat surprising that within the ARC nucleus, CART is co-expressed with the POMC-derived antifeeding peptide α-melanocyte stimulating hormone (α-MSH) whereas in the DMH/LH CART is expressed with the pro-feeding peptide MCH [18,49,51]. (Although not a focus of this review, it is interesting that MCH appears to have pro drug-seeking effects within the NAC [52]. These findings raise the possibility that different populations of CART neurons may subserve different functions. Beyond the hypothalamus, CART peptide-expressing neurons are found in the NAC, VTA and amygdala [50,53]. Interestingly, approximately 15% of CART terminals in the VTA contain MCH, whilst CART peptides also co-localise, albeit to a lesser extent, with GABA and dynorphin in both the VTA and substantia nigra [53]. CART-cell bodies are also found in the peripheral nervous system, including myenteric neurons in the gastrointestinal tract, sympathetic preganglionic neurons and in the adrenal glands [54,55]. In keeping with this broad distribution and diverse neurochemical phenotypes, CART has been shown to be involved in a number of functions, including regulation of food intake, maintenance of body weight, reward and endocrine functions [56].
CART and feeding behaviour
Early studies investigating the effects of CART on reward-seeking firmly established a role for this peptide in the regulation of feeding behaviour. Acute i.c.v. CART administration dose-dependently suppresses feeding behavior in rats [57,58], whilst chronic CART treatment prevents body weight gain in both lean and obese animals [48]. Because the identity of the CART receptor is unknown, pharmacological manipulations to antagonize the CART system have been limited to the use of antibodies raised against the active peptide sequence. Thus, central administration of CART antiserum increases feeding, corroborating findings that CART peptide injections exert an inhibitory influence on feeding behaviour [57,58]. Consistent with these functional effects, food deprived animals show a marked decrease in CART mRNA expression in the ARC nucleus and CART mRNA is almost completely absent from obese animals with disrupted leptin signalling [58]. Somewhat surprisingly though, intra-DMH infusions of CART have been shown to increase feeding [59], which may be attributed to a disinhibitory effect of CART in the DMH [60].
CART and addiction-relevant behaviours
A role for CART in drug-motivated behaviours has been evident since the original report by Douglass and colleagues [46] who showed that acute cocaine and amphetamine administration increases CART mRNA in the striatum of the rat brain. This finding has been difficult to replicate [61] and some studies have suggested that binge/repeated, rather than acute, cocaine administration is required to reliably increase CART transcript expression in the brain [62,63]. Despite these technical caveats, acute cocaine has been shown increase the proportion of Fos-immunoreactive CART neurons in the NAC, even under conditions that do not produce changes in CART mRNA levels [64]. Finally, strong evidence for a role of CART in psychostimulant use comes from reports that CART mRNA levels are increased in the VTA and NAC of human cocaine overdose cases [65,66]. In addition, alcoholism has been shown to be associated with a mutation in the CART gene in a Korean population [67] and acute administration of ethanol increases CART mRNA and peptide expression in the rat NAC [68].
Pre-clinical studies have demonstrated a clear, but complicated, role for CART in meditating the effects of psychostimulants. Administration of CART into the VTA attenuates cocaine induced locomotor activation, especially at higher doses [69], whereas infusions of CART into the NAC have no effect on locomotor activity [70]. However, NAC injections of CART immediately prior to systemic cocaine or amphetamine treatment attenuates the locomotor activating effects of these drugs [70] and prevents the expression of conditioned hyper-locomotion [71].
Further, direct co-administration of CART and dopamine into NAC reduces the cocaine-like locomotor-activating effects of dopamine [72]. Consistent with this effect, injections of CART into the VP, one of the main nuclei that receive accumbal efferents, also attenuate cocaine-induced locomotion [73]. In contrast to this evidence for a CART-mediated inhibitory effect on addiction-like behaviors, infusion of CART 55-102 peptide fragment into the VTA causes an efflux of dopamine in the NAC resulting in a cocaine-like increase in locomotor activity and can even produce a CONDITIONED PLACED PREFERENCE (CPP) [74]. Finally, Rademacher et al. [75] showed that at low doses (2μg/side), intra-basolateral amygdala injections of CART produced CPP, whilst higher doses (4μg/side) produced CONDITIONED PLACE AVERSION (CPA). Together, this work clearly establishes a role for CART in addiction-like behaviour, but also suggests that the underlying mechanisms are complex.
With respect to reinstatement of drug-seeking behaviour, a potential role for CART was first alluded to in the study of Mattson and Morrell [76]. These authors showed that conditioned cues associated with passive cocaine administration increased the number of CART-positive neurons in the NAC. Since this report, a number of subsequent studies have provided evidence for the role of hypothalamic CART cells in regulating drug-seeking behaviours. For example, Dayas et al. showed that exposure to drug-associated cues and ethanol-seeking behaviour were associated with an increase in Fos/CART-positive cells within the ARC nucleus, but not the DMH/ LH [1]. Interestingly, Millan et al. [41] showed that reinstatement of alcohol-seeking elicited by inactivation of the NAC shell is associated with an increase in PeF hypothalamic CART cells expressing c-Fos.
As discussed above, we previously proposed that the PVT might be a central site through which hypothalamic CART activity is relayed to critical reinstatement-related brain regions. Consistent with this suggestion, alcohol-associated cues activate cells within the PVT that are closely apposed to CART terminals [1]. We recently provided further support for this hypothesis by demonstrating that intra-PVT infusions of the CART 55-102 peptide fragment dose-dependently decreased drug-primed reinstatement of cocaine-seeking [43]. To date, only one other study has examined the functional effects of CNS infusions of CART on reinstatement behaviour. Consistent with our findings, King and colleagues [77] showed that i.c.v. infusion of CART 55-102 prevents context-elicited renewal of alcohol-seeking. While it is unclear from this study where CART acts to inhibit context-elicited reinstatement, the PVT is considered a potential candidate given its known reactivity to drug-associated cues and contexts [37,42]. Indeed, preliminary findings from our laboratory suggest that intra- PVT infusions of CART 55-102 attenuate cue-induced reinstatement of cocaine-seeking. Importantly, there also exists a possible role for CART in stress-induced reinstatement. CART mRNA and peptide is expressed throughout the hypothalamic-pituitary-adrenal (HPA) axis and other regions of the brain known to regulate the HPA axis, including the prefrontal cortex and amygdala [46,50,78], while i.c.v and intra-PVN administration of CART increases circulating ACTH and corticosterone levels [79]. Future studies must therefore determine how CART signaling within the PVT and other stress-related regions may influence stress-induced reinstatement of drug-seeking, as based on the literature above, it seems plausible that CART-peptide injections into the PVT or stress-responsive regions may have prodrug- seeking effects.
With respect to how CART within the PVT may modulate the brain relapse circuitry, anatomical studies show that glutamatergic efferents from the PVT are closely associated with dopamine immunoreactive terminals in the NAC shell. Moreover, stimulation of the PVT increases dopamine release within this part of the ventral striatum [34]. The NAC is critically involved in the reinstatement of drug-seeking [80,81] and dopamine signaling in this region is necessary for drug-primed [82] and context-elicited [8] reinstatement. Taken together, CART signaling within the PVT may act to inhibit reinstatement by modulating the responsivity of NAC neurons to cocaine-induced dopamine release [43].
Further to its role in the PVT, CART neurons also project to the VTA and their interaction with dopamine neurons suggests they may influence reinstatement. In the VTA, nerve terminals that contain CART mRNA and peptide contact dopamine neurons as well as GABAergic interneurons [50,53]. It has been proposed that CART may therefore regulate dopamine release from the VTA either directly or via the disinhibition of GABA interneurons [83]. Further, CART is also expressed in NAC, particularly within the medial part of the shell [84], which corresponds with the region of the ventral striatum that receives the strongest innervation by dopaminergic neurons [85]. TH-positive terminals make contact with CART peptide-containing GABAergic neurons in the NAC [84], suggesting that a functional relationship exists between midbrain dopaminergic cells and CARTpeptide containing striatal neurons. Finally, MCH neurons in the LH project to the NacS [86] and MCH terminals exist in the NacS [86]. Given that LH MCH neurons also express CART [49], it is probable that a direct CART projection from LH to NacS exists (Haemmerle, Bittencort and Dayas, personal communication, 2012) - although it is currently not known if CART is released from these MCH-expressing LH-projection neurons.
Work using an alcoholic beer RENEWAL model has also provided data supporting a role for CART signaling pathway in the expression of EXTINCTION through a circuit involving the PVT, the NAC shell (NACsh) and hypothalamus [41]. Specifically, McNally and colleagues showed that extinction in this model appeared to recruit neurons in the NACsh and possibly the infralimbic prefrontal cortex (IL-PFc) that project to the mediodorsal hypothalamus [45]. Further, Marchant et al. [44] showed that expression of extinction involved a subset of CART sensitive neurons in the MDH that express dynorphin and project to the PVT. Consistent with this circuitry, infusing the κ-opioid receptor agonist U50488 into the PVT promoted the expression of extinction - i.e. prevented drug-seeking behaviour.
In summary, given the above literature, a role for CART in addiction is now well established, however, a number of questions remain to be answered before pharmacological manipulation of this system can be realistically evaluated. For example, it will be important for future studies to determine the role of CART in mediating both cue- and stress-induced reinstatement, as well as the central site(s) of action in these processes. Further, the role for CART during extinction versus reinstatement warrants further investigation. Moreover, a greater understanding of the role of CART in Addiction related behaviours and indeed reward-seeking in general, presumably awaits the characterization of the CART receptor(s).
The Orexin Neuropeptide System
Orexin-expressing neurons are located in the DMH, PF and LH areas and secrete two peptides (orexin A & orexin B) derived from the same precursor gene (prepro-orexin) [87]. It should be noted that two research groups independently identified these peptides almost concurrently, with one group naming these peptides orexins [87] and the other naming them hypocretins [88]. The orexin peptides are highly conserved between humans and rodents, with identical orexin-A sequences and just two amino acid substitutions in orexin-B. Unlike the CART system, where the search for an endogenous receptor continues, orexin peptides have been shown to interact with two G-protein-coupled receptors; Orexin receptor 1 (OXR1) and orexinreceptor 2 (OXR2). Orexin A binds to both OXR1 and OXR2 with equal affinity, whereas orexin B binds to OXR2 with a higher affinity than OXR1 [89]. OXR1s are found in the PFC IL, hippocampus, PVT, VTA, VMH, dorsal raphe nucleus and locus coeruleus. OXR2s are expressed in regions including the prefrontal and insular cortices septal nuclei, hippocampus, medial thalamic groups, VTA, raphe nuclei and hypothalamic nuclei including the tuberomammillary nucleus, dorsomedial nucleus, paraventricular nucleus (PVN) and ventral premammillary nucleus [90-92]. Consistent with this distribution, the function of orexin peptides is diverse. Thus, in addition to reward-seeking, orexins have been implicated in the regulation of sleep [93], energy metabolism and the maintenance of arousal [94,95]. Interestingly, nearly all orexin neurons co-express dynorphin-A [96] and glutamate [97]. It is also important to note that vagal and spinal primary afferent neurons, enteric neurons and endocrine cells in both the gut and pancreas display orexin- and orexin Receptor like immunoreactivity [98] and exogenous orexin administration has been shown to excite secretomotor neurons, modulate intestinal motility and secretion [98-100] and influence hormone release from pancreatic endocrine cells [101].
Orexin and feeding behaviour
Consistent with its anatomical location within the LH Reward seeking zone, central administration of both orexin A and B has been found to dose-dependently initiate food-seeking [87,102,103] while systemic administration of the OXR1 antagonist SB-334867 has the opposite effect [104-107]. Furthermore, prepro-orexin mRNA is upregulated following fasting [87], whereas obese mice (ob/ob and db/db) show decreased prepro-orexin gene expression [108]. It is important to recognize the possibility however, that the effects of orexin on feeding behaviour may reflect the role of orexin in the maintenance of arousal and locomotor activity, both of which are essential for food-seeking behaviour following periods of fasting [18] (but see [109]). In contrast, SB-334867 has no effect on reinstatement of high-fat food seeking elicited by i.c.v. administration of orexin A, pellet-priming or yohimbine, suggesting that the OXR1 plays a minimal role in reinstatement of food-seeking [110].
Orexin and addiction
An interesting series of observations highlighting the potential role for orexin in addiction includes the low CSF orexin-A levels in patients with the neurological disorder narcolepsy [111-113] and the lack of dependence in these patients despite receiving amphetamine treatment [114]. The first experiment directly implicating orexin in addiction was the demonstration that orexin knock-out mice display attenuated morphine dependence [115]. More recent studies have showed that blockade of the OXR1 reduces self-administration of alcohol [116-118], nicotine [119,120] and high-fat food [110]. With respect to psychostimulants, OXR1 blockade does not appear to reduce cocaine self-administration under normal conditions, but does reduce the extent to which rats are willing to work for cocaine reward under a PROGRESSIVE RATIO SCHEDULE [121]. These data suggest that orexin may not play a large role in the primary rewarding effects of psychostimulants, but may modulate circuitry that drives the motivation of goal-directed behavior [121,122]. Interestingly however, orexin signaling does appear to modulate the rewarding properties of psychostimulants under some experimental conditions, as treatment with SB-334867 reduces the acquisition and expression of Cocaine conditioned reinforcement and the expression of Amphetamine induced CPP [123]. A clear role for OXR1 signaling has also been shown in the development of BEHAVIOURAL SENSITISATION to psychostimulants. For example, SB-334867 treatment prevents sensitization following repeated cocaine and amphetamine treatment [124,125]. In comparison, OXR2 signaling appears to primarily mediate wakefulness and arousal and play less of a role in mediating reward seeking, however, infusions of orexin B (presumably acting on orexin-2 receptor) into the VTA increases preference for morphine [126] and repeated cocaine exposure produces an up-regulation of OXR2 levels in the NAC [127]. It is also worth highlighting recent evidence that blockade of the OXR2 can prevent ethanol Self-administration, place preference and reinstatement [128].
With respect to drug-seeking behaviour, activation of orexin cells, as assessed by Fos-protein, has been associated with reinstatement of drug-seeking using different procedures. Importantly, Harris et al. [129] showed that re-exposure to cocaine- and morphine- associated contexts in a CPP paradigm increased the percentage of LH orexin neurons that express Fos. Further, the proportion of Fos-positive LH orexin neurons was strongly correlated with the propensity for reinstatement of CPP [129]. These authors also provided evidence that Fos-protein expression increased specifically within LH orexin neurons, as correlations between Fos expression and reinstatement of CPP was not observed with PeF or DMH orexin neuron populations, nor for non-orexin neurons within the LH [129]. Dayas et al. [1] showed in an operant self-administration reinstatement procedure that re-exposure to alcohol-associated cues increases the numbers of DMH and PF/LH Fos-positive orexin neurons. Interestingly, Hamlin et al. [130] also implicated PeF orexin neurons in the processes that are required for drug-seeking but perhaps not the drug-seeking response itself. These findings demonstrate the importance of understanding the role of distinct subpopulations of orexin neurons within the DMH, PFA and LH. Indeed, recent evidence suggests that there are two distinct subgroups of orexin cells with unique firing properties and morphologies [131] and it will be important to further understand how these different populations may differentially influence drugseeking behaviour.
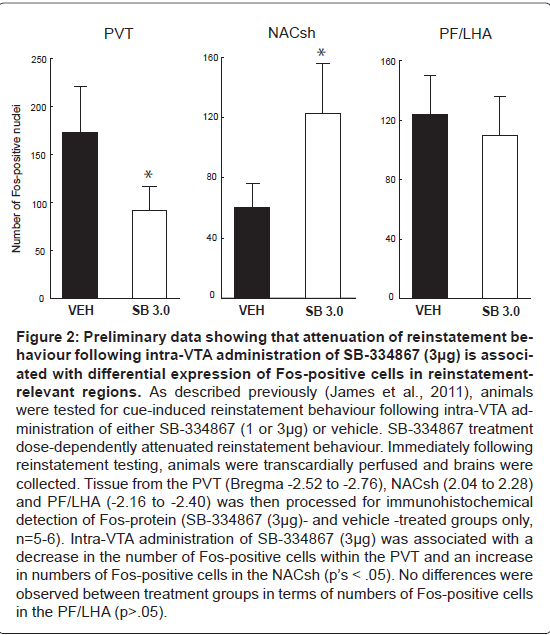
Figure 2:Preliminary data showing that attenuation of reinstatement behaviour following intra-VTA administration of SB-334867 (3�?µg) is associated with differential expression of Fos-positive cells in reinstatementrelevant regions. As described previously (James et al., 2011), animals were tested for cue-induced reinstatement behaviour following intra-VTA administration of either SB-334867 (1 or 3�?µg) or vehicle. SB-334867 treatment dose-dependently attenuated reinstatement behaviour. Immediately following reinstatement testing, animals were transcardially perfused and brains were collected. Tissue from the PVT (Bregma -2.52 to -2.76), NACsh (2.04 to 2.28) and PF/LHA (-2.16 to -2.40) was then processed for immunohistochemical detection of Fos-protein (SB-334867 (3�?µg)- and vehicle -treated groups only, n=5-6). Intra-VTA administration of SB-334867 (3�?µg) was associated with a decrease in the number of Fos-positive cells within the PVT and an increase in numbers of Fos-positive cells in the NACsh (pâ�?�?s < .05). No differences were observed between treatment groups in terms of numbers of Fos-positive cells in the PF/LHA (p>.05).
In order to confirm a functional role for orexin cell activation in reinstatement of CPP, Harris and colleagues [129] microinfused the Y4 receptor agonist rat pancreatic polypeptide (rPP) directly into the LH. As predicted by the expression of Y4 receptors in orexin cells within LH, this manipulation induced Fos expression in LH orexin neurons and robustly reinstated extinguished morphine CPP. A functional role for orexin signaling in psychostimulant reinstatement has also been well established by experiments using operant based procedures. In a key study, Boutrel and colleagues [132] demonstrated that i.c.v. infusion of orexin-A reinstated extinguished cocaine-seeking and that the OXR1 antagonist SB-334867 reduced reinstatement elicited by exposure to acute footshock stress. Similarly, Lawrence et al. [116] found that pretreatment with SB-334867 (i.p.) abolished olfactory cue-induced reinstatement of alcohol-seeking behaviour. Since these seminal studies, the orexin system has been shown to mediate various forms of reinstatement. For example, systemic administration of the OXR1 antagonist SB-334867 (10-30mg/kg) blocks reinstatement of extinguished cocaine-seeking elicited by both discrete cues [133] and discriminative contexts previously associated with cocaine availability [134]. These findings extend to non-extinguished cocaine-seeking, as SB-334867 pretreatment (10-30mg/kg) attenuates drug-seeking in cocaine-associated contexts following two weeks of abstinence in the home cage [134].
The orexin system also appears to interact with stress pathways to regulate footshock-induced reinstatement [132]. Specifically, in this paradigm administration of the corticotropin-releasing factor (CRF) anatagonist D-Phe- CRF (12-41) and the α2-noradrenergic agonist clonidine, prevents reinstatement induced by i.c.v. orexin, suggesting that orexins may mediate footshock-induced reinstatement by recruiting these stress systems. Importantly, antagonism of OXR1 does not appear to block the priming effects of psychostimulants on reinstatement behaviour, as pretreatment with SB-334867 (10-30mg/ kg, i.p.) had no effect on reinstatement of cocaine-seeking induced by an acute cocaine injection [135].
As discussed above, the PVT has been proposed as an important recipient site for hypothalamic orexin signaling in reward-seeking (eg. [25]). Indeed, we proposed that orexin signaling within the PVT might be critical to reinstatement of drug-seeking. Consistent with this suggestion, drug-associated cues activate PVT neurons that are closely apposed to orexin fibres [1] and orexin terminals make putative contact with PVT neurons that project to the NAC [34]. Somewhat surprisingly therefore, we recently reported that intra-PVT infusion of SB-334867 has no effect on cue-induced reinstatement of Cocaine seeking [136], suggesting that orexin signaling, at least through OXR1, in this region is not critical to reinstatement behaviour. Consistent with these data, subsequent preliminary work from our laboratory suggest that infusions of orexin-A into the PVT does not reinstate extinguished drug-seeking behavior. However recent evidence from another group has shown that at small doses, cocaine-seeking can be reinstated by intra-PVT infusions of orexin-A [137]. In addition to these recent findings, a role for PVT-based orexin signaling in reinstatement behaviour cannot be ruled out, as this region also strongly expresses OXR2 [90] and orexin B has been shown to produce stronger actions of PVT neurons than orexin A [138]. Further, the PVT is known to play an important role in reactivity to psychological stress [24] and orexin signaling in the PVT has been shown to modulate anxiety-like behaviour through CRF and κ-opioid receptors [139]. Finally, OXR1signaling in the PVT has been shown to be important for the ability to adapt to repeated stress [140]. Therefore, it will be for future studies to determine the function of OXR2 signaling in this region, as well as to investigate the potential role for the PVT in Stress induced drug-seeking.
The VTA is another reward pathway structure that appears to be a possible target of addiction-relevant orexin signaling. Although very few ‘classic’ synaptic contacts between orexin axons and VTA dopamine neurons exist [141], both OXR1 and OXR2 have been identified in VTA dopamine cells [142]. Importantly, functional electrophysiological studies have repeatedly shown that orexins modulate dopaminergic neuronal activity within the VTA [143,144]. For example, Korotkova et al. [144] showed that orexins increase the firing rate and can also elicit burst firing of VTA dopamine neurons. These authors also showed that subpopulations of dopamine neurons appear to respond to orexin A versus B and can express OXR1, OXR2, or both receptor subtypes. In work that described a potential pathway for orexins to enhance VTA dopamine neuron activity, Borgland et al. [124] reported that orexin-A potentiates NMDAR currents in this population through protein-kinase C-dependent trafficking of NMDARs. Systemic SB-334867 has also been shown to block cocaine sensitization and to elevate the AMPA/NMDA ratio in VTA DA neurons – a surrogate measure of synaptic plasticity [145]. More recent studies have shown that the orexin-induced changes in VTA dopamine neuron activity are also thought to involve pre-synaptic mechanisms, as orexin potentiates excitatory inputs onto VTA neurons in Cocaine trained animals [121]. Interestingly, orexin-induced presynaptic changes were also seen in animals that self-administered high fat but not regular food. Consistent with these electrophysiological findings, in vivo studies using microdialysis have shown that local infusion of orexin-A increases extrasynaptic+ VTA dopamine and glutamate levels [146] and enhances the effects of cocaine on VTA dopamine signaling [147]. Similarly, using in vivo recordings in rats, Moorman & Aston-Jones [145] demonstrated that orexin application in the VTA increases baseline DA neuron activity and augmented DA neuron activity evoked by mPFC stimulation, a region known to play a key role in mediating reinstatement.
Supporting these anatomical and physiological data, there is now a significant body of behavioural evidence to suggest that orexin signaling in the VTA plays a key role in mediating drug-seeking. For example, intra-VTA injections of orexin-A reinstated morphine preference in a CPP model [129] and extinguished cocaine-seeking behaviour in a self-administration paradigm [147], whereas intra VTA SB-334867 administration suppressed morphine CPP [143]. Consistent with these findings, work from our laboratory has implicated VTA orexin signaling in cue-induced reinstatement of cocaine seeking [148]. In this report, we showed that unilateral infusions of SB- 334867 (3μg) into the VTA prior to reinstatement testing suppressed reinstatement of drug-seeking elicited by discriminative cues previously associated with cocaine availability and that this effect was independent of any effects on locomotor activity [136]. Preliminary evidence suggests that this attenuation of drug-seeking is associated with a reduction in the number of Fos-positive neurons within the PVT (Figure 2). It is plausible that OXR1 antagonism reduced the activity of VTA dopamine neurons that project to the PVT. This is consistent with previous studies showing dopamine-immunoreactive fibers in the PVT [149-151]. Further, PVT neurons express D3 receptor mRNA [152] and the DA transporter has been shown to be present in the midline thalamic nuclei [153]. However, a recent retrograde tracing study indicates that VTA dopamine projections to the PVT may not be as strong as previously thought [154]. Future studies will need to investigate the specific pathways via which VTA OXR1 signaling in the VTA modulates PVT activity in response to Drug related cues. Alternatively, it is possible that OXR1 signalling in the VTA may influence PVT activity via an indirect route. Further, blockade of the VTA OXR1 signaling also produced an increase in the number of Fos-positive cells in the NacS; an interesting finding in light of recent reports that the NacSh mediates the expression of extinction via projections to the hypothalamus [41].
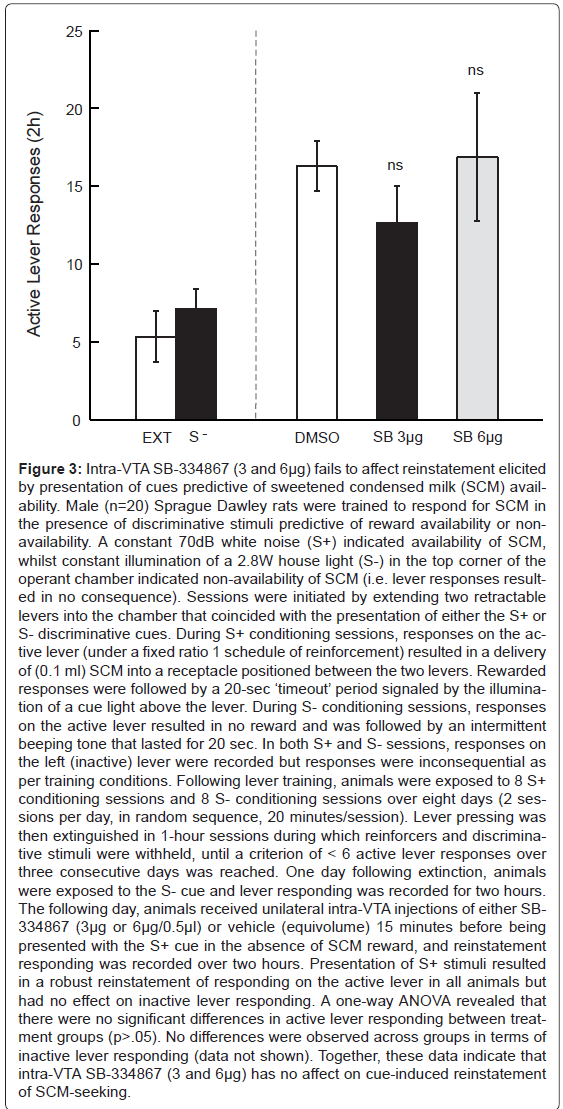
Figure 3:Intra-VTA SB-334867 (3 and 6�?µg) fails to affect reinstatement elicited by presentation of cues predictive of sweetened condensed milk (SCM) availability. Male (n=20) Sprague Dawley rats were trained to respond for SCM in the presence of discriminative stimuli predictive of reward availability or nonavailability. A constant 70dB white noise (S+) indicated availability of SCM, whilst constant illumination of a 2.8W house light (S-) in the top corner of the operant chamber indicated non-availability of SCM (i.e. lever responses resulted in no consequence). Sessions were initiated by extending two retractable levers into the chamber that coincided with the presentation of either the S+ or S- discriminative cues. During S+ conditioning sessions, responses on the active lever (under a fixed ratio 1 schedule of reinforcement) resulted in a delivery of (0.1 ml) SCM into a receptacle positioned between the two levers. Rewarded responses were followed by a 20-sec â�?�?timeoutâ�?�? period signaled by the illumination of a cue light above the lever. During S- conditioning sessions, responses on the active lever resulted in no reward and was followed by an intermittent beeping tone that lasted for 20 sec. In both S+ and S- sessions, responses on the left (inactive) lever were recorded but responses were inconsequential as per training conditions. Following lever training, animals were exposed to 8 S+ conditioning sessions and 8 S- conditioning sessions over eight days (2 sessions per day, in random sequence, 20 minutes/session). Lever pressing was then extinguished in 1-hour sessions during which reinforcers and discriminative stimuli were withheld, until a criterion of < 6 active lever responses over three consecutive days was reached. One day following extinction, animals were exposed to the S- cue and lever responding was recorded for two hours. The following day, animals received unilateral intra-VTA injections of either SB- 334867 (3�?µg or 6�?µg/0.5�?µl) or vehicle (equivolume) 15 minutes before being presented with the S+ cue in the absence of SCM reward, and reinstatement responding was recorded over two hours. Presentation of S+ stimuli resulted in a robust reinstatement of responding on the active lever in all animals but had no effect on inactive lever responding. A one-way ANOVA revealed that there were no significant differences in active lever responding between treatment groups (p>.05). No differences were observed across groups in terms of inactive lever responding (data not shown). Together, these data indicate that intra-VTA SB-334867 (3 and 6�?µg) has no affect on cue-induced reinstatement of SCM-seeking.
More recently, we sought to investigate whether the dose of SB- 334867, effective in reducing cocaine-seeking, also reduced natural reward-seeking behaviour. To achieve this aim, rats were trained to self-administer sweetened condensed milk (SCM) in the presence of discriminative cues using identical procedures as for cocaine-trained animals. While presentation of SCM-associated cues following extinction elicited reward-seeking, intra-VTA administration of SB- 334867 did not alter this behavior, even at a dose higher than what was effective in suppressing cocaine-seeking (Figure 3). These data are in keeping with a report that higher systemic doses of SB-334867 are required to reduce SCM compared to cocaine-seeking [137] and together suggest that at lower doses, SB-334867 can reduce Drug seeking without altering natural reward-seeking.
| Reinforcer | Animals | Treatment | Reinstatement Stimuli | Effect | Reference |
|---|---|---|---|---|---|
| Cocaine (SA) | Male, SD | Intra-PVT administration CART 55-102 (0.625µg and 2.5µg) |
Cocaine Prime (10mg/kg) | 2.5µg dose significantly attenuated reinstatement | James et al. (2010) + |
| Cocaine (SA) | Male, SD rat | SB-334867 (10, 20, 30mg/kg; i.p.) 4PT (10, 30mg/kg; i.p.) | Tone + cue lights paired with cocaine infusions | SB-334867: Dose-dependent decrease in reinstatement. 4PT: No effect on reinstatement | Smith et al. (2009) |
| Cocaine (SA) | Male, SD, rat | SB-334867 (10, 20, 30mg/kg; i.p.) | Re-exposure to SA environment following abstinence Context (visual, auditory, olfactory and tactile cues) | Dose-dependently decreased reinstatement following abstinence and context-induced reinstatement | Smith et al. (2010) |
| Cocaine (SA) | Male, SD rat | Intra-VTA SB-334867 (1µg and 3µg/0.5µl, unilaterally) | S+ (Background white noise) | Decreased reinstatement (3µg) | James et al. (2010) |
| Cocaine (SA) | Male, Wistar rat | 1. SB-334867 (15, 30mg/kg; i.p.)
2. Orexin-A (0.3, 0.75, 1.5nmol; i.c.v.) |
•Footshock (15mins, 0.5mA, variable interval 40s) •None |
•Dose-dependent decrease in reinstatement •0.75 and 1.5nmol doses induced reinstatement (dose-dependently) |
Boutrel et al. (2005) |
| Cocaine (SA) | Male, LE rat | 1. Intra-VTA Hcrt-1 and Hcrt-2 (10µm) 2. Intra-VTA SB-408124 | 1. None 2. Footshock (20mins, 0.4-0.6mA, variable interval 40 + 30s) |
1. Hcrt-1: induced reinstatement. Hcrt-2: No effect
2. No effect |
Wang et al. (2009) |
+ Only one other study has investigated the functional role of CART on reinstatement behaviour. King et al. (2010) showed that i.c.v. administration of CART 55-102 (0.625μg and 1.25μg), but not CART 1-27 (1.25μg), dose-dependently decreased reinstatement alcoholic beer-seeking in a renewal model. * SA: Self-administration, SB-334867 and SB-408124 are selective OXR1 antagonists. 4PT is a selective OXR2 antagonist.
Table 2: Summary of studies investigating the functional effects of CART and orexin on reinstatement of psychostimulant-seeking behaviour.
Importantly, the role of OXR1 signaling in the VTA appears to be limited to cue-induced reinstatement, as infusion of the OXR1 antagonist SB-408124 has no effect on footshock-elicited reinstatement of cocaine-seeking [147]. Accordingly, it remains to be determined where in the brain orexin acts to mediate stress-induced reinstatement. As outlined above, it is possible that orexin signaling in the PVT is important for stress-induced reinstatement. Another candidate region is the bed nucleus of the stria terminalis (BNST) known to be critical for drug-seeking elicited by stress [155-158] and a brain region densely innervated by hypothalamic orexin neurons.
To date, most of the work aimed at understanding orexin function in addiction has been focused on downstream projection targets of this system, like the PVT or VTA. Indeed, limited work has been directed at determining whether drug-induced changes occur within the hypothalamus itself, which might affect the recruitment of orexin neurons by stress, cues or drug. A study by Ahmed and colleagues [159] first alluded to this possibility by demonstrating by microarray that the hypothalamus is the most transcriptionally responsive to extended cocaine exposure compared to brain regions such as the prefrontal cortex or accumbens. Interestingly, many of the changes that were identified in this study were for genes encoding molecules implicated in pre and post-synaptic plasticity. Our group has recently addressed whether cocaine modulates hypothalamic orexin circuitry by using anatomical and electrophysiological techniques. Rats given seven days of passive cocaine exposure exhibited increased excitatory, vesicular glutamate transported 2 (VGLUT2)-positive puncta closely apposed to orexin neurons, whereas vesicular γ-aminobutyric acid (GABA) transporter (VGAT)-positive inputs were unchanged [136]. This finding was reinforced by electrophysiological studies that identified an increased frequency, but not amplitude, of miniature excitatory post-synaptic currents (mEPSCs) in PF/LHA of Cocaine treated rats compared to saline-treated animals. Importantly, similar electrophysiological differences were observed between animals trained to self-administer cocaine for two weeks versus food-trained rats. In addition, we found that the AMPA/NMDA ratio of evoked excitatory postsynaptic currents was unchanged in PF/LHA and orexin neurons from cocaine-trained rats whereas the paired-pulse ratio of these inputs was reduced in the cocaine-trained group. Together these data provide evidence that passive or self-administered cocaine increased pre but not post-synaptic plasticity in hypothalamic circuitry that may control orexin neuron excitability. One limitation of this work was that most recordings were made from unidentified PF/ LHA neurons. Furthermore, these assessments of synaptic function were made at a single time point in the self-administration paradigm. Thus, it will be important to repeat these studies in mice expressing GFP in orexin neurons, as well as to determine whether these changes persist into withdrawal. Our study also did not determine the mechanisms responsible for this pre-synaptic plasticity. Although this work has not yet determined the mechanisms underlying plasticity at excitatory inputs to orexin neurons, a potential candidate is cocaine-induced changes to Group III metabotropic glutamate receptor (mGluR) function. Previous work has shown that this system maintains background levels of tonic inhibition of excitatory synaptic input onto hypothalamic orexin neurons [160]. Accordingly, a loss in function of these receptors may contribute to plasticity changes that would enhance the activity of excitatory inputs. This postulate raises the possibility that mGluR receptor modulators, already showing promise in pre-clinical studies [161], may prove to be beneficial in reversing drug-induced plasticity that affects orexin cell excitability.
Considerations for Future Pharmacotherapies Targeting the Orexin and CART Systems for Relapse Prevention
Although pre-clinical studies support the potential therapeutic benefits of targeting orexin and CART to reduce relapse risk following psychostimulant abuse, it is important to acknowledge the challenges and limitations that such an approach faces [22]. In the case of orexin, the importance of this peptide in mediating a number of important physiological processes, including arousal, raises the possibility of ‘off-target’ effects. Despite this, and consistent with the differential roles of the orexin receptors, blockade of the OXR1 does appear to specifically attenuate reward-seeking behaviour while not affecting overall arousal levels. Thus, doses of SB-334867 that attenuate both cue- and stress-induced reinstatement do not affect extinction responding [133] or locomotor activity in a drug free state [136,162]. Regarding OXR2 antagonism, although a recent study reported that the selective OXR2 antagonist JNJ-10397049 attenuates reinstatement of alcohol CPP [128], the OXR2 antagonist 4-pyridylmethyl (S)-tertleucyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline [4PT] had no effect on cue-induced reinstatement of cocaine-seeking and produced marked impairments in locomotor activity [133]. Additionally, while JNJ-10397049 significantly alters sleep-wake states, the OXR1 antagonists SB-334867 and SB-408124 do not affect these parameters [163,164]. Importantly however, the orexin system has recently been implicated in vestibular motor control through effects on the lateral vestibular nucleus and there is some evidence that SB-334867 attenuates motor performance in tasks that involve a motor challenge [165]. Therefore, only after a comprehensive analysis of the CNS effects of OXR1 antagonists will it become clear if targeting the orexin system has the clinical efficacy and target selectivity required to be a realistic pharmacotherapy.
With respect to CART, a number of reports have described disrupted locomotor activity as a result of CART administration. For example, i.c.v. CART 55-102 peptide (1-2μg) causes a flattened body posture and movement-associated tremor [58,59,166,167]. Central administration of CART (2μg) has also been reported to reduce water intake [167] and alter licking patterns [166]. Importantly however, these effects appear to be less pronounced when CART is administered into discrete brain regions. For example, injections of CART (2.5μg) into the PVT has no noticeable effect on locomotor activity [43]. Further, intra-VTA infusions of CART have no effect on movement, except at high doses (bilateral/5μg/side; [74]). Thus, the challenge for a pharmacotherapeutic approach based on the CART system may center on the ability to selectively manipulate specific components of this system and avoid off target effects.
Another important consideration with respect to targeting both the orexin and CART systems is the effects on feeding behaviour and the potential for weight loss. Relatively high doses of SB-334867 reduce progressive ratio responding for both cocaine and natural rewards such as food [110,121,168,169]. There is some evidence however, that SB-334867 can reduce drug-seeking without affecting feeding behaviour. For example, Hollander et al. [119] showed that SB-334867 (4mg/kg, i.p.) blocks fixed ratio responding for nicotine but not food, whilst Jupp et al. [118] showed that SB-334867 (5mg/ kg) reduces progressive ratio responding for alcohol without affecting responding for sucrose pellets. Moreover, our data presented earlier in this review, along with that of Martin-Fardon et al. [137], suggests that it is possible to administer SB-334867 either systemically or directly into the VTA at doses that attenuate cue-induced reinstatement of cocaine-seeking but not natural reward-seeking.
At present it is less clear whether ‘therapeutic doses’ of CART that reduce drug-seeking would be free of anorectic effects. For example, a 1.25μg dose of CART administered i.c.v. reduces progressive ratio responding and context-induced reinstatement for alcohol [77], whereas small doses (1μg) injected i.c.v. significantly inhibit feeding [58]. Given that substance abuse is often associated with weight loss and has a high comorbidity with eating disorders [170], it will be important for future studies to determine whether it is possible to administer CART at a dose that will suppress drug-seeking without affecting feeding behaviour.
Summary
The results reviewed above indicate critical but generally opposing roles for orexin and CART peptides in the regulation of psychostimulant-motivated behaviours, including reinstatement. Although the exact mechanisms through which orexin and CART mediate reinstatement behaviour are not completely understood, it appears that both peptides exert their influence through a number of key reward-related regions. With respect to CART, the PVT appears to be a central site involved in the anti-drug seeking effects of this peptide. Indeed, we have shown that CART signaling in this region is important for drug-primed reinstatement of cocaine-seeking and preliminary evidence suggests that this might also be true for Cue induced reinstatement. Future studies are required to determine whether CART signaling in the PVT is also critical to stress-induced reinstatement. Further, it will also be important to investigate whether CART may also exert its inhibitory effects on drug-seeking through other ‘classic’ reward regions, such as the VTA and NAC.
We had previously proposed that the PVT might also be critical to reinstatement-relevant orexin signaling. Contrary to this suggestion, we recently reported that OXR1 signaling in this region is not necessary for cue-induced reinstatement of cocaine-seeking. Future studies should address whether OXR2 signaling in the PVT is involved in this form of reinstatement, as well as whether orexin signaling at either OXR1 or OXR2 may contribute to stress-induced drug-seeking. In contrast, the VTA appears to be an important site responsible for mediating the pro-drug-seeking effects of orexin. Considerable anatomical and electrophysiological evidence suggests that orexin might mediate drug-seeking via VTA dopamine neuron activity and we have recently shown that blockade of the OXR1 in this region blocks cue-induced reinstatement of cocaine-seeking, but not natural reward-seeking. It will be important to determine whether other orexin receptors in brain regions implicated by Fos-mapping can also regulate drug-seeking [117]. Importantly, orexin signaling in the VTA does not appear to be critical to stress-induced reinstatement and it will therefore be important that future studies investigate alternative loci that might be involved in the known role of orexin in this form of reinstatement. Finally, recent evidence from our laboratory suggests that cocaine-induces pre-synaptic plasticity within the hypothalamic circuitry that may affect orexin cell recruitment by relapse-evoking stimuli. A more detailed characterization of these changes may lead to new insights for developing pharmacological agents to reduce psychostimulant relapse.
Acknowledgements
This work was supported by project grants from the National Health and Medical Research Council of Australia and the Hunter Medical Research Institute to C.V.D. We would also like to thank Ms Erin Campbell, Ms Sarahanne Field and Ms Emily Levi for their assistance with the sweetened condensed milk experiments.
References
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F (2008) Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry 63: 152-157.
- Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16: 974-986.
- McGinty VB, Hayden BY, Heilbronner SR, Dumont EC, Graves SM, et al. (2011) Emerging, reemerging, and forgotten brain areas of the reward circuit: Notes from the 2010 Motivational Neural Networks conference. Behav Brain Res 225: 348-357.
- Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168: 44-56.
- Kalivas PW, Volkow ND (2005) The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry 162: 1403-1413.
- McFarland K, Kalivas PW (2001) The Circuitry Mediating Cocaine-Induced Reinstatement of Drug-Seeking Behavior. J Neurosci 21: 8655-8663.
- McFarland K, Davidge SB, Lapish CC, Kalivas PW (2004) Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci 24: 1551-1560.
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y (2007) Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci 27: 12655-12663.
- Wise RA (2009) Roles for nigrostriatal--not just mesocorticolimbic--dopamine in reward and addiction. Trends Neurosci 32: 517-524.
- Nauta WJ, Smith GP, Faull RL, Domesick VB (1978) Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience 3: 385-401.
- Fallon JH, Moore RY (1978) Catecholamine innervation of the basal forebrain. IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol 180: 545-580.
- Beckstead RM, Domesick VB, Nauta WJ (1979) Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res 175: 191-217.
- Phillipson OT, Griffiths AC (1985) The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16: 275-296.
- Ikemoto S (2007) Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev 56: 27-78.
- Anand BK, Brobeck JR (1951) Localization of a "feeding center" in the hypothalamus of the rat. Proc Soc Exp Biol Med 77: 323-324.
- Brobeck JR (1946) Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev 26: 541-559.
- Elmquist JK, Elias CF, Saper CB (1999) From lesions to leptin: hypothalamic control of food intake and body weight. Neuron 22: 221-232.
- Kishi T, Elmquist JK (2005) Body weight is regulated by the brain: a link between feeding and emotion. Mol Psychiatry 10: 132-146.
- Olds J, Milner P (1954) Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol 47: 419-427.
- Swanson LW (2000) Cerebral hemisphere regulation of motivated behavior. Brain Res 886: 113-164.
- DiLeone RJ, Georgescu D, Nestler EJ (2003) Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci 73: 759-768.
- Scammell TE, Saper CB (2007) Orexins: looking forward to sleep, back at addiction. Nat Med 13: 126-128.
- Groenewegen HJ, Berendse HW (1994) The specificity of the 'nonspecific' midline and intralaminar thalamic nuclei. Trends Neurosci 17: 52-57.
- Spencer SJ, Fox JC, Day TA (2004) Thalamic paraventricular nucleus lesions facilitate central amygdala neuronal responses to acute psychological stress. Brain Res 997: 234-237.
- Kelley AE, Baldo BA, Pratt WE (2005) A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol 493: 72-85.
- Kirouac GJ, Parsons MP, Li S (2005) Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res 1059: 179-188.
- Kirouac GJ, Parsons MP, Li S (2006) Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol 497: 155-165.
- Parsons MP, Li S, Kirouac GJ (2006) The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse 59: 480-490.
- Van der Werf YD, Witter MP, Groenewegen HJ (2002) The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev 39: 107-140.
- Bubser M, Deutch AY (1998) Thalamic paraventricular nucleus neurons collateralize to innervate the prefrontal cortex and nucleus accumbens. Brain Res 787: 304-310.
- Li S, Kirouac GJ (2008) Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 506: 263-287.
- Vertes RP, Hoover WB (2008) Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol 508: 212-237.
- Otake K, Nakamura Y (1998) Single midline thalamic neurons projecting to both the ventral striatum and the prefrontal cortex in the rat. Neuroscience 86: 635-649.
- Parsons MP, Li S, Kirouac GJ (2007) Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol 500: 1050-1063.
- Berendse HW, Groenewegen HJ (1991) Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42: 73-102.
- Moga MM, Weis RP, Moore RY (1995) Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol 359: 221-238.
- Hamlin AS, Clemens KJ, Choi EA, McNally GP (2009) Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci 29: 802-812.
- Young CD, Deutch AY (1998) The effects of thalamic paraventricular nucleus lesions on cocaine-induced locomotor activity and sensitization. Pharmacol Biochem Behav 60: 753-758.
- Brown EE, Robertson GS, Fibiger HC (1992) Evidence for conditional neuronal activation following exposure to a cocaine-paired environment: role of forebrain limbic structures. J Neurosci 12: 4112-4121.
- Franklin TR, Druhan JP (2000) Expression of Fos-related antigens in the nucleus accumbens and associated regions following exposure to a cocaine-paired environment. Eur J Neurosci 12: 2097-2106.
- Millan EZ, Furlong TM, McNally GP (2010) Accumbens shell-hypothalamus interactions mediate extinction of alcohol seeking. J Neurosci 30: 4626-4635.
- James MH, Charnley JL, Flynn JR, Smith DW, Dayas CV (2011) Propensity to 'relapse' following exposure to cocaine cues is associated with the recruitment of specific thalamic and epithalamic nuclei. Neuroscience 199: 235-242.
- James MH, Charnley JL, Jones E, Levi EM, Yeoh JW, et al. (2010) Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One 5: e12980.
- Marchant NJ, Furlong TM, McNally GP (2010) Medial dorsal hypothalamus mediates the inhibition of reward seeking after extinction. J Neurosci 30: 14102-14115.
- Millan EZ, Marchant NJ, McNally GP (2011) Extinction of drug seeking. Behav Brain Res 217: 454-462.
- Douglass J, Daoud S (1996) Characterization of the human cDNA and genomic DNA encoding CART: a cocaine- and amphetamine-regulated transcript. Gene 169: 241-245.
- Spiess J, Villarreal J, Vale W (1981) Isolation and sequence analysis of a somatostatin-like polypeptide from ovine hypothalamus. Biochemistry 20: 1982-1988.
- Larsen PJ, Vrang N, Petersen PC, Kristensen P (2000) Chronic intracerebroventricular administration of recombinant CART(42-89) peptide inhibits and causes weight loss in lean and obese Zucker (fa/fa) rats. Obes Res 8: 590-596.
- Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, et al. (2001) Characterization of CART neurons in the rat and human hypothalamus. J Comp Neurol 432: 1-19.
- Koylu EO, Couceyro PR, Lambert PD, Kuhar MJ (1998) Cocaine- and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J Comp Neurol 391: 115-132.
- Broberger C (1999) Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res 848: 101-113.
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, et al. (2009) The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci USA 106: 6772-6777.
- Dallvechia-Adams S, Kuhar MJ, Smith Y (2002) Cocaine- and amphetamine-regulated transcript peptide projections in the ventral midbrain: colocalization with gamma-aminobutyric acid, melanin-concentrating hormone, dynorphin, and synaptic interactions with dopamine neurons. J Comp Neurol 448: 360-372.
- Ekblad E, Kuhar M, Wierup N, Sundler F (2003) Cocaine- and amphetamine-regulated transcript: distribution and function in rat gastrointestinal tract. Neurogastroenterol Motil 15: 545-557.
- Dun SL, Chianca DA, Jr., Dun NJ, Yang J, Chang JK (2000) Differential expression of cocaine- and amphetamine-regulated transcript-immunoreactivity in the rat spinal preganglionic nuclei. Neurosci Lett 294: 143-146.
- Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ (2008) CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci 9: 747-758.
- Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, et al. (1998) CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse 29: 293-298.
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, et al. (1998) Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 393: 72-76.
- Abbott CR, Rossi M, Wren AM, Murphy KG, Kennedy AR, et al. (2001) Evidence of an Orexigenic Role for Cocaine- and Amphetamine-Regulated Transcript after Administration into Discrete Hypothalamic Nuclei. Endocrinology 142: 3457-3463.
- Marchant NJ, Millan EZ, McNally GP (2011) The hypothalamus and the neurobiology of drug seeking. Cell Mol Life Sci 69: 581-597.
- Marie-Claire C, Laurendeau I, Canestrelli C, Courtin C, Vidaud M, et al. (2003) Fos but not Cart (cocaine and amphetamine regulated transcript) is overexpressed by several drugs of abuse: a comparative study using real-time quantitative polymerase chain reaction in rat brain. Neurosci Lett 345: 77-80.
- Fagergren P, Hurd YL (1999) Mesolimbic gender differences in peptide CART mRNA expression: effects of cocaine. Neuroreport 10: 3449-3452.
- Hunter RG, Vicentic A, Rogge G, Kuhar MJ (2005) The effects of cocaine on CART expression in the rat nucleus accumbens: a possible role for corticosterone. Eur J Pharmacol 517: 45-50.
- Hubert GW, Kuhar MJ (2008) Cocaine administration increases the fraction of CART cells in the rat nucleus accumbens that co-immunostain for c-Fos. Neuropeptides 42: 339-343.
- Tang WX, Fasulo WH, Mash DC, Hemby SE (2003) Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem 85: 911-924.
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, et al. (2004) Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem 88: 1211-1219.
- Jung SK, Hong M-S, Suh G-J, Jin S-Y, Lee HJ, et al. (2004) Association between polymorphism in intron 1 of cocaine- and amphetamine-regulated transcript gene with alcoholism, but not with bipolar disorder and schizophrenia in Korean population. Neurosci Lett 365: 54-57.
- Salinas A, Wilde JD, Maldve RE (2006) Ethanol enhancement of cocaine- and amphetamine-regulated transcript mRNA and peptide expression in the nucleus accumbens. J Neurochem 97: 408-415.
- Jaworski JN, Kimmel HL, Mitrano DA, Tallarida RJ, Kuhar MJ (2007) Intra-VTA CART 55-102 reduces the locomotor effect of systemic cocaine in rats: an isobolographic analysis. Neuropeptides 41: 65-72.
- Jaworski JN, Kozel MA, Philpot KB, Kuhar MJ (2003) Intra-accumbal injection of CART (cocaine-amphetamine regulated transcript) peptide reduces cocaine-induced locomotor activity. J Pharmacol Exp Ther 307: 1038-1044.
- Yoon HS, Kim WY, Kim JH (2010) Microinjection of CART peptide 55-102 into the nucleus accumbens core inhibits the expression of conditioned hyperactivity in a cocaine-associated environment. Behav Brain Res 207: 169-173.
- Jaworski JN, Vicentic A, Hunter RG, Kimmel HL, Kuhar MJ (2003) CART peptides are modulators of mesolimbic dopamine and psychostimulants. Life Sci 73: 741-747.
- Hubert GW, Manvich DF, Kuhar MJ (2010) Cocaine and amphetamine-regulated transcript-containing neurons in the nucleus accumbens project to the ventral pallidum in the rat and may inhibit cocaine-induced locomotion. Neuroscience 165: 179-187.
- Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ (2000) Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55-102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther 294: 784-792.
- Rademacher D, Sullivan E, Figge D (2010) The effects of infusions of CART 55-102 into the basolateral amygdala on amphetamine-induced conditioned place preference in rats. Psychopharmacology 208: 499-509.
- Mattson BJ, Morrell JI (2005) Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine-regulated transcript in lactating, maternal rodents. Neuroscience 135: 315-328.
- King BJ, Furlong TM, McNally GP (2010) Cocaine and amphetamine related transcript (CART) inhibits context induced reinstatement of reward seeking. Behav Neurosci 124: 423-427.
- Couceyro PR, Koylu EO, Kuhar MJ (1997) Further studies on the anatomical distribution of CART by in situ hybridization. J Chem Neuroanat 12: 229-241.
- Stanley SA, Small CJ, Murphy KG, Rayes E, Abbott CR, et al. (2001) Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res 893: 186-194.
- Dayas CV, Liu X, Simms JA, Weiss F (2007) Distinct patterns of neural activation associated with ethanol seeking: effects of naltrexone. Biol Psychiatry 61: 979-989.
- Ghitza UE, Fabbricatore AT, Prokopenko V, Pawlak AP, West MO (2003) Persistent cue-evoked activity of accumbens neurons after prolonged abstinence from self-administered cocaine. J Neurosci 23: 7239-7245.
- Anderson SM, Schmidt HD, Pierce RC (2005) Administration of the D2 Dopamine Receptor Antagonist Sulpiride into the Shell, but not the Core, of the Nucleus Accumbens Attenuates Cocaine Priming-Induced Reinstatement of Drug Seeking. Neuropsychopharmacology 31: 1452-1461.
- Jaworski JN, Jones DC (2006) The role of CART in the reward/reinforcing properties of psychostimulants. Peptides 27: 1993-2004.
- Smith Y, Kieval J, Couceyro PR, Kuhar MJ (1999) CART peptide-immunoreactive neurones in the nucleus accumbens in monkeys: ultrastructural analysis, colocalization studies, and synaptic interactions with dopaminergic afferents. J Comp Neurol 407: 491-511.
- Ikemoto K, Satoh K, Kitahama K, Geffard M, Maeda T (1996) Electron-microscopic study of dopaminergic structures in the medial subdivision of the monkey nucleus accumbens. Exp Brain Res 111: 41-50.
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, et al. (1992) The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol 319: 218-245.
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, et al. (1998) Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior. Cell 92: 573-585.
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95: 322-327.
- Mieda M, Yanagisawa M (2002) Sleep, feeding, and neuropeptides: roles of orexins and orexin receptors. Curr Opin Neurobiol 12: 339-345.
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, et al. (2001) Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol 435: 6-25.
- Lu X-Y, Bagnol D, Burke S, Akil H, Watson SJ (2000) Differential Distribution and Regulation of OX1 and OX2 Orexin/Hypocretin Receptor Messenger RNA in the Brain upon Fasting. Horm Behav 37: 335-344.
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM (1998) Distribution of orexin receptor mRNA in the rat brain. FEBS Lett 438: 71-75.
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, et al. (1999) Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98: 437-451.
- Taheri S, Zeitzer JM, Mignot E (2002) The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci 25: 283-313.
- Sutcliffe JG, de Lecea L (2002) The hypocretins: setting the arousal threshold. Nat Rev Neurosci 3: 339-349.
- Chou TC, Lee CE, Lu J, Elmquist JK, Hara J, et al. (2001) Orexin (hypocretin) neurons contain dynorphin. J Neurosci 21: RC168.
- Abrahamson EE, Moore RY (2001) The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res 889: 1-22.
- Kirchgessner AL, Liu M (1999) Orexin synthesis and response in the gut. Neuron 24: 941-951.
- Naslund E, Ehrstrom M, Ma J, Hellstrom PM, Kirchgessner AL (2002) Localization and effects of orexin on fasting motility in the rat duodenum. Am J Physiol Gastrointest Liver Physiol 282: G470-479.
- Ljung T, Hellstrom PM (1999) Vasoactive intestinal peptide suppresses migrating myoelectric complex of rat small intestine independent of nitric oxide. Acta Physiol Scand 165: 225-231.
- Nowak KW, Mackowiak P, Switonska MM, Fabis M, Malendowicz LK (2000) Acute orexin effects on insulin secretion in the rat: in vivo and in vitro studies. Life Sci 66: 449-454.
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, et al. (2000) Dose-response effects of orexin-A on food intake and the behavioural satiety sequence in rats. Regul Pept 96: 71-84.
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K (1999) Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Res 849: 248-252.
- Haynes AC, Jackson B, Overend P, Buckingham RE, Wilson S, et al. (1999) Effects of single and chronic intracerebroventricular administration of the orexins on feeding in the rat. Peptides 20: 1099-1105.
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, et al. (2004) Differential effects of the selective orexin-1 receptor antagonist SB-334867 and lithium chloride on the behavioural satiety sequence in rats. Physiol Behav 81: 129-140.
- Ishii Y, Blundell JE, Halford JC, Upton N, Porter R, et al. (2005) Anorexia and weight loss in male rats 24 h following single dose treatment with orexin-1 receptor antagonist SB-334867. Behav Brain Res 157: 331-341.
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, et al. (2001) SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci 13: 1444-1452.
- Yamamoto Y, Ueta Y, Date Y, Nakazato M, Hara Y, et al. (1999) Down regulation of the prepro-orexin gene expression in genetically obese mice. Brain Res Mol Brain Res 65: 14-22.
- Sakurai T (2002) Roles of orexins in regulation of feeding and wakefulness. Neuroreport 13: 987-995.
- Nair SG, Golden SA, Shaham Y (2008) Differential effects of the hypocretin 1 receptor antagonist SB 334867 on high-fat food self-administration and reinstatement of food seeking in rats. Br J Pharmacol 154: 406-416.
- Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, et al. (2001) Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol 50: 381-388.
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E (2000) Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355: 39-40.
- Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, et al. (2002) The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 59: 1553-1562.
- Sakurai T (2007) The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci 8: 171-181.
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, et al. (2003) Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23: 3106-3111.
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148: 752-759.
- Jupp B, Krstew E, Dezsi G, Lawrence AJ (2011) Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin receptors. Br J Pharmacol 162: 880-889.
- Jupp B, Krivdic B, Krstew E, Lawrence AJ (2011) The orexin receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats. Brain Res 1391: 54-59.
- Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ (2008) Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci U S A 105: 19480-19485.
- LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA (2010) Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA. Psychopharmacology (Berl) 209: 203-212.
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, et al. (2009) Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29: 11215-11225.
- Boutrel B, Cannella N, de Lecea L (2010) The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res 1314: 103-111.
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, et al. (2011) Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol 22: 173-181.
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A (2006) Orexin A in the VTA Is Critical for the Induction of Synaptic Plasticity and Behavioral Sensitization to Cocaine. Neuron 49: 589-601.
- Quarta D, Valerio E, Hutcheson DM, Hedou G, Heidbreder C (2010) The orexin-1 receptor antagonist SB-334867 reduces amphetamine-evoked dopamine outflow in the shell of the nucleus accumbens and decreases the expression of amphetamine sensitization. Neurochem Int 56: 11-15.
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, et al. (2006) Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26: 398-405.
- Zhang GC, Mao LM, Liu XY, Wang JQ (2007) Long-lasting up-regulation of orexin receptor type 2 protein levels in the rat nucleus accumbens after chronic cocaine administration. J Neurochem 103: 400-407.
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, et al. (2011) Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl) 215: 191-203.
- Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556-559.
- Hamlin AS, Clemens KJ, McNally GP (2008) Renewal of extinguished cocaine-seeking. Neuroscience 151: 659-670.
- Schone C, Venner A, Knowles D, Karnani MM, Burdakov D (2011) Dichotomous cellular properties of mouse orexin/hypocretin neurons. J Physiol 589: 2767-2779.
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, et al. (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A 102: 19168-19173.
- Smith RJ, See RE, Aston-Jones G (2009) Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci 30: 493-503.
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G (2010) Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology 58: 179-184.
- Smith RJ, See R, Aston-Jones G (2009) The orexin-1 receptor antagonist SB-334867 blocks cue induced reinstatement of cocaine-seeking. Neuroscience Meeting Planner.
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, et al. (2011) Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol 14: 684-690.
- Martin-Fardon R., Leos BN, Kerr TM, Weiss F Administration of orexin/hypocretin (Orx/Hcrt) in the paraventricular nucleus of the thalamus (PVT) produces cocaine-seeking: Comparison with natural reward-seeking. Neuroscience Meeting Planner.
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F (2010) Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res 1314: 145-161.
- Huang H, Ghosh P, van den Pol AN (2006) Prefrontal Cortex-Projecting Glutamatergic Thalamic Paraventricular Nucleus-Excited by Hypocretin: A Feedforward Circuit That May Enhance Cognitive Arousal. J Neurophysiol 95: 1656-1668.
- Li Y, Li S, Wei C, Wang H, Sui N, et al. (2010) Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 212: 251-265.
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, et al. (2011) Orexins/Hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152: 4738-4752.
- Balcita-Pedicino JJ, Sesack SR (2007) Orexin axons in the rat ventral tegmental area synapse infrequently onto dopamine and gamma-aminobutyric acid neurons. J Comp Neurol 503: 668-684.
- Narita M, Nagumo Y, Hashimoto S, Khotib J, Miyatake M, et al. (2006) Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci 26: 398-405.
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE (2003) Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci 23: 7-11.
- Moorman DE, Aston-Jones G (2010) Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. J Neurosci 30: 15585-15599.
- Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844-858.
- Wang B, You ZB, Wise RA (2009) Reinstatement of cocaine seeking by hypocretin (orexin) in the ventral tegmental area: independence from the local corticotropin-releasing factor network. Biol Psychiatry 65: 857-862.
- Espana RA, Melchior JR, Roberts DC, Jones SR (2011) Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology (Berl) 214: 415-426.
- Groenewegen HJ (1988) Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 24: 379-431.
- Freedman LJ, Cassell MD (1991) Thalamic afferents of the rat infralimbic and lateral agranular cortices. Brain Res Bull 26: 957-964.
- Takada M, Campbell KJ, Moriizumi T, Hattori T (1990) On the origin of the dopaminergic innervation of the paraventricular thalamic nucleus. Neurosci Lett 115: 33-36.
- Mansour A, Watson SJ (2000) Dopamine receptor expression in the CNS. In: Psychopharmacology: the fourth generation of progress. (Bloom F, Kupfer D, eds.) 207-219. New York: Raven.
- Fujita M, Shimada S, Fukuchi K, Tohyama M, Nishimura T (1994) Distribution of cocaine recognition sites in rat brain: in vitro and ex vivo autoradiography with [125I]RTI-55. J Chem Neuroanat 7: 13-23.
- Li S, Kirouac GJ (2012) Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct.
- Leri F, Flores J, Rodaros D, Stewart J (2002) Blockade of Stress-Induced But Not Cocaine-Induced Reinstatement by Infusion of Noradrenergic Antagonists into the Bed Nucleus of the Stria Terminalis or the Central Nucleus of the Amygdala. J Neurosci 22: 5713-5718.
- Erb S, Stewart J (1999) A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19: RC35.
- Erb S, Salmaso N, Rodaros D, Stewart J (2001) A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 158: 360-365.
- Wang J, Fang Q, Liu Z, Lu L (2006) Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 185: 19-28.
- Ahmed SH, Lutjens R, van der Stap LD, Lekic D, Romano-Spica V, et al. (2005) Gene expression evidence for remodeling of lat eral hypothalamic circuitry in cocaine addiction. Proc Natl Acad Sci U S A 102: 11533-11538.
- Acuna-Goycolea C, Li Y, Van Den Pol AN (2004) Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons. J Neurosci 24: 3013-3022.
- Knackstedt LA, Kalivas PW (2009) Glutamate and reinstatement. Curr Opin Pharmacol 9: 59-64.
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, et al. (2008) Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl) 199: 109-117.
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, et al. (2009) Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther 330: 142-151.
- Smith MI, Piper DC, Duxon MS, Upton N (2003) Evidence implicating a role for orexin-1 receptor modulation of paradoxical sleep in the rat. Neurosci Lett 341: 256-258.
- Zhang J, Li B, Yu L, He YC, Li HZ, et al. (2011) A role for orexin in central vestibular motor control. Neuron 69: 793-804.
- Aja S, Schwartz GJ, Kuhar MJ, Moran TH (2001) Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. Am J Physiol Regul Integr Comp Physiol 280: R1613-1619.
- Aja S, Robinson BM, Mills KJ, Ladenheim EE, Moran TH (2002) Fourth ventricular CART reduces food and water intake and produces a conditioned taste aversion in rats. Behav Neurosci 116: 918-921.
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, et al. (2010) The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci 31: 336-348.
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC (2010) The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167: 11-20.
- Holderness CC, Brooks-Gunn J, Warren MP (1994) Co-morbidity of eating disorders and substance abuse review of the literature. Int J Eat Disord 16: 1-34.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15579
- [From(publication date):
specialissue-2013 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 11137
- PDF downloads : 4442