Research Article Open Access
In Situ Quantification of Glucose Concentration in Airway Surface Liquid With Functionalized ZnO Nanorod-Coated Microelectrodes
Alejandro A. Pezzulo1 , Muhammad H. Asif2*, Magnus Willander2 and Joseph Zabner1*
1Department of Internal Medicine, Carver College of Medicine, University of Iowa, Iowa City, IA, USA
2Departments of Science and Technology, Campus Norrköping, Linköping University, Norrköping, Sweden
- *Corresponding Author:
- Joseph Zabner
University of Iowa 440 EMRB
Iowa City, IA, 52242
Tel: 319-3357608
Fax: 319-3357623
E-mail: joseph-zabner@uiowa.edu
- Muhammad H. Asif
Linköping University
Department of Science and Technology (ITN)
Norrköping City, SE-60174
Tel: +4611363119
Fax: +4611363270
E-mail: muhammad.asif@liu.se
Received date: June 10, 2011; Accepted date: August 10, 2011; Published date: Augutst 12, 2011
Citation: Pezzulo AA, Asif MH, Willander M, Zabner J (2011) In Situ Quantification of Glucose Concentration in Airway Surface Liquid With Functionalized ZnO Nanorod-Coated Microelectrodes. J Anal Bioanal Tech S7:002. doi:10.4172/2155-9872.S7-002
Copyright: © 2011 Pezzulo AA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The surface of the airways that conduct gases into and out of the lungs has components that protect the host from inhaled and aspirated pathogens. The thin (4-7 mm height) layer of airway surface liquid (ASL) that lines the airways has physicochemical properties that are important for normal function of these antimicrobial components. Among these properties, low glucose concentration is required for normal antimicrobial activity. Current methods for assessing the ASL have important flaws (temporal resolution, dilution factors, collection volume), which have been a recurring obstacle for understanding diseases in which ASL composition is abnormal. To circumvent these problems, microelectrodes coated with ZnO nanorods and immobilized glucose oxidase was used to determine glucose concentration in ASL of well-differentiated cultures of human airway epithelia. The sensor responded to glucose linearly over a concentration range of 0.128 to 8 mM and the effects of electroactive interferents were minimal. The measured concentration of glucose in ASL was consistent with values previously reported. This method confirms the presence of a transepithelial glucose concentration gradient in human airway epithelia and is an important step towards characterizing the physicochemical properties of ASL and understanding diseases caused by changes in ASL composition.
Introduction
The airway surface liquid (ASL) is a thin layer (3-7 mm height) of fluid lining epithelial cells in the airways that allow flow of gas between lungs and the environment. This fluid contains antimicrobial components that, along with physical clearance of particles by cough and the mucociliary escalator, keep the lungs sterile [1]. Physicochemical properties of the ASL such as concentration of divalent and monovalent cations and pH are important regulators of antimicrobial activity. Moreover, glucose concentration also affects innate immunity against bacteria [2]. Epithelial cells in the airways constantly absorb glucose, maintaining a gradient in which ASL glucose concentration is approximately one tenth that of plasma. Inhaled or aspirated bacteria encounter this ASL once they come into contact with the airways, and low ASL glucose concentration results in decreased availability of carbon sources for growth. Therefore, low ASL glucose concentration aids in maintaining sterile lungs.
The small volume of ASL makes study of its physicochemical properties difficult without altering them during measurements. This has been a recurring obstacle in research involving diseases such as cystic fibrosis, in which alterations in ASL composition are thought to be crucial elements of pathogenesis. Commonly used methods such as filter paper collection of ASL result in samples that contain a combination of ASL and submucosal fluid, confounding chemical analyses [3]. Indirect methods such as collection of exhaled breath condensate are useful in avoiding submucosal fluid sampling but have a relatively low temporal resolution and are very sensitive to calculations of dilution factors (which can reach 10,000) and to the contribution of alveolar tissue and the mouth to samples of condensate [4,5].
Direct measurement methods such as electrochemical analysis with substrate selective electrodes have been hampered in the past by very slow electrode response times, limited stability, and methods of electrode manufacturing that are complicated and time consuming. To circumvent these limitations, in this study, we have applied a recently developed sensor [6-9] consisting of glucose oxidase immobilized on ZnO nanorods [10] grown at the tip of borosilicate glass capillaries. The physical dimensions, biocompatibility, short response times, sensitivity, and ease of use of this electrode configuration [11] allowed us to directly determine the glucose concentration in the ASL of welldifferentiated cultures of human airway epithelia in situ and opens the possibility of characterizing the dynamic nature of glucose transport by airway epithelia in vivo.
Materials and Methods
The selective ASL glucose-measurement method utilizes two microelectrodes: 1) A ZnO nanorod-decorated borosilicate glass capillary coated with immobilized glucose oxidase as the working microelectrode and 2) a Ag/AgCl coated borosilicate glass capillary as the reference microelectrode. The electrochemical potentialdifference response recorded in this way measures the difference in electrochemical surface potential generated near the microelectrodes. The electrochemical interactions that generate electrode potentials in response to glucose were detailed in [10].
Microelectrode fabrication
The outer surface of borosilicate glass capillaries was uniformly coated with chromium and silver film as we have described earlier in [7-10]. The AgCl tip coating was prepared electrochemically by dipping the coated end of a capillary in 0.2M HCl solution and then by electrolyzing the silver film to form AgCl by polarizing it at 1.0 V for one minute. A 3 cm long Ag/AgCl layer was coated on the tip of the capillary to serve as a reference microelectrode. The outer end of the Ag/AgCl layer was connected with a copper wire (0.5 mm in diameter and 15 cm in length) and fixed by means of high-purity silverconductive paint
Growth and characterization of ZnO nanostructures
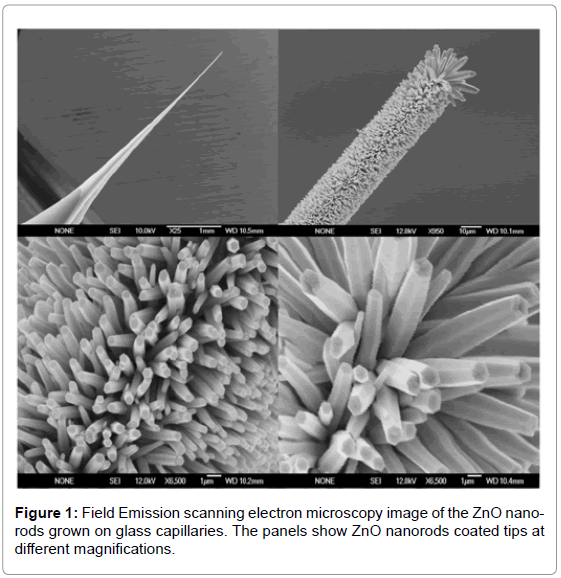
ZnO nanostructures were grown using the low-temperature growth technique (aqueous chemical growth technique) on Ag coated tips of borosilicate glass capillaries in an aqueous solution of zinc nitrate hexahydrate [Zn(NO3)2.6H2O, 99.9% purity] and hexamethylenetetramine (C6H12N4, 99.9% purity). The concentrations of both were fixed at 0.025M. All the aqueous solutions were prepared in distilled water and we restrict the results to glass tip substrate. The glass capillaries substrates were immersed into the aqueous solution and tilted against the wall of beaker. Next, the beaker was incubated at 93°C for two hours to generate aligned ZnO nanostructures, after which the substrate was removed from the solution and cleaned with de-ionized water. The grown ZnO nanorods on glass tip have been studied by field emission scanning electron microscope (FESEM) at different magnifications as shown in Figure 1.
Immobilization of glucose oxidase enzyme and microelectrode calibration
Glucose oxidase (G7141, Sigma-Aldrich, St. Louis, MO) 5 mg/ml was prepared in Phosphate Buffered Saline (PBS) containing 8.1 mM NaH2PO4-7H2O, 1.5 mM KH2PO4, 137.9 mM sodium chloride and 2.7 mM KCl (14190, Invitrogen, Carlsbad, CA). Glucose oxidase was electrostatically immobilized by dipping the tip of a borosilicate glass capillary with well-aligned ZnO nanorods into 2 mL of the enzyme solution for 15 minutes at room temperature and then air-drying for at least 20 minutes (Figure 2).
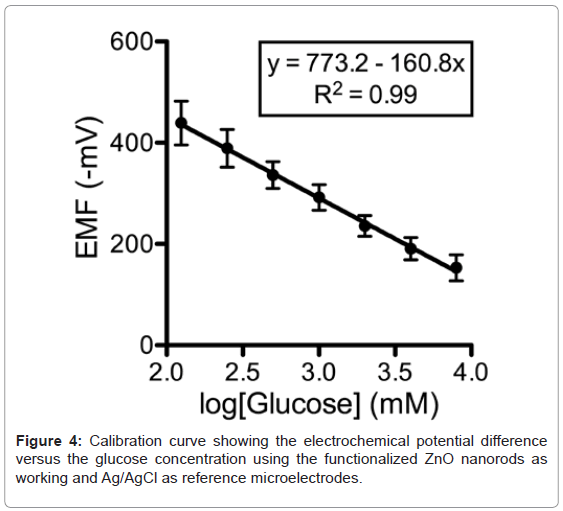
A calibration curve was generated by recording the electrochemical potential of the glucose-probe (6514, Keithley, Cleveland, OH) versus an Ag/AgCl reference microelectrode in a solution of glucose with concentrations ranging from 0.125 mM to 8 mM dissolved in glucosefree DMEM (11966, Invitrogen, Carlsbad, CA). A very fast response time was noted over the whole concentration range with 95% of the steady state voltage achieved within one second. Within 5 minutes after generating a standard curve, the microelectrode was used for determination of ASL glucose concentration.
Primary cultures of human airway epithelia and electrochemical measurements
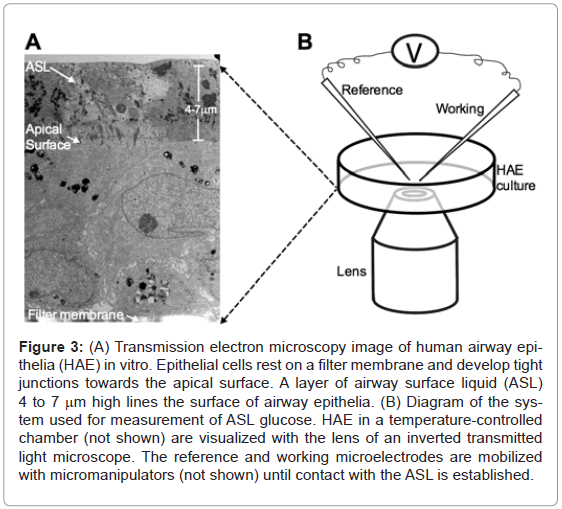
Well-differentiated cultures of human airway epithelia cells were obtained from the In Vitro Models and Cell Culture Core of the University of Iowa at least 2 weeks after cells were seeded on clear microporous membranes using the methods described in Karp et al. [12] (Figure 3A). Under these conditions, the epithelial cells polarize and develop tight junctions that define the limits between a ciliated apical surface lined with ASL that constitutes the air-liquid interface and a basolateral surface that contacts the filter membrane. The cultures were incubated for at least 3 days at 37°C in a humidified 21% O2/ 5% CO2 incubator with 5mM glucose in glucose-free DMEM as the nutrient medium. The nutrient medium was replaced every 6 hours to maintain a constant basolateral glucose concentration.
Figure 3: (A) Transmission electron microscopy image of human airway epithelia (HAE) in vitro. Epithelial cells rest on a filter membrane and develop tight junctions towards the apical surface. A layer of airway surface liquid (ASL) 4 to 7 mm high lines the surface of airway epithelia. (B) Diagram of the system used for measurement of ASL glucose. HAE in a temperature-controlled chamber (not shown) are visualized with the lens of an inverted transmitted light microscope. The reference and working microelectrodes are mobilized with micromanipulators (not shown) until contact with the ASL is established.
To measure ASL glucose concentration, the tips of both reference and working microelectrodes were applied to the surface of the epithelial cells incubated at 37°C using micromanipulators (5171, Eppendorf AG, Hamburg, Germany) while visualizing the epithelial cells using phase contrast inverted light microscopy with 20X magnification (TE200, Nikon, Tokyo, Japan). (Figure 3B). The microelectrodes were slowly directed towards the surface of the epithelial cells at a 45° angle until a meniscus could be observed around the tip of the microelectrodes when they contacted the ASL. Once the electrochemical potential generated by this maneuver was recorded, the glucose concentration was interpolated from the standard curve corresponding to the specific microelectrode used for measurement.
Results
The morphology of high density and highly aligned ZnO nanorods was investigated by FESEM (Figure 1). ZnO nanorods grown on the glass tip substrate have a rod-like shape with hexagonal cross section and are primarily aligned along the c-axis. The typical diameters of the as-grown nanorods are in the range of 60 to 80 nm and length in the range of about 1 μm.
The response of the electrochemical potential difference of the ZnO nanorods to the changes in buffer electrolyte glucose was measured for a range of 0.125 mM to 8 mM and shows that this glucose dependence is linear and has sensitivity equal to 160.8 mV/decade (Figure 4). Since the Ag/AgCl reference microelectrode can be sensitive to Cland the concentration of Cl- in the ASL can be different to that in the calibration media, the selectivity of the sensor was tested with different concentrations of Cl- to determine its relative contribution to the measurements of glucose. There was no observable effect on the glucose response (data not shown). To assess the reversibility of the microelectrode potential, we washed the microelectrode with deionized water after making the calibration curve and repeated one of the standard glucose concentration measurements. In all cases, we obtained a value within 5% of expected. Repeated measurements of the same sample with an electrode generated values within 3% of expected.
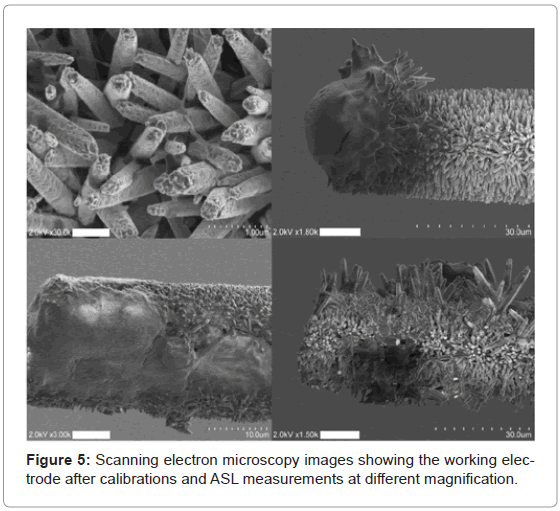
We used the micro-sensor to measure the free concentration of glucose in ASL. The glucose concentration was estimated to 380±15 μM (n=4). The microelectrodes were imaged with FESEM after use to exclude rupture of the microelectrode tips during measurements (Figure 5). In all cases included in data analyzed in this study, the microelectrode tip structure was preserved.
Discussion
Functionalized hexagonal ZnO nanorod coated microelectrodes were used as a selective sensor for the glucose concentration in ASL. The functionalization was achieved through the use of the enzyme glucose oxidase and the potential difference was found to be linear over a wide concentration range (0.125 mM to 8 mM). Our results are consistent with values obtained previously using breath condensate analysis [13,14] and to our knowledge, are the first in situ electrochemical determination of glucose in the ASL. Moreover, our results confirm the presence of a transepithelial glucose concentration gradient [2] using methodology that can be adapted for use in vivo to obtain realtime measurements. The effect of electroactive interferents under the testing conditions can be negligible and showed adequate selectivity for glucose.
These results demonstrate the capability to perform biologically relevant measurements of glucose in ASL in situ. The ZnO nanorod microelectrode thus holds promise for minimally invasive dynamic analyses of ASL analytes and is an important step towards understanding pathogenesis of diseases that present with abnormal ASL composition
Acknowledgements
We thank Peter Taft, Thomas Moninger (University of Iowa Central Microscopy Research Facility) and Phil Karp (University of Iowa In vitro models and Cell Culture Core) for excellent assistance. This work was supported by National Institutes of Health PPGPRO HL91842 (J.Z.)
References
- Bartlett JA, Fischer AJ, McCray PB (2008) Innate immune functions of the airway epithelium. Contrib Microbiol 15: 147-163.
- Pezzulo AA, Gutiérrez J, Duschner KS, McConnell KS, Taft PJ, et al. (2011) Glucose Depletion in the Airway Surface Liquid Is Essential for Sterility of the Airways. PLoS ONE 6: e16166.
- Landry JS, Eidelman DH (2001) Airway surface liquid: end of the controversy? J Gen Physiol 117: 419-422.
- Effros RM, Biller J, Foss B, Hoagland K, Dunning MB, et al. (2003) A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med 168: 1500-1505
- Effros RM, Su J, Casaburi R, Shaker R, Biller J, et al. (2005) Utility of exhaled breath condensates in chronic obstructive pulmonary disease: a critical review. Curr Opin Pulm Med 11: 135-139.
- Al-Hilli SM, Willander M, Öst A, Strålfors P (2007) ZnO nanorods as an intracellular sensor for pH measurements. J Appl Phys 102: 084304-084305.
- Asif MH, Ali SM, Nur O, Willander M, Englund UH, et al. (2010) Functionalized ZnO nanorod-based selective magnesium ion sensor for intracellular measurements. Biosens Bioelectron 26: 1118-1123.
- Asif MH, Fulati A, Nur O, Willander M, Brännmark C, et al. (2009) Functionalized zinc oxide nanorod with ionophore-membrane coating as an intracellular Ca[sup 2+] selective sensor. Appl Phys Lett 95: 023703-023703.
- Fulati A, Ali SMU, Asif MH, Alvi NuH, Willander M, et al. (2010) An intracellular glucose biosensor based on nanoflake ZnO. Sens Actuators B Chem 150: 673- 680.
- Asif MH, Ali SM, Nur O, Willander M, Brännmark C, et al. (2010) Functionalised ZnO-nanorod-based selective electrochemical sensor for intracellular glucose. Biosens Bioelectron 25: 2205-2211.
- Rahman MM, Ahammad AJS, Jin J-H, Ahn SJ, Lee J-J (2010) A Comprehensive Review of Glucose Biosensors Based on Nanostructured Metal-Oxides. Sensors 10: 4855-4886.
- Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, et al. (2002) An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188: 115-137.
- Brennan A, Gyi K, Wood D, Johnson J, Holliman R, et al. (2007) Airway glucose concentrations and effect on growth of respiratory pathogens in cystic fibrosis. J Cyst Fibros 6: 101-109.
- Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, et al. (2007) Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol 102: 1969-1975.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14515
- [From(publication date):
specialissue-2013 - Apr 01, 2025] - Breakdown by view type
- HTML page views : 9917
- PDF downloads : 4598