Commentary Open Access
Inflammation Plays Multiple Roles in Colorectal Cancer
Lisa Kozicky, Roger Jen and Laura M. Sly*
Child and Family Research Institute, BC Children’s Hospital, and The University of British Columbia, Vancouver, British Columbia, Canada
- *Corresponding Author:
- Laura M Sly
Child and Family Research Institute
BC Children’s Hospital and The University of British Columbia
Vancouver, British Columbia, Canada
Tel: 604-875-3654
E-mail: laurasly@mail.ubc.ca
Received date: June 15, 2013; Accepted date: August 03, 2013; Published date: August 06, 2013
Citation: Kozicky L, Jen R, Sly LM (2013) Inflammation Plays Multiple Roles in Colorectal Cancer. J Gastroint Dig Syst 3:127. doi:10.4172/2161-069X.1000127
Copyright: © 2013 Kozicky L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Keywords
Inflammation; Colorectal cancer; Th17 response; Epithelial barrier; Dysbiosis
Inflammation is a critical process in the generation of neoplasms. It causes genomic instability, which allows cells to acquire the functional properties of a cancer cell or the “hallmarks of cancer” [1]. Epidemiological observations highlight the utility of colorectal cancer as a paradigm for inflammation associated cancer and recent research has demonstrated multiple roles for inflammation in neoplastic transformation [2]. People with inflammatory bowel diseases, which are characterized by chronic intestinal inflammation, have a dramatically increased life-time risk of developing colorectal cancer [3]. Moreover, risk of disease increases with severity and duration of inflammation reaching as high as 40% in people diagnosed with pan colitis before 15 years of age [3]. The assumption thus far has been that inflammation leads to the production of reactive oxygen species that cause DNA damage and neoplastic transformation. While this is correct, recent work has highlighted additional, specific roles for inflammation in cancer development. This commentary will focus on insights provided by mechanistic studies in pre-clinical models of colorectal cancer that have shed light on new roles for inflammation in the development of colorectal cancer.
In 2009, Wu et al. reported that a specific bacterium, enterotoxigenic Bacteroides fragilis (ETBF), which expresses B. fragilis toxin, was a potent inducer of colonic tumors [4]. For these studies, they colonized Min mice (multiple intestinal neoplasia mice that are defective in the Apc gene), which typically develop multiple ileal polyps and colonic adenomas, with a single bacterium (monocolonization). By comparing tumour development in mice monocolonized with B. fragilis that expressed or did not express the toxin, they were able to conclude that the toxin is sufficient to drive inflammation and tumorigenesis. Mechanistically, they demonstrated that ETBF drives a specific immune response characterized by infiltration of Th17 cells. The pro- inflammatory cytokine, IL-23, supports Th17 cell development and Th17 cells produce IL–17, a potent inflammatory cytokine that exacerbates inflammation by inducing chemokine production, which leads to the infiltration of additional IL-23-producing monocytes as well as neutrophils. Blockade of either IL-23 or IL-17 was sufficient to block ETBF-induced colonic inflammation and tumour formation [4]. These data are consistent with observations made in human colorectal cancer in which a Th1 response correlates with a cytotoxic and protective anti-tumour effect caused by tumour immune surveillance [5]; whereas a Th17 dominated response correlates with a dramatic decrease in disease-free survival [6]. Furthermore, carriage rates of B. fragilis that express the enterotoxin are significantly higher in people with colorectal cancer compared to control subjects. Taken together, these studies suggest a possible role for colonization with a specific toxin-expressing bacterium in driving pathogenic inflammation that leads to the development of colorectal cancer.
A second role for inflammation in colorectal cancer is the disruption of the epithelial barrier. This enables intestinal microbes to access the tumour microenvironment promoting localized inflammation and genetic instability. Givennikov et al. also used APC/Min mice to demonstrate that bacterial colonization and IL-23 signalling are required for colorectal cancer development in these mice [7]. They further demonstrated that there was a loss of polarized expression of epithelial cell junctional proteins, JAM-A and B, in murine adenomas. They extended their observations investigating these phenomena in early human ademonas and showed a direct correlation between the disruption of tight junction proteins and high pathogenic IL-23 and IL- 17 levels [6,7]. These studies suggest that inflammation leads to barrier disruption that acts in a positive feedback loop to drive inflammation and colorectal cancer.
A notable common theme in studies discussed thus far is the requirement for intestinal microbes in initiating tumorigenic inflammatory responses. Recent work by Arthur et al. suggests that inflammation actually shapes the microbial community, which, in turn, influences cancer development [8]. IL-10 deficient mice (il10-/-) develop spontaneous colonic inflammation and colon tumors. Jobin’s group previously demonstrated that tumour development in the il10- /- mice is significantly reduced in a germ-free environment compared to conventionally housed mice, which harbour a standard microbiota [9]. This observation sparked the idea that there may be a bacteriallydriven oncogenic environment in colorectal cancer. By comparing the microbiota from wild type mice with that from il10-/- mice that were untreated or treated with the carcinogen azoxymethane, they demonstrated that inflammation, rather than cancer development, causes a shift in microbial composition, which is also known as dysbiosis [8]. Intriguingly, monocolonization of il10-/- mice with a specific Escherichia coli strain (NC101) enriched by the inflammatory environment, promoted the development of invasive carcinomas when mice were treated with azoxymethane. Increased tumorigenesis was dependent on bacterial expression of the polyketide synthase (pks) island, which encodes the genotoxin Colibactin [8]. In addition, the genotoxic effects of pks+ E. coli require bacteria-host cell contact [10] suggesting that the aforementioned inflammation induced barrier disruption may also play a critical role in this model. Correlative data from human studies has demonstrated an association between colonization with adherent and invasive E. coli and colorectal cancer suggesting that these bacteria may play a direct role in development of colorectal tumours in humans [11]. Recent work has also demonstrated increased prevalence of E. coli producing genotoxin in subjects with colon cancer compared to control subjects [12]. These studies highlight a novel role for inflammation in shaping a carcinogenic microbiome that may drive inflammation associated colon cancer.
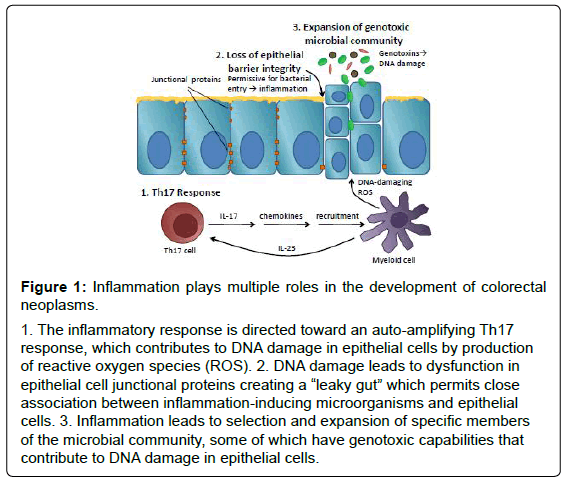
Inflammation has been implicated in development of colorectal cancer and its role has been validated by many excellent epidemiological studies. Recent work in pre-clinical models suggests that this association is not simply attributable to production of DNA-damaging reactive oxygen species but rather, that the role of inflammation in colorectal neoplasia is multi-faceted and complex. Figure 1 summarizes the multiple roles that inflammation plays in murine models of tumorigenesis. In step 1, inflammation is skewed away from protective Th1-mediated tumour surveillance to a Th17 response that auto-amplifies the inflammatory response by recruiting additional inflammatory cells to sites of inflammation. In step 2, inflammation has direct effects on epithelial barrier cell proteins and barrier function that is proportional to damaging inflammatory responses and permits close association between intestinal microorganisms and epithelial cells. Finally, in step 3, inflammation skews the microbial community, which may permit the selection and expansion of microorganisms harbouring genotoxic abilities. Furthermore, evidence suggests that these seemingly disparate roles for inflammation in colorectal cancer may cooperate to create the “perfect storm” permitting neoplastic transformation and the development of colorectal cancer. Importantly, all of these phenomena have been observed in human subjects with colorectal cancer suggesting that the lessons that we have learned from animal models may be directly relevant to human disease. Insight from these studies may lead to the development of novel strategies to prevent colorectal cancer that target inflammation directly, or its effects on epithelial barrier or the intestinal microbiota.
Figure 1: Inflammation plays multiple roles in the development of colorectal
neoplasms.
1. The inflammatory response is directed toward an auto-amplifying Th17
response, which contributes to DNA damage in epithelial cells by production
of reactive oxygen species (ROS). 2. DNA damage leads to dysfunction in
epithelial cell junctional proteins creating a “leaky gut” which permits close
association between inflammation-inducing microorganisms and epithelial
cells. 3. Inflammation leads to selection and expansion of specific members
of the microbial community, some of which have genotoxic capabilities that
contribute to DNA damage in epithelial cells.
References
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646-674.
- Danese S, Mantovani A (2010) Inflammatory bowel disease and intestinal cancer: a paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene 29: 3313-3323.
- Ekbom A, Helmick C, Zack M, Adami HO (1990) Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 323: 1228-1233.
- Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, et al. (2009) A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 15: 1016-1022.
- Gallimore AM, Godkin A (2013) Epithelial barriers, microbiota, and colorectal cancer. N Engl J Med 368: 282-284.
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, et al. (2011) Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 71: 1263-1271.
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, et al. (2012) Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491: 254-258.
- Arthur JC, Perez-Chanona E, Mühlbauer M, Tomkovich S, Uronis JM, et al. (2012) Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120-123.
- Uronis JM, Mühlbauer M, Herfarth HH, Rubinas TC, Jones GS, et al. (2009) Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One 4: e6026.
- Nougayrède JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, et al. (2006) Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313: 848-851.
- Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, et al. (2010) Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 1: 138-147.
- Buc E, Dubois D, Sauvanet P, Raisch J, Delmas J, et al. (2013) High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS One 8: e56964.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14447
- [From(publication date):
July-2013 - Apr 11, 2025] - Breakdown by view type
- HTML page views : 9825
- PDF downloads : 4622