Induction Chemotherapy Followed by Concomitant Chemoradiation in Head and Neck Squamous Cell Carcinoma: A Single Institution Experience
Received: 14-Jun-2011 / Accepted Date: 16-Nov-2011 / Published Date: 21-Nov-2011 DOI: 10.4172/2161-119X.1000108
Abstract
Objective: Phase 3 studies are underway to compare induction chemotherapy (IC) followed by concomitant chemoradiation (CRT) with CRT alone in advanced head and neck cancer. The purpose is to report the outcome of patients with advanced head and neck cancer treated at Centre Hospitalier de l’Université de Montréal (CHUM) with IC followed by CRT.
Methods: From March 1998 to December 2007, 56 consecutive patients were treated for advanced squamous cell carcinoma of the head and neck with high-dose IC followed by CRT. Sixteen patients with carcinoma of the nasopharynx, paranasal sinuses or nasal cavity were excluded. Patients presented with either T4 (60%) or N3 (60%) disease. Outcomes were computed using Kaplan-Meier curves. The number of IC cycles were compared with logrank tests.
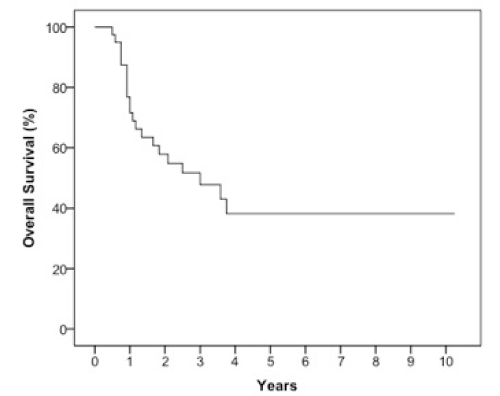
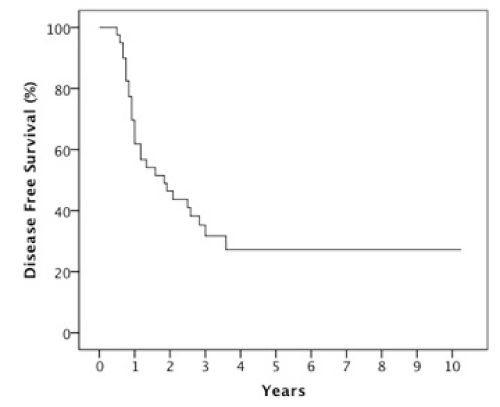
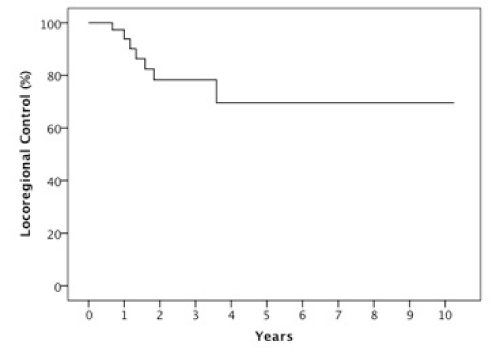
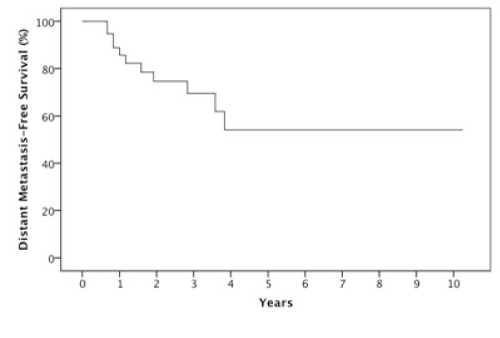
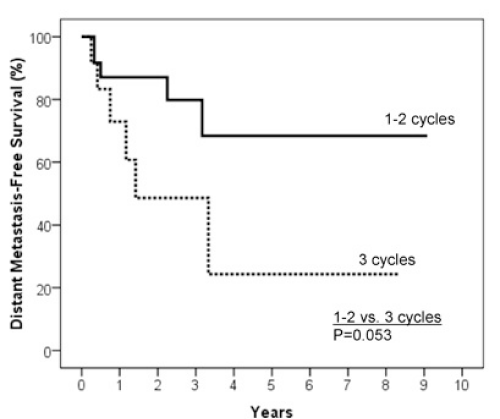
Results: The 2 year estimates of OS, DFS, LRC and DMFS rates were 58%, 46%, 78% and 75% respectively. At last follow-up, we observed 17 patients with relapse of which 10 were at a distant site. When stratified by the number of IC cycles, a DMFS rate of 87% was observed for 1-2 cycles vs 49% for 3 cycles, p=0.05.
Conclusions: Despite intensive treatment with platinum based IC and CRT, prognosis for this highly advanced population of T4 or N3 cancers is poor. The number of IC cycles seem to influence the rate of DM. Further trials are needed to answer the question regarding IC followed by CRT vs CRT alone. Targeted therapies might also yield more promising results.
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) is considered the sixth most common cancer in the world and accounts for 6% of all cancers [1]. The majority of patients present with advanced locoregional disease [2,3]. Surgery has historically been the primary treatment modality for this disease. With an exclusive locoregional treatment, locoregional relapse within two years reaches up to 60% and up to 20-30% for distant metastasis, especially among patients with N2/3 staging [4-8]. The Veterans Affairs larynx trial paved the way in changing treatment approaches by demonstrating that chemotherapy followed by radiotherapy could replace the morbid laryngectomy in selected patients without sacrificing survival rates [9]. Thus, concomitant chemoradiation therapy (CRT) has become the standard of care in the nonsurgical management of most locally advanced head and neck cancer [10-12].
In the Meta-Analysis of Chemotherapy in the Head and Neck Cancer [10,13], an absolute survival benefit of 8% at 5 years was observed when chemotherapy was administered concomitantly with radiotherapy, but no statistically significant improvement was found when chemotherapy was given before locoregional treatment (IC). However, the regimens used as IC were often suboptimal in the included studies. When analyses were restricted to trials using an IC regimen composed of cisplatin and fluorouracil, a significant survival advantage of 5% at 5 years was observed [3,14]. Furthermore, when a more intensive CRT was used [15], a local control rate of more than 90% at 3 years was observed. However, seventeen percent of these patients presented distant metastasis.
The role of IC is not yet clear as there is no published phase III trial comparing the standard treatment of CRT versus IC followed by CRT. Two individual trials have shown a survival benefit for IC followed by local treatment over local treatment alone [16-18]. However, the local treatment in those trials did not consist of CRT but rather of surgery plus radiotherapy or radiotherapy alone.
More recently, phase III studies from the EORTC/TAX study group comparing two IC regimens consisting of cisplatin and fluorouracil with or without docetaxel followed by CRT in patients with unresectable squamous cell carcinoma of the head and neck found a significant improvement in the progression free and overall survival with inclusion of docetaxel. A phase III trial GORTEC 2000-01 [19] comparing docetaxel, cisplatin and fluorouracil (TPF) with cisplatinfluorouracil as induction chemotherapy demonstrated that TPF was superior to the cisplatin– fluorouracil regimen in terms of overall RR (80.0 versus 59.2%) and 3-year actuarial larynx preservation rate (70.3 versus 57.5%). Posner et al. [20] reported comparable results in a subgroup analysis of the TAX 324 randomized trial comparing TPF with CDDP – fluorouracil, followed by carboplatin radiotherapy. These results support the use of induction TPF as a treatment option for larynx preservation.
IC has been used in clinical practice [21] and is thought to be beneficial for reducing the rate of distant metastasis, increasing organ preservation [9,22] and survival rates [16,17].
At Centre Hospitalier de l’Université de Montréal (CHUM), IC has been used since 1998 for patients with stage III or IVA-B SCCHN presenting with important symptoms caused by the tumor or rapidly progressing disease. It was also used when radiation therapy could not be started within a reasonable timeframe. IC was also preferred for patients in whom shrinkage of the tumor was desirable in order to diminish radiation fields and hence toxicities, for example in an N3 disease near the brachial plexus. In order to better define the value of IC, we initiated a retrospective review to evaluate the overall survival (OS), disease-free survival (DFS), locoregional control (LRC) and distant metastasis-free survival (DMFS) rates in our cohort of patients treated with IC followed by CRT for locally advanced, non-metastatic SCCHN.
Methods and Material
Study design
This is a single-institution, retrospective study. We reviewed the files of all consecutive patients treated at Centre hospitalier de l’Université de Montréal (CHUM), Hôpital Notre-Dame, between March 1998 and December 2007 for locally advanced head and neck cancers with IC followed by CRT. Inclusion criteria were: 1) Stage III or IVA-B squamous cell carcinoma according to the 6th edition of American Joint Committee on Cancer staging criteria; 2) Treatment with a curative intent; 3) Treatment with IC and 4) CRT following IC. Patients were excluded if treated for a recurrence, post-operatively or pre-operatively. Patients presenting with carcinoma of the nasopharynx, paranasal sinuses or nasal cavity were also excluded. The results are based on a final review of charts as of December 2009.
This study was approved by the hospital authorities in the context of a review of the quality of practice to establish institutional guidelines of therapy.
Treatment
The institutional policy at the CHUM is to offer organ-preserving treatment to all locally advanced head and neck cancers. As are all the head and neck cancer patients at CHUM, patients with either T4 or N3 disease were presented at the Tumour Board composed of surgeons, radiologists, pathologists, medical oncologists and radiation oncologists. The treatment for these patients was a nonsurgical approach combining IC followed by CRT when the delay for radiotherapy was prolonged because of dental extracts, dental healing, or of a waiting list in radiotherapy which is the case in our actual Canadian context. This regimen was also favoured when patients were presenting symptoms such as significant weight loss at presentation, neck disease near brachial plexus, uncontrolled pain or trismus, or severe dysphagia that would otherwise require a feeding tube
Neoadjuvant and concomitant chemotherapy schedule
Five regimens were primarily used as IC at CHUM in the study period: cisplatin 75 mg/m2 and docetaxel 75 mg/m2 for 4 days in continuous infusion 1g/m2 every 3 weeks or cisplatin 75 mg/m2 and docetaxel 75 mg/m2 with 5-FU for 4 days in continuous infusion 1g/m2 every 3 weeks or cisplatin 75 mg/m2 with 5FU for 5 days in continuous infusion 1g/m2 every 3 weeks or cisplatin 80-100 mg/m2 in bolus every 3 weeks or carboplatin 70mg/m2 for 4 days and 5-FU in continuous infusion 600mg/m2 for 4 days every 3 weeks. Patients were scheduled to receive 1-3 cycles of IC depending on the delay before radiotherapy.
As for the chemotherapy given concomitantly to radiation therapy, two regimens were primarily used at CHUM in the study period: carboplatin 70 mg/m2/d in bolus for 4 days, with 5FU 600 mg/m2/d as a continuous infusion for 4 days, every 3 weeks, or cisplatin 100 mg/m2 in bolus every 3 weeks, depending on the patient’s medical status or on the treating physician’s preference. As a reference, the first regimen was usually used for patients with a primary lesion of the oropharynx. The three other regimens were seldomly used: carboplatin 25 mg/m2 daily (n=5, 12.5%), cisplatin 7mg/m2 daily (n=2, 5%) and cisplatin 40mg/m2 weekly (n=1, 2.5%). Chemotherapy started with the first day of radiation therapy. Most patients were scheduled to receive 3 cycles of chemotherapy during radiation therapy. Two cycles were scheduled only for patients with specific co-morbidities and/or with an age of more than 65 years. For IC or concomitant chemotherapy, dose reductions were applied for patients receiving cisplatin for renal and haematological toxicities.
Radiation therapy
The radiation therapy dose was 70 Gy in daily fractions of 1.8 to 2.12 Gy to the primary tumor and involved nodes adjusted after IC. The prophylactic dose to adjacent nodal regions was 50 to 60 Gy. Patients were treated either with a standard fractionation consisting of 70 Gy in 35 fractions over 7 weeks or with intensity modulated radiation therapy (IMRT) consisting of 70 Gy in 33 fractions over 6.5 weeks. Treatment was delivered with a 4 or 6 MV linear accelerator.
Surgery
Patients who had residual disease at their primary or regional site at the end of their course of treatment were referred for salvage surgery. The institutional policy at CHUM being to observe patients attaining clinical or radiological complete response 8 weeks after completion of CRT, neck dissection was only offered to patients presenting partial response.
Follow-up
Patients had a physical exam every two months for the first two years, every four months for the next three years and then annually. Every patient had a computed tomography (CT) scan six to eight weeks after definitive treatment and periodically during the first two years or if the questionnaire or the physical exam was suspicious of recurrence. A chest radiograph was also performed annually.
Statistical analysis
All data were obtained prospectively, although the analysis of IC was not decided prospectively. OS, DFS, LRC and DMFS were measured using the Kaplan-Meier [23] method. Follow-up time was calculated using the inverse Kaplan-Meier method [24]. Survival was calculated from the date of diagnosis to the date of death from any cause for OS, and to the date of first event (local, regional or distant relapse, second primary or death from any cause) for disease-free survival. The number of IC cycles was compared with logrank tests. Statistical analyses were conducted using Statistical Package for Social Sciences, SPSS software version 18 (SPSS, Chicago,IL). All tests were considered to be statistically significant for p-values smaller than 0.05.
Results
Patient characteristics
Fifty-six patients were treated with IC followed by CRT for locally advanced SCCHN. Forty patients fulfilled the inclusion criteria. Sixteen patients were excluded because of nasopharynx, paranasal sinuses or nasal cavity as being the first site of disease. Patients were treated between March 1998 and December 2007. Table 1 summarizes the demographics and clinical characteristics of these patients. The mean age at diagnosis was 57 years (44-76), with men composing 93% of the patient population. The majority of patients had oropharyngeal cancer (77%). Thirty-two percent had stage III-IVA disease and 68% had stage IVB. All but one patient presented with either T4 (60%) or N3 (60%) disease.
| #Patients (percent) | |
|---|---|
| Total number of patients | 40 |
| Age | |
| Mean | 57.4 |
| Range | 44-76 |
| Sex | |
| Female | 3 (7.5) |
| Male | 37 (92.5) |
| Primary tumor sites | |
| Oropharynx | 31 (77.0) |
| Larynx | 3 (7.5) |
| Unknown primary | 3 (7.5) |
| Hypopharynx | 2 (5.0) |
| Oral cavity | 1 (2.5) |
| T (tumor stage) | |
| Tx | 3 (7.5) |
| T1 | 4 (10.0) |
| T2 | 4 (10.0) |
| T3 | 5 (12.5) |
| T4 | 24(60.0) |
| N (lymph node stage) | |
| N0 | 3 (7.5) |
| N1 | 2 (5.0) |
| N2 | 11 (27.5) |
| N3 | 24 (60.0) |
| Stage | |
| III | 1 (2.5) |
| IVa | 12 (30.0) |
| IVb | 27 (67.5) |
Table 1: Patient characteristics.
Induction chemotherapy and concomitant chemoradiation
Treatment characteristics are listed in Table 2. Patients were treated with various platinum-based regimens. Most of the patients (95%) had a cisplatin based IC regimen. Of those, 38% also had docetaxel in combination. Twenty-eight patients received 1 or 2 cycles of IC while 12 patients received 3 cycles. As for the chemotherapy given concurrently to radiation, most of the patients were treated with either carboplatin and 5-FU (55.8%) or with cisplatin every three weeks (25.6%). The number of breaks during IC and concomitant chemotherapy were 7% and 25.6%, respectively. As for the radiotherapy treatment, median dose was 70 Gy almost equally split between conventional (47.5%) and IMRT (52.5%) techniques. Ninety-eight percent of patients completed the RT as planned. Only one patient had 23.32 Gy in 11 fractions due to a voluntary interruption of treatments.
| #Patients (percent) | |
|---|---|
| Induction Chemotherapy | |
| Carbo-5FU | 2 (5.0) |
| Cisplatin | 11 (27.5) |
| Cisplatin-docetaxel | 14 (35.0) |
| Cisplatin-5FU | 12 (30.0) |
| Cisplatin-5FU-docetaxel | 1 (2.5) |
| Number of cycles | |
| 1 cycle | 11 (27.5) |
| 2 cycles | 17 (42.5) |
| 3 cycles | 12 (30.0) |
| Concomitant Chemotherapy | |
| Carbo die | 5 (12.5) |
| Carbo-5FU | 22 (55.0) |
| Cisplatin die | 2 (5.0) |
| Cisplatin q 1 week | 1 (2.5) |
| Cisplatin q 3 weeks | 10 (25.0) |
| Radiation therapy | |
| Technique | |
| IMRT | 21 (52.5) |
| Conventional | 19 (47.5) |
| Median dose | 70 Gy |
| Less than 68Gy | 1 (2.5) |
Table 2: Treatment characteristics.
Complications
Table 3 describes complications associated with IC and CRT. Only two patients (5.9%) had febrile neutropenia for which they had to be hospitalized subsequent to IC. Overall, four patients had to be hospitalized (the other two patients were hospitalized for renal insufficiency and pneumonia). After CRT, some patients developed a Grade 3 or 4 neutropenia (13.9%) and nausea and vomiting (7.7%) while the majority experienced grade 3 or 4 mucositis and dermatitis. There was a 34.2% rate of admission during CRT treatment. There was no mortality during treatment.
| IC (percent) |
CRT #Patients (percent) |
|
|---|---|---|
| Mucositis (gr3-4) | N/A | 26/35 (74.3) |
| Dermatitis (gr3-4) | N/A | 13/34 (38.2) |
| Nausea/vomitting (gr3-4) | 7/31 (22.6) | 2/26 (7.7) |
| Febrile neutropenia | 2/34 (5.9) | 5/36 (13.9) |
| Enteral feeding | 1/40(2.5) | 16/34 (47.1) |
| Hospitalization | 4/34(11.8) | 13/38 (34.2) |
| Death during treatment | 0(0) | 0 (0) |
Table 3: Acute complications during IC and CRT.
Response rates
Following IC, thirty-two patients out of 35 (91.4%) had a partial response of their primary tumor site and 34 out of 36 patients (94.4%) had a partial response at the nodal level. Following CRT treatment, 35 patients (87.5%) had a complete response at the primary site and 19 (47.5%) at the regional site. A total of 18 patients underwent neck dissection of which five presented a pathological disease. Three patients had no regional complete response but did not undergo neck dissection. Two patients were not operable because of poor performance status and one patient was lost to follow-up. Regarding the primary site, one patient underwent surgery and did not present pathological disease. Four patients had no complete response of the primary site but did not undergo surgery because of the following reasons: three patients were not operable because of poor performance status or progression of disease and one patient was lost to follow-up.
Patient outcomes
The median follow-up was 23 months (6-123 months). At two years, the estimated OS, DFS, LRC and DMFS rates were 58%, 46%, 78% and 75%, respectively for the whole population (Figures 1-4).There were a total of 17 relapses in the whole population of which three were local, five regional and ten at a distant site. Twelve deaths were recorded of which ten resulted from their carcinomas, one had a nonrelated cause (myocardial infarct) and one presented a second primary.
1-2 versus 3 cycles of IC
In order to explore the characteristics of IC that were predictive of outcome, an analysis on the number of IC cycles was performed. We compared patients who had 1-2 cycles of IC with patients having 3 cycles. Baseline characteristics of patients in both groups of these analyses were not statistically different based on age, gender, KPS, primary sites, TNM stage, grade, concomitant chemotherapy regimen and regional/primary site response (data not shown, p>0.05). There was no statistically significant difference in two-year OS (p=0.46), twoyear DFS (p=0.12) and two-year LRC (p=0.49) between the two groups of patients. There was however a statistical significant difference as for the two-year DMFS (87.1% vs. 48.6%; p=0.05, Figure 5).
Discussion
Induction chemotherapy has several theoretical advantages including the delivery of doses of chemotherapy to untreated, well vascularised tumors as well as the eradication of micrometastatic disease. Moreover, induction chemotherapy reduces tumor burden before local treatment. In addition, a patient might better tolerate a chemotherapy treatment if not administered immediately with radiation therapy. Our aim was to report our clinical experience on outcomes of patients followed at CHUM for advanced head and neck cancer (T4 or N3) treated with CRT with 2 or 3 cycles of high dose cisplatin. This study was retrospective in design, although the data were all obtained prospectively.
Previously reported studies have shown comparable two years rates for OS (61-66%), LRC (71-76%) and DMFS (79-91%) [25-27]. However, these studies had larger inclusion criteria such as inclusion of nasopharynx, inclusion of T1N2 or T3N1 patients whereas our study only had one patient with stage III disease, and all others with T4 or N3 disease. For example, in the SWOG study where T4 tumors were excluded, the 3-year overall survival rate was 64% and the 3-year progression-free survival with organ preservation was 52% [28]. Of note, 25% of patients developed distant metastasis during follow-up in our study.
Several phase II studies confirmed that IC achieved objective tumor regression in 60-90% of the patients with a clinical complete response in 20-50% of them [29,30]. In our study, IC followed by CRT resulted in an 87.5% complete primary response and a 47.5% complete regional response.
Phase II studies have shown the benefit of combining docetaxel to cisplatin and fluorouracil [30,31]. In 2007, Vermorken et al. [32] published the results of a phase III trial (EORTC 24971/TAX 323 Study Group) comparing IC regimens of cisplatin and fluorouracil with or without docetaxel followed by radiotherapy alone and concluded that as compared with the standard regimen of cisplatin and fluorouracil, the same IC with the addition of docetaxel significantly improved PFS and OS. However, concomitant chemoradiation therapy was not used in the TAX 323 Study and it is therefore difficult to draw conclusions for the benefit of taxanes. At CHUM, taxanes were part of the IC regimen since 2004 but analysis could not be done in view of only forty patients in our cohort. Furthermore, Posner and al. [33] also conducted a randomised phase III study (TAX 324 Study Group) demonstrating that patients who received docetaxel plus cisplatin and fluorouracil IC followed by CRT had a significantly longer survival than did patients who received cisplatin and fluorouracil IC followed by CRT. However, the concomitant regimen used weekly carboplatin, a therapy that is not standard and unproven by a phase III trial, and could be potentially suboptimal therefore underestimating the results associated with the arm without docetaxel in the IC regimen. In many published trials [12,34-38], head and neck tumors are treated by CRT with 2-3 cycles of high dose cisplatin. In fact, many ongoing trials use cisplatin as the standard arm in randomized trials of head and neck cancer where concurrent chemoradiation is part of the definitive treatment. Moreover, Brizel and Vokes [39] suggested that the benefit of taxanes may diminish as the intensity of local-regional therapy increases.
In a recent meta-analysis by Su et al. [40], it has been shown that IC in SCCHN patients, with the cisplatin and fluorouracil regimen, had no effect on locoregional relapse but a small significant benefit was shown in reducing distant metastasis and improving the overall survival. In our study, we had a 42.5% relapse rate with 59% being at a distant site. We also had a total of 12 deaths with 83% being related to their carcinoma.
The present study shows that patients receiving only 1-2 cycles of IC had statistically significant improvement in two year DMFS compared with patients receiving 3 cycles of IC. This could be due to the fact that CRT, being the treatment of choice for advanced SCCHN, is delayed because of a third cycle of IC. In a phase II study by Urba et al. [41] a single cycle of IC was given to stage III-IV squamous cell carcinoma of the larynx. Patients with less than 50% response underwent total laryngectomy and the remainder underwent CRT. They obtained a 70% rate of larynx preservation. They believe and our results concur with the premise that speed of tumor response to neoadjuvant chemotherapy is an important prognostic factor. Reducing overall treatment time to diminish accelerated repopulation of surviving clonogens is an important radiobiology concept. Although response to IC was not well documented in patients’ files, we assume that patients who received 3 cycles were not responding to IC as well as those receiving 1-2 cycles. Further research with biological markers might answer the question as to why patients with the same baseline characteristics respond differently to treatment.
There might be predictive biomarkers that would help in choosing between an IC option or not. At the moment, in our institution, only those where an expected delay for the planning of radiotherapy such as dental work, waiting list or where a shrinkage of the tumor is necessary for radiotherapy fields to be safe are offered IC. In such, we believe that if IC must be given, it should be restrained to only one or two cycles.
As for treatment related toxicities, patients in our study presented morbidities comparable to the literature. Febrile neutropenia was present in 5.9% patients due to IC and in 13.9% following CRT which is no different from the TAX 323 Study. Aside from mucositis and dermatitis, the treatments were well tolerated and there were no related deaths. The toxicities from CRT post IC do not seem to differ from the toxicities usually encountered by patients treated with CRT exclusively in our institution (data not shown).
The limitations of our study include its retrospective nature and the relatively small number of patient meeting the inclusion criteria. Moreover, no systematic criteria were used in determining the need for IC. Patients in this study were treated in different era in terms of general care and planning techniques, and there was no standard IC regimen until 2004 where cisplatin, 5FU and docetaxel became the standard regimen unless a patient was not eligible.
Conclusions
In this single-center retrospective analysis, despite intensive treatment with platinum based IC and CRT, prognosis for this highly advanced population of T4 or N3 cancers is poor. Our cohort of patients resulted in fair LRC and OS rates but with significant DM rates. The number of IC cycles seems to influence the rate of DM. The role of IC followed by CRT is currently under evaluation in three prospective randomized trials in North America [8]. The ongoing DeCIDE (University of Chicago) and PARADIGM (Dana Faber Cancer Center) trials are large studies that are designed to test the benefit of cisplatin, 5FU and docetaxel IC followed by CRT against CRT alone. Their outcomes will refine our understanding of the optimal nonsurgical treatment for the management of advanced SCCHN. Also, novel targeted agents, such as EGFR antagonists are showing promise in the treatment of patients with both locoregionally advanced and recurrent-metastatic SCCHN and might also yield more promising results when used in the regimen of IC [42]. Research is also warranted for prognostic biomarkers and for factors which could help in selecting patients who might benefit from IC.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, et al. (2007) Cancer statistics, 2007. CA Cancer J Clin 57:43-66.
- Patel SG, Shah JP (2005) TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin 55:242-258.
- Monnerat C, Faivre S, Temam S, Bourhis J, Raymond E (2002) End points for new agents in induction chemotherapy for locally advanced head and neck cancers. Ann Oncol 13:995-1006.
- Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, et al. (2004) Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 22:69-76.
- Forastiere AA, Trotti A, Pfister DG, Grandis JR (2006) Head and neck cancer: recent advances and new standards of care. J Clin Oncol 24:2603-2605.
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, et al. (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567-578.
- Posner MR, Haddad RI, Wirth L, Norris CM, Goguen LA, et al. (2004) Induction chemotherapy in locally advanced squamous cell cancer of the head and neck: evolution of the sequential treatment approach. Semin Oncol 31:778-785.
- Adelstein DJ, Leblanc M (2006) Does induction chemotherapy have a role in the management of locoregionally advanced squamous cell head and neck cancer? J Clin Oncol 24:2624-2628.
- (1991) Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. N Engl J Med 324:1685-1690.
- Pignon JP, Bourhis J, Domenge C, Designé L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-Analysis of Chemotherapy on Head and Neck Cancer. Lancet 355:949-955.
- Garden AS, Asper JA, Morrison WH, Schechter NR, Glisson BS, et al. (2004) Is concurrent chemoradiation the treatment of choice for all patients with Stage III or IV head and neck carcinoma? Cancer 100:1171-1178.
- Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, et al. (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091-2098.
- Pignon JP, le Maitre A, Bourhis J (2007) Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): an update. Int J Radiat Oncol Biol Phys 69:S112-S114.
- Weaver A, Fleming S, Ensley J, Kish JA, Jacobs J, et al. (1984) Superior clinical response and survival rates with initial bolus of cisplatin and 120 hour infusion of 5-fluorouracil before definitive therapy for locally advanced head and neck cancer. Am J Surg 148:525-529.
- Vokes EE, Kies MS, Haraf DJ, Stenson K, List M, et al. (2000) Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer. J Clin Oncol 18:1652-1661.
- Paccagnella A, Orlando A, Marchiori C, Zorat PL, Cavaniglia G, et al. (1994) Phase III trial of initial chemotherapy in stage III or IV head and neck cancers: a study by the Gruppo di Studio sui Tumori della Testa e del Collo. J Natl Cancer Inst 86:265-272.
- Zorat PL, Paccagnella A, Cavaniglia G, Loreggian L, Gava A, et al. (2004) Randomized phase III trial of neoadjuvant chemotherapy in head and neck cancer: 10-year follow-up. J Natl Cancer Inst 96:1714-1717.
- Domenge C, Hill C, Lefebvre JL, De Raucourt D, Rhein B, et al. (2000) Randomized trial of neoadjuvant chemotherapy in oropharyngeal carcinoma. French Groupe d'Etude des Tumeurs de la Tete et du Cou (GETTEC). Br J Cancer 83:1594-1598.
- Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, et al. (2009) Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 101:498-506.
- Posner MR, Norris CM, Wirth LJ, Shin DM, Cullen KJ, et al. (2009) Sequential therapy for the locally advanced larynx and hypopharynx cancer subgroup in TAX 324: survival, surgery, and organ preservation. Ann Oncol 20:921-927.
- Harari PM (1997) Why has induction chemotherapy for advanced head and neck cancer become a United States community standard of practice? J Clin Oncol 15:2050-2055.
- Lefebvre JL, Chevalier D, Luboinski B, Kirkpatrick A, Collette L, et al. (1996) Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst 88:890-899.
- Kaplan EL, Meier P (1958) Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 53:457-481.
- Schemper M, Smith TL (1996) A note on quantifying follow-up in studies of failure time. Control Clin Trials 17:343-346.
- Psyrri A, Kwong M, DiStasio S, Lekakis L, Kassar M, et al. (2004) Cisplatin, fluorouracil, and leucovorin induction chemotherapy followed by concurrent cisplatin chemoradiotherapy for organ preservation and cure in patients with advanced head and neck cancer: long-term follow-up. J Clin Oncol 22:3061-3069.
- Hitt R, Lopez-Pousa A, Martinez-Trufero J, Escrig V, Carles J, et al. (2005) Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 23: 8636-8645.
- Bhide SA, Ahmed M, Barbachano Y, Newbold K, Harrington KJ, et al. (2008) Sequential induction chemotherapy followed by radical chemo-radiation in the treatment of locoregionally advanced head-and-neck cancer. Br J Cancer 99: 57-62.
- Urba SG, Moon J, Giri PG, Adelstein DJ, Hanna E, et al. (2005) Organ preservation for advanced resectable cancer of the base of tongue and hypopharynx: a Southwest Oncology Group Trial. J Clin Oncol 23: 88-95.
- Adelstein DJ (2008) Redefining the role of induction chemotherapy in head and neck cancer. J Clin Oncol 26: 3117-3119.
- Posner MR, Glisson B, Frenette G, Al-Sarraf M, Colevas AD, et al. (2001) Multicenter phase I-II trial of docetaxel, cisplatin, and fluorouracil induction chemotherapy for patients with locally advanced squamous cell cancer of the head and neck. J Clin Oncol 19: 1096-1104.
- Haddad R, Colevas AD, Tishler R, Tishler R, Busse P, et al. (2003) Docetaxel, cisplatin, and 5-fluorouracil-based induction chemotherapy in patients with locally advanced squamous cell carcinoma of the head and neck: the Dana Farber Cancer Institute experience. Cancer 97: 412-418.
- Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, et al. (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357: 1695-1704.
- Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, et al. (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357: 1705-1715.
- Huguenin P, Beer KT, Allal A, Rufibach K, Friedli C, et al. (2004) Concomitant cisplatin significantly improves locoregional control in advanced head and neck cancers treated with hyperfractionated radiotherapy. J Clin Oncol 22: 4665-4673.
- Adelstein DJ, Li Y, Adams GL, Wagner H, Kish J A, et al. (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21: 92-98.
- Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefèbvre JL, et al. (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350: 1945-1952.
- Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, et al. (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350: 1937-1944.
- Cooper JS, Ang KK (2005) Concomitant chemotherapy and radiation therapy certainly improves local control. Int J Radiat Oncol Biol Phys 61: 7-9.
- Brizel DM, Vokes EE (2009) Induction chemotherapy: to use or not to use? That is the question. Semin Radiat Oncol 19: 11-16.
- Su YX, Zheng JW, Zheng GS, Qing LG, yuan ZZ, et al. (2008) Neoadjuvant chemotherapy of cisplatin and fluorouracil regimen in head and neck squamous cell carcinoma: a meta-analysis. Chin Med J (Engl) 121: 1939-1944.
- Urba S, Wolf G, Eisbruch A, Worden F, Lee J, et al. (2006) Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol 24: 593-598.
- Kies MS, Holsinger FC, Lee JJ, William WN Jr, Glisson BS, et al. (2010) Induction chemotherapy and cetuximab for locally advanced squamous cell carcinoma of the head and neck: results from a phase II prospective trial. J Clin Oncol 28: 8-14.
Citation: Delouya G, Clavel S, El-Bared N, Soulières D, Fortin B, et al. (2012) Induction Chemotherapy Followed by Concomitant Chemoradiation in Head and Neck Squamous Cell Carcinoma: A Single Institution Experience. Otolaryngol 1:108. DOI: 10.4172/2161-119X.1000108
Copyright: © 2012 Delouya G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 14457
- [From(publication date): 3-2012 - Apr 07, 2025]
- Breakdown by view type
- HTML page views: 9844
- PDF downloads: 4613