Review Article Open Access
Inflammatory Bowel Disease Associated Colorectal Neoplasia
| Michelle Vu1, Jyh-Yau Chang2, Jeremy Chen2 and David Q. Shih2* | |
| 1Department of Medicine, Cedars-Sinai Medical Center, Los Angeles, CA, USA | |
| 2Inflammatory Bowel & Immunobiology Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA 90048, USA | |
| Corresponding Author : | David Q. Shih, MD, PhD Cedars-Sinai Inflammatory Bowel and Immunobiology Research Institute 8700 Beverly Blvd, Suite 4066 Los Angeles, CA 90048, USA Tel: 310-423-7722 Fax: 310-423-0224 E-mail: david.shih@csmc.edu |
| Received October 31, 2011; Accepted January 21, 2012; Published January 23, 2012 | |
| Citation: Vu M, Chang JY, Chen J, Shih DQ (2012) Inflammatory Bowel Disease Associated Colorectal Neoplasia. J Gastroint Dig Syst S8:002. doi:10.4172/2161-069X.S8-002 | |
| Copyright: © 2012 Vila JJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Patients with ulcerative colitis (UC) or Crohn’s colitis have a greater risk for developing colorectal cancer (CRC). Many studies have described the evolving epidemiology and risk factors for CRC in patients with inflammatory bowel disease (IBD). Recent evidence indicates that the incidence has been decreasing with the advancement of medical and surgical therapies, and surveillance has emerged as the foundation of prevention. Chemoprophylaxis is another area of research; however, given the limited efficacy of these agents, they are only being used in conjunction with endoscopic surveillance. Our ability to formulate effective strategies for the prevention of this dreaded complication expands as more is discovered of the molecular events underlying IBD carcinogenesis. Management strategies are constantly updated as new evidence and endoscopic techniques emerge. In this paper, we review the literature regarding epidemiology, pathogenesis, risk factors and chemoprophylaxis as well as the latest consensus guidelines for management of dysplasia and neoplasia in IBD patients.
| Keywords |
| IBD; Neoplasia; Ulcerative colitis; Crohn’s disease; Chemoprophylaxis |
| Abbreviations |
| ALMs: Adenoma-like Lesion or Mass; ALDH1: Aldehyde Dehydrogenase isoform 1; ASA: Aminosalicylates; CHI3L1: Chitinase 3-Like-1; CRC: Colorectal Cancer; CCFA: Crohn’s and Colitis Foundation Of America; CD: Crohn’s Disease; DALMs: Dysplasia Associated Lesion or Mass; IBD: Inflammatory Bowel Disease; MMP-9: Matrix Metalloproteinase-9; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; PSC: Primary Sclerosing Cholangitis; TLR4: Toll-Like Receptor 4; UC: Ulcerative Colitis; UDCA: Ursodeoxycholic Acid; WT: Wild Type |
| Introduction |
| Inflammatory bowel disease (IBD) carries an increased risk of developing colorectal cancer (CRC). Many molecular anomalies responsible for sporadic CRC are also seen in colitis-associated CRC, but differences in timing and frequency of these events indicate an alternate pathophysiology within these chronic inflammatory states. The risk of CRC increases with duration of colitis, extent of disease involvement, degree of histologic inflammation, family history of CRC, and concomitant primary sclerosing cholangitis (PSC). The decreasing incidence of this feared complication has been attributed to heightened surveillance and advances in medical therapy. Chemoprophylactic agents, including aminosalycilates (ASA), ursodeoxycholic acid (UDCA), and possibly folic acid, dampen the inflammatory milieu which predisposes to dysplasia. Colonoscopy with biopsies is the standard mode of dysplasia detection, and consensus guidelines afford structure to the surveillance schedule. In this article, we review the pathogenesis as well as current consensus guidelines for screening and surveillance of CRC in IBD. |
| Epidemiology |
| The increased risk of CRC in IBD has been established for decades. Varying data has compounded the challenge of quantifying this risk. Differences in study design, length of follow-up, case definitions, environmental diversity, treatment strategies and referral center bias contribute to the wide range of risk seen in the literature. |
| The reported risk of developing CRC in the background of ulcerative colitis (UC) ranges from no increased probability versus the general population to a 34% chance of developing CRC after 25 years disease duration [1-8]. Initial studies from tertiary referral centers often overestimated the CRC risk in IBD, as patients from these institutions often present with more severe illness or later in the disease course [2-6]. Because of potential miscalculation and variation of CRC risk in hospital-based populations, there was a subsequent shift of focus toward population-based studies that potentially afford greater external validity. These results were more conservative and demonstrated CRC rates that approached that of the general population. A 2001 meta-analysis attempted to sort through the data in 116 studies and found the overall prevalence of CRC in UC patients to be 3.7% (95% confidence interval (CI) 3.2-4.2), with an increase to 5.4% in patients with pancolitis. The cumulative risk of developing CRC in any UC patient was estimated at 2% at 10 years of disease duration, 8% at 20 years, and 18% at 30 years [9]. |
| An association between Crohn’s disease (CD) and CRC has been suggested, with results ranging from no increase in risk to a 20 times increased risk [3,8,10]. The first long-term study investigating CRC risk in CD followed 449 patients with CD symptoms prior to 21 years of age. All cases of CRC occurred in patients with colonic disease, and the risk of CRC was estimated to be 20 times greater than in the general population [3]. A later hospital-based study examining CRC risk in CD patients with an intact colon showed relative risk (RR) increase of 4.3, with RR increasing further to 23.8 when patients with extensive colonic disease were examined [10]. A 2006 meta-analysis found that in six studies examining the difference of CRC risk in CD patients versus general population, the overall RR for developing CRC in CD patients was 2.5 (95% CI, 1.3-4.7, p=0.004). In patients with Crohn’s colitis, the RR was greater at 4.5 (95% CI, 1.3-14.9, p<0.015). Sub-analysis based on referral location (primary, secondary or tertiary center) showed no difference in risk. The cumulative risk of developing CRC in CD at any site was calculated to be 2.9% at 10 years of disease duration, 5.6% at 20 years and 8.3% at 30 years [11]. |
| Later studies examined the risk of CRC in both UC and CD patients, and found less drastic numbers but a persistently increased incidence of CRC in the IBD patient population. A 2001 study of CRC in IBD patients showed similar increase in RR for CRC in UC (colon cancer 2.75; 95% CI, 1.91-3.97; rectal cancer 2.64; 95% CI, 1.69-4.12) and CD (CRC 2.64; 95% CI, 1.69-4.12) [12]. A 2006 population-based study of patients in Olmsted County, Minnesota showed no increase in risk for CRC in any UC patients with a standardized incidence ratio (SIR)1.1 (95% CI, 0.4-2.4), but a secondary analysis demonstrated a greater CRC risk in the setting of extensive colitis with a SIR 2.4 (95% CI, 0.6-6.0). The cumulative incidence of CRC in UC patients was estimated to be at 2.0% at 25 years after diagnosis. Based on extent of disease, the CRC risk was lowest in proctitis patients (SIR 0.0; 95% CI, 0.0-3.5) and greatest in patients with extensive colitis (SIR 2.4; 95% CI, 0.6-6.0) [13]. This decreasing incidence of CRC over the past few decades is likely a testament to the success of constant surveillance and more successful control over disease activity with the advent of new therapeutics. |
| Molecular Events |
| The pathogenesis of IBD involves immune system dysregulation in response to gut flora in a genetically predisposed individual, leading to a state of chronic intestinal inflammation [14]. Normal immune activation triggers a tumor suppressive response, but it is believed that this security system fails in the face of chronic inflammation. The altered immune response allows for proliferation of dysplastic cells, which are stimulated by proinflammatory cytokines and growth factors [15,16]. |
| UC and CD are driven by different T helper cell mediated immune responses, which result in these two distinct phenotypes. Duration and degree of intestinal inflammation is a risk factor for CRC in IBD, and the immune system and cytokines that mediate inflammation have been investigated regarding disease pathogenesis. While UC consists of a Th2 response with high IL-5 and IL-13, CD is primarily a Th1 response with elevated TNFα and IFNγ [17]. In models of sporadic CRC, a Th2 response is associated with further disease progression, while a Th1 response is considered protective [18,19]. Recent research has implicated a coexisting Th17 response in CD as the reason for the higher CRC risk seen in observed in CD patients [20,21]. The mechanisms underlying these distinct conditions and implications on the development of colorectal neoplasia have yet to be fully elucidated. |
| The contribution of innate immune activity in IBD carcinogenesis is further suggested by recent research implicating epithelial toll-like receptor 4 (TLR4) as a potential marker for dysplasia and target for preventing CRC in colitis [22]. Transgenic murine models of colitis with elevated epithelial TLR4 developed severe acute inflammation, greater colitis-associated neoplasia, and elevated levels of inflammatory and pro-oncogenic factors (TNFα, COX-2, PGE-2) versus wild type (WT) mice [23,24]. When the epithelial TLR4 signaling pathway was blocked in WT mice with colitis-associated tumors, there were fewer colonic polyps. Furthermore, in UC patients with associated dysplasia or neoplasia, the level of TLR4 expression increased with dysplastic grade [22]. |
| While sporadic CRC typically sprouts from mucosal polyps, morphologic changes in colitis-associated CRC are heterogeneous and may present as flat or raised lesions. Despite the challenge of identifying these changes in a chronically inflamed colon, detection of these changes is imperative as IBD patients carry a higher risk of developing CRC. Sporadic CRC arises because of genomic instability and the two major types, chromosomal instability (CSI) and microsatellite instability (MSI), account for approximately 85% and 15% of sporadic CRC respectively. While these mechanisms are also observed in colitisassociated CRC, the timing and frequency is different in IBD [25]. |
| Chromosomal instability, a function of abnormal chromosomal segregation and DNA aneuploidy, is the reason for most p53 and APC dysfunction. APC dysfunction is typically an early event in the adenoma-carcinoma pathway of sporadic CRC, while p53 mutations take place at later stages of disease. In colitis-associated CRC, this is reversed- APC defects are less frequent and seen later in disease course, whereas p53 chromosomal abnormalities observed in earlier stages [26-28]. This finding may account for the flat morphology of dysplasia observed in IBD-associated CRC, as APC mutations are considered the reason for polyp formation. Errors in the p53 tumor suppressor gene, which is contained within chromosome 17p, occur earlier and more frequently even in nondysplastic mucosa, suggesting that underlying CSIs provide the background for CRC development in IBD patients [29]. The molecular events behind p53 dysfunction have been explored in animal models of colitis. One proposed mechanism is that matrix metalloproteinase-9 (MMP-9) has a tumor suppressive effect through Notch1 and subsequent p53 activation. Notch1 signaling in sporadic colon cancer induces adenoma formation in animal models [30-32] whereas studies have found a protective role against tumor formation in colitis-an effect theorized attributed to its inflamed environment [33]. Murine models deficient in MMP-9, which activates the transcription factor Notch1, have increased susceptibility to CRC [34]. The downstream effects of MMP-9 were elucidated when Notch1 inhibition led to lower levels of p53 and higher levels of tumor in and dysplasia compared to control mice [33]. MSI involves failure of the DNA mismatch repair system, which is comprised of proteins that coordinate DNA repair. In sporadic CRC, transcriptional silencing of the hMLH1 promoter via methylation accounts for a major part of MSI CRCs [35,36]. IBD patients with high levels of DNA MSI more often have heterogenous defects in hMLH1, hMSH2, hMSH6 and hPMS2 mismatch repair genes, and less frequently had hMLH1 promoter methylation [37]. Chronic inflammation may predispose the colorectal mucosa of IBD patients to genomic instability, thus accounting for the progression of the genetic changes of colitis-associated CRC. Unlike sporadic CRC, dysplasia and neoplasia in IBD patients typically arise from many discrete areas of a colitic environment. |
| Biologic markers have been explored for their role in carcinogenesis and potential value in identifying dysplasia in an inflammatory milieu. Chitinase 3-like-1 (CHI3L1) is one of these factors expressed in colonic tumors [38,39], and levels of CHI3L1 mRNA are also greater in patients with active IBD [38,40,41]. Specifically, CHI3L1 is elevated only in colonic epithelium of colitic areas and this has prompted investigations into the role of CHI3L1 in IBD carcinogenesis. UC patients with remote dysplasia were found to have significantly higher CHI3L1 mRNA expression than UC patients without remote dysplasia (p=0.05) [42]. When compared to normal individuals, its expression was 20 times greater in UC patients with remote dysplasia (p >0.001). These results have implications for the future use of this biomarker in distinguishing IBD patients at greatest risk for malignancy. Aldehyde dehydrogenase isoform 1 (ALDH1) has been established as a colonic stem cell marker, and is upregulated in colonic stem cells [43]. In cancer stem cells, ALDH1 is similarly active and protects against cell destruction [43]. Staining for ALDH1 to distinguish inflammatory from premalignant changes yielded a high sensitivity (92% for CRC or HGD, 95% for LGD), but poor specificity (55% for dysplasia) as many inflammatory changes were also ALDH1 positive [44]. The presence of high ALDH1 implicates colonic stem cells in the spectrum of processes, including inflammation and dysplasia, in IBD carcinogenesis. |
| Risk Factors |
| Association of an increased CRC risk in the background of IBD has been described extensively in the setting of UC, and more recently has been demonstrated in certain CD patients. Environmental, genetic and individual factors synergistically increase the risk of colorectal dysplasia and neoplasia in these patients. Summary of the IBD associated CRC risk factors are listed in Table 1. |
| Cumulative cancer risk increases with age in the general population; in IBD patients, this hazard is further compounded by specific disease characteristics including disease duration, area of colonic involvement, and degree of inflammation. A longer duration of colorectal inflammation has consistently been correlated with higher rates of CRC in IBD patients, although this rate has decreased with the advent of surveillance colonoscopy [9,11,45-47]. Extent of disease is another independent risk factor for development of CRC. A study of Swedish UC patients demonstrated a RR of CRC of 1.7 in ulcerative proctitis, 2.8 in left-sided colitis, and 14.8 in extensive colitis [8]. The 19-fold increased risk of CRC in patients with extensive UC was reproduced in patients with extensive Crohn’s colitis, who demonstrated a 20-fold excess risk [2]. A few recent studies have shown that greater degree of histologic inflammation, when controlling for duration and extent of disease, also predicts higher risk of CRC in UC patients [48,49]. The presence of backwash ileitis (BWI) in UC patients with pancolitis has been investigated for its predictive value for CRC [50]. Ulcerative pancolitis patients with BWI had a greater odds ratio (OR) of CRC than those without BWI (OR 19.36 vs. 9.58, p<0.001), although a confounding association of BWI with primary sclerosing cholangitis (PSC) was also noted [51,52]. Interestingly, higher disease activity as indicated by more frequent clinical exacerbations was not found to be a risk factor for development of CRC [53]. |
| Other studies have shown that CRC as a complication of IBD is greater in patients with an earlier age at IBD diagnosis, regardless of duration of colitis. Crohn’s patients with colonic involvement diagnosed at a younger age had higher rates of CRC [54,55]. In a Swedish population, the RR of CRC was 20.9 in Crohn’s colitis patients diagnosed before 30 years old versus those diagnosed at later ages (RR 2.2) [54]. In this same population, UC patients with pancolitis diagnosed prior to 15 years old had an absolute CRC risk of 40 percent after 35 years of disease, versus 30 percent in all age groups [8]. The results of this population-based study are similar to a referral center study of CRC in UC patients diagnosed prior to 15 years old which yielded a cumulative incidence of 43 percent by 35 years disease duration. |
| A positive family history of CRC, regardless of family history of IBD, is independently associated with increased CRC risk [56,57]. One Mayo clinic study showed that UC patients with a first-degree relative of CRC had a 2 times greater risk of developing CRC, irrespective of extent and duration of colitis [57]. The presence of PSC further adds to the CRC risk in IBD patients [51,52,58,59]. A meta-analysis of eleven studies showed that UC patients with concomitant PSC have an increased risk of CRC, with an OR of 4.09 (95% CI, 2.89-5.76) versus UC patients without PSC [60]. In these higher-risk groups, earlier screening and aggressive surveillance guidelines have been instituted to mitigate this hazard and, if necessary, perform colectomy. |
| Smoking tobacco has been shown to exacerbate CD, but it exerts a protective effect in UC [61-63]. The increased risk of CRC in cigarette smokers and the positive effect of cigarette smoking on the disease course of UC have been well-established. Animal models of colitis that were exposed to cigarette smoke had a greater rate of colonic adenoma and adenocarcinoma [64]; however, the degree to which cigarette smoking in UC patients alters molecular events in IBD carcinogenesis is still unknown. A subgroup analysis in a case-control study of UC patients did reveal an association with smoking and a lower risk of CRC versus nonsmokers, but further investigation is warranted [65]. |
| Surveillance |
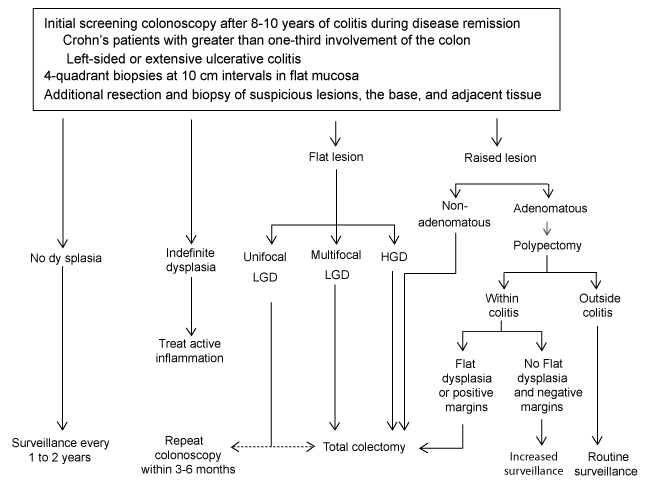
| Dysplasia is a known precursor to CRC and is defined as epithelial neoplasia confined to the basement membrane [66]. The goal of surveillance is to identify these premalignant changes and allow for intervention that will forestall the development of CRC and its dreaded complications. Regular colonoscopy is the mainstay of surveillance. In the general population, premalignant lesions present as prominent colonic polyps which can be resected during colonoscopy, and these patients are thereafter subjected to more rigorous surveillance. Detection of colorectal dysplasia in high-risk IBD patients presents an even greater challenge. Figure 1 outlines the current surveillance guideline for IBD patients. |
| The macroscopic appearance of dysplasia in IBD patients is classified as flat or raised. Flat dysplasia can occur within areas of normal colonic mucosa, and newer methods have been aimed at detection of this elusive entity. In addition to flat dysplasia, dysplasia can be found in plaques and lesions (dysplasia associated lesion or mass, DALMs) or resemble sporadic adenomas surrounded by normal appearing colonic mucosa (adenoma-like lesion or mass, ALMs). While ALMs have low malignant potential and may be removed via polypectomy, the presence of DALM is associated with concomitant CRC [67-69]. In a review of 10 surveillance programs, CRC was found in 43% of colectomies performed because of a DALM with any dysplasia grade [70]. |
| Dysplasia is classified histologically as indefinite, low-grade and high-grade [66]. While this system is commonly used, it is subject to poor interobserver agreement in histopathologic interpretation [71]. One study found only 60% agreement in diagnosing LGD [72], although very high levels of agreement has been observed in negative specimens or in HGD (kappa statistic for gastrointestinal pathologists 0.30, 95% CI, 0.26-0.34; for general pathologists 0.28, 95% CI, 0.23-0.32) [73]. |
| In UC patients with dysplasia who underwent colectomy, CRC was found in 19% of patients with LGD and 42% of patients with HGD [70]. Further analysis revealed that 29% of patients with LGD had eventual progression to HGD, DALM or CRC. Some evidence indicates that flat LGD in particular has a tendency to transform into HGD or cancer, with the rate of neoplastic transformation of flat LGD being 53% after 5 years [74-76]. A meta-analysis of twenty surveillance studies found that LGD carried a 9 times increased risk of developing CRC (OR 9.0, 95% 4.0-20.5), and a 12 times increased risk of developing any advanced lesion (OR 11.9, 95% CI, 5.2-27) [77]. The association with dysplasia and colitis secondary to CD also holds true. Small series of CD patients with CRC report similarly high rates of concomitant highgrade dysplasia [78,79]. General consensus is that whenever DALM or HGD is identified, a colectomy should be strongly recommended [70]. Patients with LGD should likewise be considered for colectomy given the risk of malignant transformation [80]. |
| Although colonoscopy is widely accepted as standard method to detect dysplasia and neoplasia in IBD, it has several limitations. These include incomplete patient and practitioner adherence, invasiveness, high cost, endoscopic sampling variations and poor interobserver agreement of histologic analysis. The major question is whether or not surveillance actually has an impact on mortality rates. A Cochrane review of three studies concluded that there was no concrete evidence for improved survival in patients with extensive colitis (RR 0.81, 95% CI, 0.17-3.83), although there was a trend toward earlier stage of CRC diagnosis which has been associated with improved outcomes [81]. |
| Surveillance should begin 8-10 years after symptom onset in pancolitis, and should be done annually or biannually with 2-4 random biopsies at 10 cm intervals and additional samples from suspicious areas. |
| Despite the known potential for conversion of dysplasia to neoplasia and the possibility of surveillance failure, many patients opt for surveillance over prophylactic colectomy with the understanding that they are at increased risk for cancer and warrant heightened surveillance. This is not unreasonable, as dysplasia can potentially regress or remain stable over time [82]. Because the risk of CRC in Crohn’s colitis is comparable to UC, guidelines are similar for both processes. Surveillance colonoscopy should be initiated 8-10 years after symptom onset in pancolitis and while disease is in remission, as active inflammation may obscure the distinction between reactive atypia and dysplasia [83,84]. One study calculated that in order to achieve 90% confidence of dysplasia detection, 33 random biopsies should be obtained [85]. To achieve 95% confidence of dysplasia detection, the authors found that 64 random biopsy specimens are required. The 2005 Crohn’s and Colitis Foundation of America (CCFA) Consensus advocates four-quadrant biopsies at 10 cm intervals, with additional samples in suspicious areas or in the lower sigmoid and rectum [86]. The CCFA recommends surveillance every 1 to 2 years in patients with extensive or left-sided UC and patients with Crohn’s colitis with one third colonic involvement. After two negative colonoscopies, surveillance should occur every 1 to 3 years, and then every 1 to 2 years once the disease has been present for 20 years [86]. There are no strict guidelines stating the optimal surveillance interval, but because the risk of cancer increases with time, the frequency of screening should likewise increase with each decade of disease [87,88]. |
| Recommendations have been further refined in UC patients. Studies in surveillance programs have uncovered a predilection for distal neoplasa, with 47-68% of CRC occurring in the rectosigmoid region [80,89]. In a recent 2011 study, Goldstone et al noted that in 103 patients with extensive colitis, neoplasia occurred in the rectosigmoid colon significantly more than other regions (p<0.0007). This also remained true in subgroup analyses of patients with advanced and flat neoplasia [87]. Patients with PSC should undergo surveillance at the time of diagnosis and annually thereafter [86]. |
| Prophylaxis |
| The mainstay of chemoprophylaxis is the attenuation of CRC risk by identifying and treating reversible risk factors. Some of these factors that play a role in development of dysplasia and neoplasia include chronic inflammation, vitamin deficiencies and environmental exposures. Chemoprevention has been investigated as an adjunct to surveillance, as even optimal surveillance does not afford complete protection against development of CRC. |
| Chronic, uncontrolled inflammation is a factor in malignant transformation, and anti-inflammatory medications have been investigated for a possible role in primary chemopropylaxis. Aminosalicylates (ASA) block the NFкB pathway which results in apoptosis and reduction of tumor [90]. Nonsteroidal antiinflammatory drugs (NSAIDs) decrease the risk of sporadic CRC by induction of apoptosis and inhibition of colonic epithelial growth in sporadic polyps. These compounds have been assessed in the setting of IBD for a protective effect against CRC. While there have been no prospective trials evaluating the beneficial effect of ASA on CRC in IBD patients, these compounds have long been used for their therapeutic and potential chemoprophylactic effects. A case control study of 102 cases of CRC in UC patients showed a 75% decreased risk of CRC in patients who used any ASA compounds [53]. The greatest protective effect against CRC was seen with mesalamine at doses greater than 1.2 grams/day (OR 0.09, 95% CI, 0.03-0.28). A 2005 meta-analysis of 9 studies demonstrated a protective association with the regular use of 5-ASA and dysplasia or CRC (OR 0.51, 95% CI, 0.38-0.69) [91]. |
| Other therapeutics with anti-inflammatory properties has been evaluated for a chemopreventive effect, without much success. While systemic and topical steroids are associated with reduction in CRC risk, their long-term use for chemoprophylaxis is not feasible given the extensive side effect profile [53,92]. Biologics such as azathioprine and 6-mercaptopurine do not appear to have any role in reduction of CRC risk [93]. |
| Folate deficiency has been implicated in development of CRC via induction of DNA strand breaks in the p53 tumor suppressor gene [94]. One small study found red blood cell folate levels, which reflect body stores, to be significantly lower in UC patients with CRC versus patients without CRC [95]. The CRC risk has been reported to be as high as 17 times greater in IBD patients with hyperhomocysteinemia and folate deficiency [96]. A retrospective study did reveal a trend for dose-dependent protective effect of folate supplementation against neoplasia in UC [97], but a significant effect of folate supplementation on CRC development has yet to be reliably reproduced. While folate is not considered an effective chemoprophylactic agent, clinicians will typically correct this deficiency in IBD patients. |
| Patients with PSC and IBD may benefit from ursodeoxycholic acid, which decreases colonic exposure to carcinogenic bile acids. A retrospective cross-sectional study of patients with PSC and UC showed that regular use of UDCA was associated with a decreased risk of colonic dysplasia (OR 0.18, 95% CI, 0.05-0.61). This was reproduced in a prospective randomized placebo-controlled trial in which patients with PSC and UC who were taking UDCA had a RR of 0.26 (95% CI, 0.06-0.92) for developing CRC [98]. |
| Undergoing a total colectomy would obviate the need for surveillance as it completely eliminates the chance of CRC. The efficacy of a total colectomy in prevention of CRC can be inferred by from a 2006 population-based study from Copenhagen which reported a 0.06% annual risk of CRC in UC patients, and a 30-year cumulative CRC risk of only 2.1%. The authors of this study attributed their low results to use of 5-ASA maintenance therapy in 70% of patients and an aggressive surgical approach to treatment in their population [1]. In patients with a single, flat low-grade dysplastic lesion on their first surveillance colonoscopy, immediate colectomy has been found to be preferable in terms of costs-effectiveness and quality-adjusted-lifeyears ratios versus continued surveillance [99]. |
| Conclusion |
| CRC risk is increased in IBD patients. The risk is related to the duration and anatomic extent of the disease. Many molecular anomalies responsible for sporadic CRC are also seen in colitis-associated CRC, but differences in timing and frequency of these events indicate an alternate pathophysiology within these chronic inflammatory states. Although no large controlled trials have proven that surveillance reduces mortality, surveillance is widely practiced and recommended by major gastroenterology societies. |
References
- Winther KV, Jess T, Langholz E, Munkholm P, Binder V (2004) Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol 2: 1088-1095.
- Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN (1994) Ulcerative colitis and Crohn's disease: a comparison of the colorectal cancer risk in extensive colitis. Gut 35: 1590-1592.
- Weedon DD, Shorter RG, Ilstrup DM, Huizenga KA, Taylor WF (1973) Crohn's disease and cancer. N Engl J Med 289: 1099-1103.
- Devroede GJ, Taylor WF, Sauer WG, Jackman RJ, Stickler GB (1971) Cancer risk and life expectancy of children with ulcerative colitis. N Engl J Med 285: 17-21.
- Gyde SN, Prior P, Allan RN, Stevens A, Jewell DP, et al. (1988) Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut 29: 206-217.
- Greenstein AJ, Sachar DB, Smith H, Janowitz HD, Aufses AH (1981) A comparison of cancer risk in Crohn's disease and ulcerative colitis. Cancer 48: 2742-2745.
- Kewenter J, Ahlman H, Hulten L (1978) Cancer risk in extensive ulcerative colitis. Ann Surg 188: 824-828.
- Ekbom A, Helmick C, Zack M, Adami HO (1990) Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 323: 1228-1233.
- Eaden JA, Abrams KR, Mayberry JF (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48: 526-535.
- Gyde SN, Prior P, Macartney JC, Thompson H, Waterhouse JA, et al. (1980) Malignancy in Crohn's disease. Gut 21: 1024-1029.
- Canavan C, Abrams KR, Mayberry J (2006) Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther 23: 1097-1104.
- Bernstein CN, Blanchard JF, Kliewer E, Wajda A (2001) Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 91: 854-862.
- Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, et al. (2006) Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology 130: 1039-1046.
- Shih DQ, Targan SR (2009) Insights into IBD Pathogenesis. Curr Gastroenterol Rep 11: 473-480.
- Xie J, Itzkowitz SH (2008) Cancer in inflammatory bowel disease. World J Gastroenterol 14: 378-389.
- Rizzo A, Pallone F, Monteleone G, Fantini MC (2011) Intestinal inflammation and colorectal cancer: a double-edged sword? World J Gastroenterol 17: 3092-3100.
- Fuss IJ, Neurath M, Boirivant M, Klein JS, de la Motte C, et al. (1996) Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn's disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol 157: 1261-1270.
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, et al. (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353: 2654-2666.
- Shibata M, Nezu T, Kanou H, Abe H, Takekawa M, et al. (2002) Decreased production of interleukin-12 and type 2 immune responses are marked in cachectic patients with colorectal and gastric cancer. J Clin Gastroenterol 34: 416-420.
- Sakuraba A, Sato T, Kamada N, Kitazume M, Sugita A, et al. (2009) Th1/Th17 immune response is induced by mesenteric lymph node dendritic cells in Crohn's disease. Gastroenterology 137: 1736-1745.
- Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, et al. (2008) Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn's disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm Bowel Dis 14: 437-445.
- Fukata M, Shang L, Santaolalla R, Sotolongo J, Pastorini C, et al. (2011) Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis 17: 1464-1473.
- Shang L, Fukata M, Thirunarayanan N, Martin AP, Arnaboldi P, et al. (2008) Toll-like receptor signaling in small intestinal epithelium promotes B-cell recruitment and IgA production in lamina propria. Gastroenterology 135: 529-538.
- Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, et al. (2006) Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology 131: 862-877.
- Willenbucher RF, Aust DE, Chang CG, Zelman SJ, Ferrell LD, et al. (1999) Genomic instability is an early event during the progression pathway of ulcerative-colitis-related neoplasia. Am J Pathol 154: 1825-1830.
- Tarmin L, Yin J, Harpaz N, Kozam M, Noordzij J, et al. (1995) Adenomatous polyposis coli gene mutations in ulcerative colitis-associated dysplasias and cancers versus sporadic colon neoplasms. Cancer Res 55: 2035-2038.
- Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, et al. (2002) The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer 94: 1421-1427.
- Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, et al. (1994) Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology 107: 369-378.
- Rabinovitch PS, Dziadon S, Brentnall TA, Emond MJ, Crispin DA, et al. (1999) Pancolonic chromosomal instability precedes dysplasia and cancer in ulcerative colitis. Cancer Res 59: 5148-5153.
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, et al. (1997) Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science 275: 1784-1787.
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, et al. (2005) Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964-968.
- Reedijk M, Odorcic S, Zhang H, Chetty R, Tennert C, et al. (2008) Activation of Notch signaling in human colon adenocarcinoma. Int J Oncol 33: 1223-1229.
- Garg P, Jeppsson S, Dalmasso G, Ghaleb AM, McConnell BB, et al. (2011) Notch1 regulates the effects of matrix metalloproteinase-9 on colitis-associated cancer in mice. Gastroenterology 141: 1381-1392.
- Garg P, Sarma D, Jeppsson S, Patel NR, Gewirtz AT, et al. (2010) Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res 70: 792-801.
- Redston M (2001) Carcinogenesis in the GI tract: from morphology to genetics and back again. Mod Pathol 14: 236-245.
- Kuismanen SA, Holmberg MT, Salovaara R, de la Chapelle A, Peltomaki P (2000) Genetic and epigenetic modification of MLH1 accounts for a major share of microsatellite-unstable colorectal cancers. Am J Pathol 156: 1773-1779.
- Svrcek M, El-Bchiri J, Chalastanis A, Capel E, Dumont S, et al. (2007) Specific clinical and biological features characterize inflammatory bowel disease associated colorectal cancers showing microsatellite instability. J Clin Oncol 25: 4231-4238.
- Johansen JS (2006) Studies on serum YKL-40 as a biomarker in diseases with inflammation, tissue remodelling, fibroses and cancer. Dan Med Bull 53: 172-209.
- Cintin C, Johansen JS, Christensen IJ, Price PA, Sorensen S, et al. (1999) Serum YKL-40 and colorectal cancer. Br J Cancer 79: 1494-1499.
- Mizoguchi E (2006) Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology 130: 398-411.
- Vind I, Johansen JS, Price PA, Munkholm P (2003) Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol 38: 599-605.
- Chen CC, Pekow J, Llado V, Kanneganti M, Lau CW, et al. (2011) Chitinase 3-like-1 expression in colonic epithelial cells as a potentially novel marker for colitis-associated neoplasia. Am J Pathol 179: 1494-1503.
- Deng S, Yang X, Lassus H, Liang S, Kaur S, et al. (2010) Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One 5: e10277.
- Toll AD, Boman BM, Palazzo JP (2011) Dysplastic lesions in inflammatory bowel disease show increased positivity for the stem cell marker aldehyde dehydrogenase. Hum Pathol 43: 238-242.
- Munkholm P, Langholz E, Davidsen M, Binder V (1993) Intestinal cancer risk and mortality in patients with Crohn's disease. Gastroenterology 105: 1716-1723.
- Lakatos L, Mester G, Erdelyi Z, David G, Pandur T, et al. (2006) Risk factors for ulcerative colitis-associated colorectal cancer in a Hungarian cohort of patients with ulcerative colitis: results of a population-based study. Inflamm Bowel Dis 12: 205-211.
- Langholz E, Munkholm P, Davidsen M, Binder V (1992) Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology 103: 1444-1451.
- Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, et al. (2004) Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology 126: 451-459.
- Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, et al. (2007) Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology 133: 1099-1105.
- Heuschen UA, Hinz U, Allemeyer EH, Stern J, Lucas M, et al. (2001) Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology 120: 841-847.
- Shetty K, Rybicki L, Brzezinski A, Carey WD, Lashner BA (1999) The risk for cancer or dysplasia in ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol 94: 1643-1649.
- Marchesa P, Lashner BA, Lavery IC, Milsom J, Hull TL, et al. (1997) The risk of cancer and dysplasia among ulcerative colitis patients with primary sclerosing cholangitis. Am J Gastroenterol 92: 1285-1288.
- Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J (2000) Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther 14: 145-153.
- Ekbom A, Helmick C, Zack M, Adami HO (1990) Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet 336: 357-359.
- Gillen CD, Andrews HA, Prior P, Allan RN (1994) Crohn's disease and colorectal cancer. Gut 35: 651-655.
- Askling J, Dickman PW, Karlen P, Brostrom O, Lapidus A, et al. (2001) Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology 120: 1356-1362.
- Nuako KW, Ahlquist DA, Mahoney DW, Schaid DJ, Siems DM, et al. (1998) Familial predisposition for colorectal cancer in chronic ulcerative colitis: a case-control study. Gastroenterology 115: 1079-1083.
- Thackeray EW, Charatcharoenwitthaya P, Elfaki D, Sinakos E, Lindor KD (2011) Colon neoplasms develop early in the course of inflammatory bowel disease and primary sclerosing cholangitis. Clin Gastroenterol Hepatol 9: 52-56.
- Sokol H, Cosnes J, Chazouilleres O, Beaugerie L, Tiret E, et al. (2008) Disease activity and cancer risk in inflammatory bowel disease associated with primary sclerosing cholangitis. World J Gastroenterol 14: 3497-3503.
- Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO (2002) Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc 56: 48-54.
- Holdstock G, Savage D, Harman M, Wright R (1984) Should patients with inflammatory bowel disease smoke? Br Med J (Clin Res Ed) 288: 362.
- Harries AD, Baird A, Rhodes J (1982) Non-smoking: a feature of ulcerative colitis. Br Med J (Clin Res Ed) 284: 706.
- Calkins BM (1989) A meta-analysis of the role of smoking in inflammatory bowel disease. Dig Dis Sci 34: 1841-1854.
- Liu ES, Ye YN, Shin VY, Yuen ST, Leung SY, et al. (2003) Cigarette smoke exposure increases ulcerative colitis-associated colonic adenoma formation in mice. Carcinogenesis 24: 1407-1413.
- Pinczowski D, Ekbom A, Baron J, Yuen J, Adami HO (1994) Risk factors for colorectal cancer in patients with ulcerative colitis: a case-control study. Gastroenterology 107: 117-120.
- Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, et al. (1983) Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 14: 931-968.
- Engelsgjerd M, Farraye FA, Odze RD (1999) Polypectomy may be adequate treatment for adenoma-like dysplastic lesions in chronic ulcerative colitis. Gastroenterology 117: 1288-1294.
- Odze RD, Farraye FA, Hecht JL, Hornick JL (2004) Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol 2: 534-541.
- Friedman S, Odze RD, Farraye FA (2003) Management of neoplastic polyps in inflammatory bowel disease. Inflamm Bowel Dis 9: 260-266.
- Bernstein CN, Shanahan F, Weinstein WM (1994) Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet 343: 71-74.
- Riddell RH (1995) Grading of dysplasia. Eur J Cancer 31: 1169-1170.
- Melville DM, Jass JR, Morson BC, Pollock DJ, Richman PI, et al. (1989) Observer study of the grading of dysplasia in ulcerative colitis: comparison with clinical outcome. Hum Pathol 20: 1008-1014.
- Eaden J, Abrams K, McKay H, Denley H, Mayberry J (2001) Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol 194: 152-157.
- Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S (2003) Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology 125: 1311-1319.
- Lim CH, Dixon MF, Vail A, Forman D, Lynch DA, et al. (2003) Ten year follow up of ulcerative colitis patients with and without low grade dysplasia. Gut 52: 1127-1132.
- Bernstein CN (2004) Ulcerative colitis with low-grade dysplasia. Gastroenterology 127: 950-956.
- Thomas T, Abrams KA, Robinson RJ, Mayberry JF (2007) Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther 25: 657-668.
- Petras RE, Mir-Madjlessi SH, Farmer RG (1987) Crohn's disease and intestinal carcinoma. A report of 11 cases with emphasis on associated epithelial dysplasia. Gastroenterology 93: 1307-1314.
- Hamilton SR (1985) Colorectal carcinoma in patients with Crohn's disease. Gastroenterology 89: 398-407.
- Woolrich AJ, DaSilva MD, Korelitz BI (1992) Surveillance in the routine management of ulcerative colitis: the predictive value of low-grade dysplasia. Gastroenterology 103: 431-438.
- Collins PD, Mpofu C, Watson AJ, Rhodes JM (2006) Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev: CD000279.
- Axon AT (1994) Cancer surveillance in ulcerative colitis--a time for reappraisal. Gut 35: 587-589.
- Kornbluth A, Sachar DB (2010) Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol 105: 501-523.
- Eaden JA, Mayberry JF (2002) Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut 51: 10-12.
- Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, et al. (1992) DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology 103: 1611-1620.
- Itzkowitz SH, Present DH (2005) Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis 11: 314-321.
- Goldstone R, Itzkowitz S, Harpaz N, Ullman T (2011) Dysplasia is more common in the distal than proximal colon in ulcerative colitis surveillance. Inflamm Bowel Dis.
- Farraye FA, Odze RD, Eaden J, Itzkowitz SH (2010) AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 138: 746-774.
- Choi PM (1993) Predominance of rectosigmoid neoplasia in ulcerative colitis and its implication on cancer surveillance. Gastroenterology 104: 666-667.
- Munkholm P, Loftus EV, Reinacher-Schick A, Kornbluth A, Mittmann U, et al. (2006) Prevention of colorectal cancer in inflammatory bowel disease: value of screening and 5-aminosalicylates. Digestion 73: 11-19.
- Velayos FS, Terdiman JP, Walsh JM (2005) Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol 100: 1345-1353.
- Velayos FS, Loftus EV, Jess T, Harmsen WS, Bida J, et al. (2006) Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology 130: 1941-1949.
- Matula S, Croog V, Itzkowitz S, Harpaz N, Bodian C, et al. (2005) Chemoprevention of colorectal neoplasia in ulcerative colitis: the effect of 6-mercaptopurine. Clin Gastroenterol Hepatol 3: 1015-1021.
- Kim YI, Pogribny IP, Basnakian AG, Miller JW, Selhub J, et al. (1997) Folate deficiency in rats induces DNA strand breaks and hypomethylation within the p53 tumor suppressor gene. Am J Clin Nutr 65: 46-52.
- Lashner BA (1993) Red blood cell folate is associated with the development of dysplasia and cancer in ulcerative colitis. J Cancer Res Clin Oncol 119: 549-554.
- Phelip JM, Ducros V, Faucheron JL, Flourie B, Roblin X (2008) Association of hyperhomocysteinemia and folate deficiency with colon tumors in patients with inflammatory bowel disease. Inflamm Bowel Dis 14: 242-248.
- Lashner BA, Provencher KS, Seidner DL, Knesebeck A, Brzezinski A (1997) The effect of folic acid supplementation on the risk for cancer or dysplasia in ulcerative colitis. Gastroenterology 112: 29-32.
- Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD (2003) Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology 124: 889-893.
- Nguyen GC, Frick KD, Dassopoulos T (2009) Medical decision analysis for the management of unifocal, flat, low-grade dysplasia in ulcerative colitis. Gastrointest Endosc 69: 1299-1310.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14206
- [From(publication date):
specialissue-2012 - Oct 02, 2025] - Breakdown by view type
- HTML page views : 9427
- PDF downloads : 4779
