Research Article Open Access
In vivo Efficacy of PAMAM-Dendrimer-Cisplatin Complexes in SKOV-3 Xenografted Balb/C Nude Mice
Venkata K Yellepeddi, Kiran Kumar Vangara and Srinath Palakurthi*Irma Lerma Rangel College of Pharmacy, Texas A&M Health Science Center, 1010 W Ave B, Kingsville, Texas, 78363, USA
- Corresponding Author:
- Srinath Palakurthi
Irma Lerma Rangel College of Pharmacy
Texas A&M Health Science Center
1010 W Ave B, Kingsville, Texas-78363, USA
Tel: +1 3612210748
Fax: +1 3612210793
E-mail: palakurthi@pharmacy.tamhsc.edu
Received date: November 20, 2012; Accepted date: November 27, 2012; Published date: November 27, 2012
Citation: Yellepeddi VK, Vangara KK, Palakurthi S (2012) In vivo Efficacy of PAMAM-Dendrimer-Cisplatin Complexes in SKOV-3 Xenografted Balb/C Nude Mice. J Biotechnol Biomaterial S13:003. doi:10.4172/2155-952X.S13-003
Copyright: © 2012 Yellepeddi VK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Loco-regional delivery of cisplatin by Intraperitoneal (IP) administration in ovarian cancer is highly advantageous, since it exposes high concentrations of cisplatin to tumors in the peritoneal cavity. PAMAM-dendrimer-cisplatin complexes are expected to enhance the penetration of tumors in the peritoneal cavity, and aid in enhancing antitumor efficacy of cisplatin. In the present study, PAMAM G4 NH2 and Biotin PAMAM G4 NH2 dendrimer cisplatin complexes were administered intraperitoneally to SKOV xenografted athymic nude mice at a dose of 4 mg/kg. The increase in lifespan of tumor bearing mice and biodistribution of cisplatin in various organs was evaluated after IP administration of cisplatin and dendrimer-cisplatin complexes. The lifespan of mice increased by 41.93% and 25.8%, after treatment with PAMAM G4 NH2 and Biotin PAMAM G4 NH2 dendrimer cisplatin complexes, respectively. A 3-fold increase in the levels of platinum in plasma was observed, after administration of both PAMAM G4 NH2 and Biotin PAMAM G4 NH2 dendrimer cisplatin complexes. Biodistribution studies have shown higher accumulation of platinum in liver, spleen and kidneys with PAMAM G4 NH2 dendrimer-cisplatin complexes. The increased plasma concentration and enhanced tissue accumulation after IP administration of dendrimer-cisplatin complexes offer advantage of administering low dose of cisplatin, which would reduce the dose-dependent toxic effects of cisplatin.
Keywords
Cisplatin; PAMAM; Biotinylated dendrimer-cisplatin
Introduction
In the United States, ovarian cancer ranks ninth among most common cancers and ranks fifth in cancer deaths, among women. It is estimated that approximately 22,280 women will be diagnosed and 15,500 women will die from ovarian cancer in 2012 [1]. It is also estimated that ovarian cancer will occur in 190,000 women and causes death in 114,000 patients annually worldwide [2]. According to the American Cancer Society, the rate at which women are diagnosed with ovarian cancer has been reduced in the past 20 years. However, the 5-year survival rate is still dismal at 46%. It was also reported that if ovarian cancer was detected and treated before it metastasizes; the 5-year survival rate will be around 93%. Unfortunately, less than 20% of all ovarian cancers are detected at this early stage [3]. The first-line treatment of ovarian cancer involves use of platinum based chemotherapeutic agents, such as cisplatin along with other anti-cancer drugs such as paclitaxel [4-6]. Cisplatin is widely used for the treatment of peritoneal carcinomatosis by Intraperitoneal (IP) administration [7,8]. Although clinical studies have demonstrated the superior efficacy of IP chemotherapy of cisplatin compared to Intravenous (IV) chemotherapy, IP cisplatin chemotherapy suffers from major drawbacks, such as poor penetration of cisplatin into peritoneal tumors and dose-limiting systemic toxicity due to infiltration of cisplatin into systemic circulation through peritoneum.
The first report on the potential of Intraperitoneal (IP) administration in ovarian cancer was published by Dedrick et. al. [9], in Cancer Treatment Reports. A theoretical modeling study supporting the examination of IP antineoplastic drug delivery for ovarian cancer was presented, and suggested that tumors in the peritoneal cavity should be exposed to higher cytotoxic drug concentrations with regional IP treatment [9]. Because of its low molecular weight and water solubility, cisplatin was reported to enter the systemic circulation rapidly after IP injection, leading to low concentrations in the peritoneal cavity and increasing risk of systemic toxicity [10]. The other limitation is that IP chemotherapy of cisplatin is limited to patients whose residual tumor nodules are less than 0.5 cm after a surgical debulking [11]. Poor penetration of cisplatin into the inner core within large tumor masses is also a reason for failure of IP chemotherapy of cisplatin [12]. To improve cisplatin penetration, either cisplatin should be in contact with the tumor for a longer period of time, or should be administered at very high concentrations. Both of these strategies have very high propensity of resulting in systemic side effects such as nephrotoxicity and myelotoxicity [13]. One attractive strategy to improve drug delivery to the peritoneal cavity and to reduce toxicity is use of tumor targeted drug delivery systems.
We have previously reported the potential of biotinylated PAMAM dendrimers as targeted drug delivery systems for cisplatin to ovarian cancer [14,15]. Biotinylated dendrimer-cisplatin complexes were shown to be taken up avidly by ovarian cancer cells because of great appetite of cancer cells for vitamins like biotin [16]. So far biotinylated dendrimercisplatin complexes were not evaluated for IP chemotherapy. Herein we report the in vivo biodistribution of dendrimer-cisplatin complexes prepared using simple PAMAMG4 and biotinylated PAMAM dendrimers, and their efficacy in prolonging the life span in a clinically relevant SKOV-3 xenografted murine ovarian cancer model.
Materials and Methods
Materials
PAMAM dendrimers (G4 NH2 ) were purchased from Dendritic Nanotechnologies Inc., (Mount Pleasant, MI, USA). Cisplatin, 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl tetrazolium bromide (MTT), 2,5 Dihydroxybenzoic acid (DHB), trifluoroacetic acid, nickel chloride (NiCl2) and insulin (recombinant human) were purchased from Sigma- Aldrich (St. Louis, MO, USA). Biotinylating reagents Sulfo-NHS-LC- Biotin (Mr 556), Morpholinoethane Sulfonic acid-(MES) buffered saline packs were purchased from Pierce Chemical Co., (Rockford, IL, USA). Sodium Siethyldithiocarbamate Trihydrate (DDTC) was purchased from Fluka Chemicals, (Steinheim, Switzerland). Dialysis membranes were purchased from Spectrum Laboratories (Rancho Dominguez, CA, USA). Omni Trace® nitric acid was purchased from EMD Biosciences (Gibbstown, New Jersey, USA). The Plasma Cal tune A, tune B, platinum standard (1000 μg/mL) and iridium standard (1000 μg/mL) were purchased from SCP Science (Baie D“Urfé, Quebec, Canada).
Synthesis and characterization of biotinylated PAMAM dendrimers
Biotin-conjugated PAMAM dendrimers were prepared, as reported previously [15,17]. PAMAM G4 NH2 dendrimers (10 mg/mL) dissolved in phosphate buffer pH 9.0 were added to sulfo-NHS-LC-biotin at 1:30 molar ratio, and stirred for 2 h at room temperature. The unconjugated biotin was separated by dialyzing reaction mixture against deionized water and lyophilized (Free zone 2.5. Labconco Corporation, Kansas City, MO, USA), to yield a white product. The final product was characterized for attachment of biotin by proton nuclear magnetic resonance (1H NMR, Bruker AMX-400 spectrometer, Bruker, Rheinstetten, Germany), using D2O as solvent at a concentration of 5 mg/mL. Degree of biotinylation was calculated using Bruker MALDI-TOF (matrix assisted laser desorption/ionization-time-of flight) using 2, 5 dihydroxybenzoic acid in 0.1 M trifluoroacetic acid as the matrix [15].
Preparation of dendrimer-cisplatin complexes
Dendrimer-cisplatin complexes were prepared by using a method developed in our lab previously [14]. Briefly, cisplatin was completely dissolved in 7.5 mL of water at 2 mg/mL concentration in a round bottomed flask. To this solution 20 mg of biotinylated and unconjugated dendrimers were added dropwise under stirring. The mixture was reacted for 4 hrs and dialyzed (3.5 kDa MW cutoff) against deionized water for 24 hrs. The final reaction product was lyophilized to yield a white product. Drug loading efficiency of dendrimer-cisplatin complexes was determined using inductively couple plasma-mass spectroscopy (ICP-MS). Briefly, 1 mg of dendrimer-cisplatin complex was dissolved in 1 mL of 0.9% Nacl solution. 100 μL of this solution was aliquoted and added to 5 mL of 1% HNO3 with 10 ng/mL iridium as the internal standard.
Quantification of cisplatin by ICP-MS
The ICP-MS system was comprised of an X Series ICP-MS (Thermo Electron Corporation, Madison, WI, USA) equipped with a Cetac 500 auto sampler (Cetac Technologies, Omaha, NB, USA). Analysis was performed in X-series default mode using 2.Xi+screen. The results were analyzed using PlasmaLab software (Thermo Electron Corporation). Major isotopes of platinum and iridium were monitored at m/z 195 and 193, respectively. Sample 6 nebulization was performed using a concentric nebulizer and detection modes for both isotopes were “scanning”. Details of ICP-MS operating conditions are given in table 1. Quantification was based on the mean (n=3) intensity ratios for platinum and iridium against a calibration curve using linear regression analysis. All standards and samples were prepared in 1% OmniTrace® nitric acid. A standard platinum calibration curve was prepared with concentrations from 0.1 to 1000 ng/mL. In all standard solutions iridium was added to get a final concentration of 10 ng/mL.
| Flow Conditions (L min-1) | Torch alignment (mm) | Ion optics (volts) | |
|---|---|---|---|
| Plasma flow 18.0 Auxillary flow 0.90 Sheath gas 0.25 Nebulizer flow 0.95 |
Sampling depth 130 | Lens 1 Lens 2 Lens 3 Pole Bias Hexapole Bias Extraction |
0.28 -29.02 -195.29 0.51 -3.02 -235.29 |
Table 1: ICP-MS instrument settings.
Sample preparation for biodistribution studies
Plasma (100 μL) was separated from freshly collected blood (200 μL) by centrifugation at 4000 rpm. Plasma samples were digested with concentrated nitric acid (HNO3) (70%) and after digestion samples were dried at 85°C overnight. Similarly, isolated tissues were homogenized using an Omni 2000 Tissue Homogenizer, Omni international, GA, USA, and were digested with concentrated HNO3 (70%) and dried at 85°C overnight. Both plasma and tissue dried samples were reconstituted with 1% HNO3 and 10 ng/mL of iridium was added as internal standard prior to analysis by ICP-MS. The standard graph of cisplatin was prepared by spiking cisplatin with blank plasma and tissues using concentrations, ranging from 0.1 ng/mL to 1000 ng/mL.
Cell culture
SKOV cells (passages 32-46) were obtained from ATCC and grown in DMEM (Dulbecco“s modified eagle medium) supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin solution. All cells were maintained at 37°C in a humidified incubator containing 5% CO2.
In vivo anti-tumor efficacy of dendrimer-cisplatin complexes
Animals: Female athymic nude mice 4 weeks old were procured from Harlan Laboratories (Indianapolis, IN, USA) and were housed at Pharmaceutical Research Facility, Texas A&M Health Science Center, Kingsville, Texas. All mice were housed and maintained under specific pathogen-free conditions according to guidelines of the American Association for Accreditation of Laboratory Animal Care (AAALAC), and the facility was operated in accordance with current regulations and standards of the U.S. Department of Agriculture (USDA), U.S. Department of Health and Human Services, and NIH. All protocols were approved by the institutional animal care and use committee of the College of Pharmacy (Protocol No. 10039). The mice were maintained on a standardized 12-h light and 12-h dark cycle and were provided access to food and water ad libitum. Animals were fed with irradiated 5053 PicoLab® rodent diet 20, PMI Nutrition International, St. Louis, MO, USA.
SKOV xenografted murine ovarian cancer model
SKOV tumor model was chosen because SKOV cells have the ability to get implanted directly on the peritoneal wall, resulting in numerous visible microtumor nodules and parietal thickening, resembling disease in humans [18]. Tumors were initiated by IP injection of SKOV cells (5×106), and mice were separated into four groups of 8 mice each for survival studies and three groups of 10 mice each for biodistribution studies. Tumors established by IP injection of cells reflect the growth pattern of advanced epithelial ovarian cancer and is widely used as a clinically relevant model for studying epithelial ovarian cancer [19]. Mice were evaluated weekly for tumor growth and/or ascites fluid accumulation. The animals were considered to have developed ascites if they showed clinical signs of tumor formation such as reduced movement, slightly hunched back, matted fur and reduction in animal weight. All animals in survival studies were sacrificed by 50 days after tumor initiation.
Treatment protocol
Cisplatin, PAMAM G4 NH2 cisplatin and Biotin-PAMAM G4 NH2 cisplatin, were administered by IP injection (500 μL) with PBS as the vehicle. The control groups of animals were administered PBS and other groups received 4 mg/kg equivalent of cisplatin either in solution or as dendrimer-cisplatin complexes four times with an interval of a week between each administration. Animals were euthanized using CO2 at 4 hrs and 24 hrs and blood was collected by cardiac puncture. In the biodistribution study brain, lung, heart, liver, kidney, and spleen, were isolated.
Statistical analysis
Statistical analyses were performed by Graph Pad Prism 5.0® and evaluated for statistically significant differences by one-way ANOVA and F<0.05 was considered statistically significant. Percentage of survival was analyzed using Kaplan-Meier analysis and compared using the long-rank test.
Results and Discussion
Synthesis and characterization of Biotin-PAMAM dendrimers
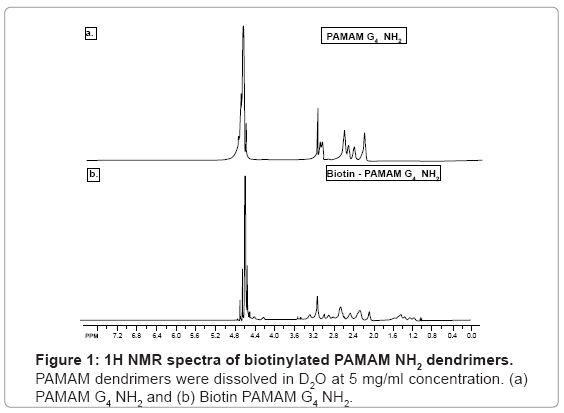
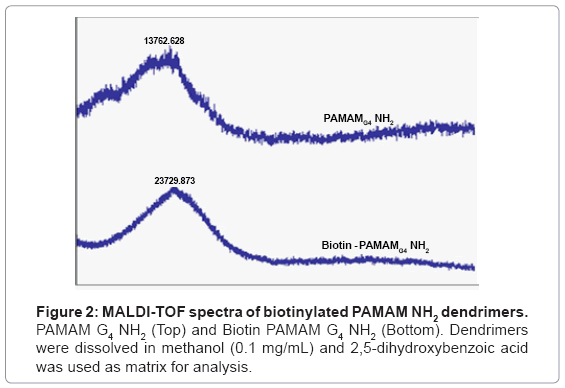
Dendrimers were biotinylated resulting in a product with high yield (>85%). Biotinylation was confirmed by 1H NMR by identifying biotin ring juncture protons at δ=4.4 and 4.6 ppm which were absent in the native PAMAM dendrimers (Figure 1) [15].To further confirm the conjugation and degree of biotinylation, biotinylated PAMAM dendrimers were analyzed using MALDI-TOF spectroscopy. MALDI-TOF depicted that approximately 27 biotin molecules were conjugated to PAMAM G4 NH2 dendrimers (Figure 2) [15].
Preparation of biotinylated dendrimer-cisplatin complexes
The drug loading efficiency of cisplatin as determined by ICP-MS was 102.9 μg per 1 mg of dendrimer (10.29%). The optimization of reaction medium was performed using two different solvents 0.9% sodium chloride and deionized water. The loading efficiency was very low (~0.1-2%), when 0.9% sodium chloride was used as reaction medium. However, the loading efficiency increased to ~10-11% when deionized was used as reaction medium. The high chloride ion concentration in 0.9% sodium chloride which inhibits the hydrolysis (aquation) of cisplatin and henceforth its reactivity, with dendrimers might be the reason for low loading. Our data corroborates with recent report by Kirkpatrick et al. [20], which showed that improved hydrolysis (aquation) of cisplatin using silver nitrate resulted in improved loading of cisplatin to dendrimers. The advantage of our dendrimer based nanocarrier system is evident as our loading efficiency is significantly higher than other nanocarrier formulations of cisplatin such as LipoplatinTM (8.9%) (a liposomal cisplatin formulation) [21], and PLGA-mPEG cisplatin nanoparticles (1.99%) [22].
Optimization of suitable analytical method for estimation of cisplatin
Various analytical methods for cisplatin quantification in plasma and tissues were developed using UV, HPLC and ICP-MS. The suitable analytical method was chosen based on parameters such as ease of sample preparation, robustness and sensitivity. Data showed that UV and HPLC methods were not sensitive (LLOQ 1 μg/mL). Moreover, derivatization of cisplatin by DDTC in those methods made the method cumbersome. Both UV and HPLC methods were not robust as the results were inconsistent when pH of the sample matrix was changed. Based on these observations ICP-MS was chosen as suitable method for estimation of platinum in plasma and tissues, as it is very sensitive (LLOQ 0.1 ng/mL), robust and convenient.
In vivo anti-tumor efficacy of dendrimer-cisplatin complexes
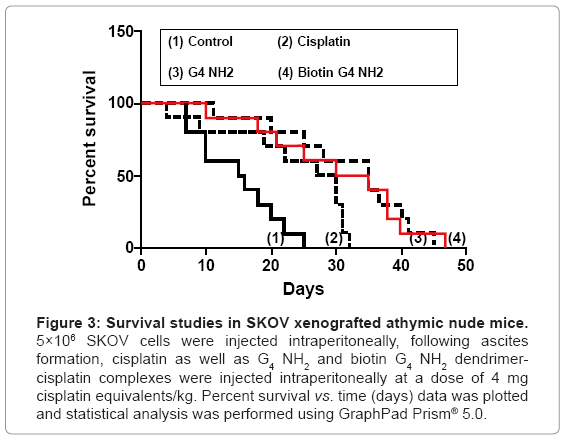
PAMAM G4 NH2 and Biotin PAMAM G4 NH2 dendrimer-cisplatin complexes were investigated for in vivo anti-tumor efficacy by evaluating their ability to prolong survival of tumor bearing mice. Procedures involving SKOV xenograft mouse tumor model were conducted in conformity with guidelines of the American Association for Accreditation of Laboratory Animal Care (AAALAC). Mice were monitored daily for body weight and signs of tumor progression such as abdominal distension, hunched back and matted fur. For survival studies, animals were sacrificed after reaching a predetermined endpoint characterized by weight loss exceeding 15% associated with anorexia, and/or diarrhea. Survival curves for the control and the treatment groups are shown in figure 3. All survival curves for treatment groups were significantly different from the control groups (p<0.033), which were associated with median survival times of 28.5 to 32.5 days (Table 2). Treatment with IP PAMAM G4 NH2-cisplatin and Biotin PAMAM G4 NH2-cisplatin resulted in a 41.9 % and 25.8 % increase in lifespan, when compared with treatment of cisplatin alone. Moreover, treatment with IP PAMAM G4 NH2- and Biotin PAMAM G4 NH2 dendrimer-cisplatin did not reduce the body weight in comparison with cisplatin (data not shown). Survival studies clearly indicate that eventhough biotinylation enhances the targeting ability of PAMAM dendrimers, it is limiting the cytotoxic efficacy of platinum probably by interfering with their interaction with DNA in cancer cells.
Figure 3: Survival studies in SKOV xenografted athymic nude mice. 5×106 SKOV cells were injected intraperitoneally, following ascites formation, cisplatin as well as G4 NH2 and biotin G4 NH2 dendrimercisplatin complexes were injected intraperitoneally at a dose of 4 mg cisplatin equivalents/kg. Percent survival vs. time (days) data was plotted and statistical analysis was performed using GraphPad Prism® 5.0.
| Group | Treatment | Median Survival§ (Days) | % Change in Lifespan † | Maximum Survival (Days) |
|---|---|---|---|---|
| 1 | Control: intraperitoneal saline | 15.5 | 25 | |
| 2 | Cisplatin (4 mg/kg): Intraperitoneally 1 X / Week X 4 | 28.5 | 83.87 | 32 |
| 3 | G4 NH2 Cisplatin (4 mg/kg): Intraperitoneally 1 X / Week X 4 | 35 | 125.80 | 45 |
| 4 | Biotin G4 NH2 Cisplatin (4 mg/kg): Intraperitoneally, 1 X / Week X 4 | 32.5 | 109.67 | 47 |
§-Median survival was calculated using Kaplan-Meier analysis. Log-rank test (Mantel-Cox test) indicated significant differences amongst all treatment groups.
†-Percentage change in lifespan, calculated as: [(median survival time in treated animals/median survival time in controls)–1]×100.
*-Statistical analyses were performed by Graph Pad Prism 5.0®, and evaluated for statistically significant differences by one-way ANOVA and F<0.05 was considered statistically significant. Percentage of survival was analyzed using Kaplan-Meier analysis and compared using the long-rank test.
Table 2: Statistical analysis of the results obtained from survival studies.
Biodistribution studies
The platinum concentration in the plasma and tissues was determined by ICP-MS. The calibration curve was linear in the concentration range from 0.1 ng/mL to 1000 ng/mL (R2=0.999), with a limit of detection of 0.05 ng/mL and limit of quantification of 0.1 ng/mL (5% standard deviation). Quality control samples, 0.1 ng/mL, 1 ng/mL and 10 ng/mL were used for method validation for accuracy and precision and the percentage coefficient of variation was <5% for all the data. The platinum recovery from cisplatin spiked tissues was plasma-93.4 ± 3.5% (mean ± SD); liver-84.5 ± 2.3 %; lungs-90.2 ± 1.2 %; heart-96.2 ± 5.3 %; kidneys - 97.9 ± 4.3 %; spleen - 96.8 ± 2.6 %; ascites-95.7 ± 5.2 %.
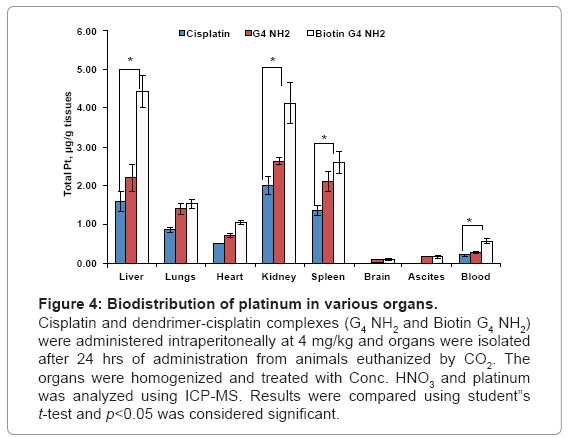
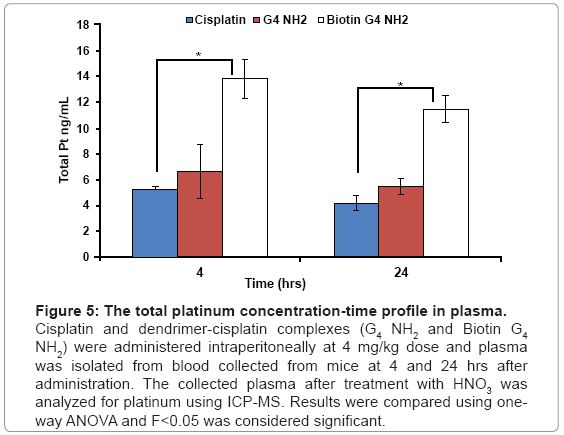
The biodistribution data showed that the biotin-G4 NH2 dendrimer-cisplatin complexes accumulated in significantly high concentrations in liver, heart, spleen, kidneys when compared with PAMAM G4 NH2 cisplatin and cisplatin only, with no increase in accumulation in lungs, brain and ascites. Our data corroborates with a report by Malik et al. who reported that PAMAM NH2 dendrimers of generation 4 showed high accumulation in liver [23]. As shown in figure 4, there was a 2.5 fold and 0.6 fold increased accumulation of platinum in in liver with biotin-G4 NH2- and G4 NH2 dendrimer-cisplatin complexes respectively, when compared with cisplatin alone. Liver and spleen, which are the organs of the mononuclear phagocytic system, have been shown to have a greater tendency to accumulate nanoparticulate formulations including dendrimers [24]. Moreover, IP administration of dendrimer-cisplatin complexes might have resulted in increased penetration of cisplatin through peritoneum and resulted in higher accumulation in various organs. Although a statistically significant increase in cisplatin accumulation in ascites was observed with dendrimer-cisplatin complexes as compared to cisplatin alone, the results were not as good as expected, possibly because of excessive adherence of dendrimers to the peritoneal cavity wall, probably mediated by surface charge. The smaller size and surface charge of the dendrimers, which are the cardinal aspects for enhancing tumor penetrating ability, might have led to increased diffusion of cisplatin through the peritoneum into systemic circulation. As shown in figure 5, plasma concentrations of platinum after 4 hr and 24 hr were also higher with IP administration of dendrimer-cisplatin complexes as compared cisplatin alone. Overall, the pharmacokinetics of cisplatin was altered after IP administration of dendrimer-cisplatin complexes.
Figure 4: Biodistribution of platinum in various organs.
Cisplatin and dendrimer-cisplatin complexes (G4 NH2 and Biotin G4 NH2) were administered intraperitoneally at 4 mg/kg and organs were isolated after 24 hrs of administration from animals euthanized by CO2. The organs were homogenized and treated with Conc. HNO3 and platinum was analyzed using ICP-MS. Results were compared using student‟s t-test and p<0.05 was considered significant.
Figure 5: The total platinum concentration-time profile in plasma.
Cisplatin and dendrimer-cisplatin complexes (G4 NH2 and Biotin G4 NH2) were administered intraperitoneally at 4 mg/kg dose and plasma was isolated from blood collected from mice at 4 and 24 hrs after administration. The collected plasma after treatment with HNO3 was analyzed for platinum using ICP-MS. Results were compared using oneway ANOVA and F<0.05 was considered significant.
Conclusion
In conclusion, all the dendrimer-cisplatin complexes studied have shown greater anti-tumor efficacy in vivo as compared to cisplatin alone. Though toxicity experiments were not performed in the present study, higher accumulation of cisplatin in liver and kidney suggests the need to improve the retention of dendrimer-cisplatin complexes in the peritoneal cavity, may be by using external stimuli-(such as magnetic field, ultrasound, etc.) responsive nanoparticles of cisplatin. Under the control of such external stimulus, these nanoparticles may retain in the “tumor sanctuaries” interspersed across the peritoneum for extended duration and show high therapeutic activity with minimal non-target distribution and lower magnitude of side effects.
Acknowledgements
This work was supported partly by a grant from National Institutes of Health (NIH R15 CA121980-01) to S. Palakurthi. Authors would also like to thank Mr. Donald Marek, Ms. Yaneth Gamboa of environmental engineering department, Texas A&M University, Kingsville, for helping with ICP-MS.
References
- American CancerSociety (2011) Ovarian cancer-detailed guide-ovarian cancer key statistics.
- Ricciardelli C, Oehler MK (2009) Diverse molecular pathways in ovarian cancer their clinical significance. Maturitas 62: 270-275.
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60: 277-300.
- Basu A, Krishnamurthy S (2010) Cellular responses to Cisplatin-induced DNA damage. J Nucleic Acids 2010.
- Fricker SP (2007) Metal based drugs: from serendipity to design. Dalton Trans 4903-4917.
- Kelland L (2007) Broadening the clinical use of platinum drug-based chemotherapy with new analogues. Satraplatin and picoplatin. Expert Opin Investig Drugs 16: 1009-1021.
- Tamura T, Imai J, Matsumoto A, Tanimoto M, Suzuki A, et al. (2002) Organ distribution of cisplatin after intraperitoneal administration of cisplatin-loaded microspheres. Eur J Pharm Biopharm 54: 1-7.
- Echarri Gonzalez MJ, Green R, Muggia FM (2011) Intraperitoneal drug delivery for ovarian cancer: why, how, who, what, and when? Oncology (Williston Park) 25: 156-165.
- Dedrick RL, Myers CE, Bungay PM, DeVita VT Jr (1978) Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep 62: 1-11.
- Rossi CR, Mocellin S, Pilati P, Foletto M, Quintieri L, et al. (2003) Pharmacokinetics of intraperitoneal cisplatin and doxorubicin. Surg Oncol Clin N Am 12: 781-794.
- Chauffert B, Favoulet P, Polycarpe E, Duvillard C, Beltramo JL, et al. (2003) Rationale supporting the use of vasoconstrictors for intraperitoneal chemotherapy with platinum derivatives. Surg Oncol Clin N Am 12: 835-848.
- Los G, Mutsaers PH, Lenglet WJ, Baldew GS, McVie JG (1990) Platinum distribution in intraperitoneal tumors after intraperitoneal cisplatin treatment. Cancer Chemother Pharmacol 25: 389-394.
- Tamura T, Imai J, Tanimoto M, Matsumoto A, Suzuki A, et al. (2002) Relation between dissolution profiles toxicity of cisplatin-loaded microspheres. Eur J Pharm Biopharm 53: 241-247.
- Yellepeddi VK, Kumar A, Maher DM, Chauhan SC, Vangara KK, et al. (2011) Biotinylated PAMAM dendrimers for intracellular delivery of cisplatin to ovarian cancer: role of SMVT. Anticancer Res 31: 897-906.
- Yellepeddi VK, Kumar A, Palakurthi S (2009) Biotinylated poly(amido)amine (PAMAM) dendrimers as carriers for drug delivery to ovarian cancer cells in vitro. Anticancer Res 29: 2933-2944.
- Yellepeddi VK, Kumar A, Palakurthi S (2009) Surface modified poly(amido)amine dendrimers as diverse nanomolecules for biomedical applications. Expert Opin Drug Deliv 6: 835-850.
- Mamede M, Saga T, Kobayashi H, Ishimori T, Higashi T, et al. (2003) Radiolabeling of avidin with very high specific activity for internal radiation therapy of intraperitoneally disseminated tumors. Clin Cancer Res 9: 3756-3762.
- Etter AL, Bassi I, Germain S, Delaloye JF, Tschopp J, et al. (2007) The combination of chemotherapy and intraperitoneal MegaFas Ligand improves treatment of ovarian carcinoma. Gynecol Oncol 107: 14-21.
- Rose GS, Tocco LM, Granger GA, DiSaia PJ, Hamilton TC, et al. (1996) Development and characterization of a clinically useful animal model of epithelial ovarian cancer in the Fischer 344 rat. Am J Obstet Gynecol 175: 593-599.
- Kirkpatrick GJ, Plumb JA, Sutcliffe OB, Flint DJ, Wheate NJ (2011) Evaluation of anionic half generation 3.5-6.5 poly(amidoamine) dendrimers as delivery vehicles for the active component of the anticancer drug cisplatin. J Inorg Biochem 105: 1115-1122.
- Boulikas T (2009) Clinical overview on Lipoplatin: a successful liposomal formulation of cisplatin. Expert Opin Investig Drugs 18: 1197-1218.
- Gryparis EC, Hatziapostolou M, Papadimitriou E, Avgoustakis K (2007) Anticancer activity of cisplatin-loaded PLGA-mPEG nanoparticles on LNCaP prostate cancer cells. Eur J Pharm Biopharm 67: 1-8.
- Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, et al. (2000) Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release 65: 133-148.
- Wilbur DS, Pathare PM, Hamlin DK, Buhler KR, Vessella RL (1998) Biotin reagents for antibody pretargeting. 3. Synthesis, radioiodination, and evaluation of biotinylated starburst dendrimers. Bioconjug Chem 9: 813-825.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15064
- [From(publication date):
specialissue-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10412
- PDF downloads : 4652