Research Article Open Access
Impact of Sex and Gonadal Hormones on Cocaine and Food Reinforcement Paradigms
Kerry A. Kerstetter1* and Tod E. Kippin1,21Department of Psychological and Brain Sciences, USA
2Neuroscience Research Institute, University of California at Santa Barbara, Santa Barbara, CA 93106-9660, USA
- *Corresponding Author:
- Kerry A. Kerstetter
Department of Psychological and Brain Sciences
University of California, Santa Barbara, Santa Barbara
California 93106-9660, USA
Tel: (805) 893-2459
Fax: (805) 893-4303
E-mail: kerstetter@psych.ucsb.edu
Received September 20, 2011; Accepted December 15, 2011; Published December 20, 2011
Citation: Kerstetter KA, Kippin TE (2011) Impact of Sex and Gonadal Hormones on Cocaine and Food Reinforcement Paradigms. J Addict Res Ther S4:002. doi:10.4172/2155-6105.S4-002
Copyright: © 2011 Kerstetter KA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Men and women express sexually dimorphic patterns of cocaine abuse, such that women progress faster from initially trying cocaine to becoming dependent upon the drug and display a greater incidence of relapse. Sex differences in response to cocaine are also seen in the laboratory in both humans and animal models. In this review, animal models of cocaine abuse that have reported sex differences in appetitive reinforcement are discussed. In both human and animal studies, sex differences in the subjective and behavioral effects of cocaine are often related to the female reproductive cycle and ovarian hormones. As a comparison, food reinforcement studies have shown the opposite profile of sex differences and the impact of sex steroids on food intake and response rate. In contrast, limited attention has been given to “choice” models in rodents of either sex, however, our recent studies have indicated a role of sex and estrogen in cocaine choice over food with intact females, and OVX females treated with estrogen, choosing cocaine significantly more than males. Interestingly, estrous cycle phase does not seem to impact cocaine choice as it does response rate in single-reinforcer studies, suggesting that genomic rather than neurosteroid effects of estrogen modulate sex differences in this model. Future studies should more fully explore the impact of sex hormones on concurrent reinforcement and discrete choice models of addiction.
Keywords
Cocaine; Food; Reinforcement; Choice; Hormones; Sex
Introduction
Women, compared to men, face a more severe pattern of cocaine dependence. Sex differences in the profile of cocaine dependence have indicated that women, relative to men, transition faster from first use to entering treatment [1], which may be explained by women abusing cocaine via more addictive routes more quickly and reporting a more rapid progression of drug dependence [2]. In addition, cocaine dependent women report shorter cocaine-free periods [3], have an increased incidence of relapse [4], experience greater cocaine craving in response to psychological and physical stressors [5], and have a greater tendency to report relapses with an impulsive quality [6]. There is also evidence of sex differences in the profile of dependence on other drugs such as prescription opiates [7] and methamphetamine [8,9] that have indicated a more severe impact of substance abuse in women compared to men, including exacerbation of cognitive impairments associated with stimulant dependence [9]. Compared to cocaine-dependent men, cocaine-dependent women are also more likely to attempt suicide [10,11] and addicted women exhibit poorer nutrition than addicted men [12]. Together, these findings indicate that a critical aspect in sex differences in addiction may include altered evaluation of the choice to engage in drug taking versus nondrug experiences, such as food and other pleasurable activities.
Sex differences in psychostimulant dependence may be explained by the effects of gonadal hormones, including fluctuations across the female reproductive cycle. For women, the subjective experience of using cocaine is more or less pleasurable, depending on what phase of the menstrual cycle they are in at the time of drug intake [13,14]. In addition, progesterone administration can diminish the positive effects of cocaine in women, but not men [15,16]. Accordingly, evidence from human laboratory studies indicates that there are roles for hormones in cocaine responsiveness and that the ability of hormones to modulate cocaine responsiveness is sexually differentiated. However, the direction of sex differences in stimulant reinforcement can sometimes be dose-dependent. For instance, one study that employed a progressive-ratio schedule of reinforcement reported that a low dose of d-amphetamine functions as a reinforcer in women, but not men, whereas a high dose of d-amphetamine functioned as a reinforcer in men, but not women [17]. Sex differences in response to cocaine has also been described in rodents. For example, female rats, relative to males, display a greater propensity to acquire cocaine self-administration [18-22], increased responding under fixed and progressive ratio schedules of cocaine reinforcement [23,24], and greater drug intake during cocaine and methamphetamine Self administration [25-28]. Further, female rats show increased cocaine and methamphetamine seeking during reinstatement tests relative to male rats [24,29].
Moreover, drug abuse leads to maladaptive choice in humans [12,30] and this can be modeled in animals via concurrent reinforcement models in which the animal chooses between drug and a natural reinforcer. Research from our lab indicates that female rats are more likely to choose intravenous cocaine infusions over food than male rats, and ovarian hormones play a critical role in cocaine choice by females. Surprisingly, the reproductive cycle does not seem to modulate cocaine choice in female rats [31], whereas reproductive phase modulates rate-dependent measures examined in traditional single reinforcer experiments [24,32-35].
Role of female reproductive cycle and gonadal hormones on cocaine reinforcement
Female rats in the estrus phase (note: vaginal estrus as opposed to behavioral estrus) of the estrous cycle, a time of low progesterone and its neurosteroids, display greater cocaine seeking relative to non-estrus females [e.g. 24,34]. Furthermore, estrus phase females earn significantly more cocaine infusions during a progressive ratio schedule of cocaine reinforcement than non-estrus phase females [32,33]. Thus, the estrus phase appears to be a specific “at risk” phase of the female reproductive cycle for cocaine taking and seeking. Further, Kerstetter et al. [24] demonstrated that female rats press more for cocaine than male counterparts during maintenance, extinction, and reinstatement after prolonged abstinence, results for active lever pressing during cocaine self-administration showed that females responded significantly more than males during sessions 8-10. In addition, Kerstetter et al. [24] reported that females in estrus respond significantly more during cocaine self-administration than they did in other phases of the estrous cycle. In addition, it was recently reported that females in the estrus phase display fewer retreats in the runway model of cocaine reinforcement relative to females in the other phases [36]. These “retreat” behaviors appear to reflect the anticipation of the anxiogenic properties of cocaine, such that the rat is “conflicted” about receiving the cocaine and will repeatedly approach and retreat from the goal box where they will receive cocaine [see review, 37]. Together with the self-administration data, these findings indicate that reproductive status contributes to the motivation for cocaine and that sex differences in addiction vulnerability may be attributable to differences in the motivational impact of both the appetitive and aversive properties of cocaine. Given that the reproductive cycle seems to modulate appetitive reinforcement for cocaine and cocaine seeking (in the absence of immediate reinforcement), the estrus phase appears to model an “at risk” phase and indicates that ovarian hormones may play a role the pattern of addiction in women.
Similar to the effects of estrous cycle modulation of cocaine reinforced response rates, several animal studies have demonstrated that estrogen can increase, and progesterone can decrease, the level of responding for stimulants in females, but not males. Specifically, ovariectomized (OVX) rats treated with estrogen display enhanced acquisition of cocaine self-administration relative to OVX controls, and blocking estrogen receptors with tamoxifen in intact female rats reduces their ability to acquire cocaine self-administration relative to intact controls [38,19]. This effect has also been reported for methamphetamine self-administration, with estrogen treated OVX females self-administering more methamphetamine than males and vehicle treated OVX females [39]. Moreover, progesterone administration to female rats undergoing abstinence after cocaine self-administration reduces their cocaine seeking relative to Vehicle treated females during cocaine-primed reinstatement [40]. In addition, estrogen administration to OVX female rats enhances escalation of cocaine intake, and progesterone administration to OVX females reduces escalation of cocaine intake relative to vehicle controls [41]. Other reports have demonstrated that the progesterone metabolite, allopregnanolone (ALLO), is effective in reducing responding during both cocaine-primed and stress-induced reinstatement to cocaine seeking in female, but not male, rats; it has also been shown that ALLO reduces response rates more significantly than progesterone in females [e.g. 42,43]. These effects extend to other psychostimulants, as ALLO has also been shown to effectively reduce reinstatement to methamphetamine seeking in female, but not male, rats [29]. Furthermore, during a long-access schedule of cocaine reinforcement ALLO significantly reduces escalation of cocaine intake in female rats [44]. This indicates that female gonadal hormones modulate female responding for stimulants, and perhaps estrogen and progesterone have opposing effects that impact the severity of their methamphetamine and cocaine dependence. In addition, because ALLO has a more profound effect on response rates than progesterone, the ability of the female reproductive cycle to modulate cocaine reinforcement is likely due to the neurosteroid (fast-acting, non-genomic) effects of ALLO, as its levels rise during proestrus and fall during estrus. This is contrasted by the fact that gonadectomy and sex hormone treatments in males do not have a large impact on cocaine-reinforced behaviors [39,45]. This suggests that sex hormones may modulate cocaine dependence in women, but not men, and these effects should be examined further in humans.
Role of female reproductive cycle and gonadal hormones on food reinforcement
Sex and estrous cycle differences also exist for food intake and reinforcement. Sex differences are observed for response rate during food self-administration, with males pressing significantly faster over the sessions than females [46]. However, this study did not control reinforcement level for differences in body weight between males and females, and it is possible that males and females are not equivalently reinforced and/or satiated by the same number of food pellets. Future experiments should examine the impact of adjusting reinforcement level in males and females to account for body weight differences because male rats may not value the same absolute amount of food (e.g. 1 x 45 mg food pellet) the same as females. Gonadal hormones substantially impact sex differences in food motivation. Gonadectomy of male and female rats results in increased response rate and food intake in females, and decreased response rate and food intake in males [46,47] suggesting that gonadal hormones in males drive motivation for food, and gonadal hormones in females inhibit motivation for food. Consistent with this notion, testosterone treatment increases response rate for food reinforcement and intake of food in gonadectomized males and females [46,47]. Additionally, administration of estradiol, as well as, estrogen receptor agonists, reduces food intake in OVX females, and females in the estrus phase of the estrous cycle have significantly less food intake relative to females in the other stages of the cycle [48-50]. Despite the fact that estrogen is relatively low during estrus, the decrease in feeding during this time could be mediated by long-term (i.e. genomic, rather than neurosteroid) effects of estrogen, which are delayed in onset and can persist for long periods after the estrogen peak [see review, 48]. Furthermore, estrogen and estrogen receptor agonist administration decreases food intake in male rats [51]. Collectively, this evidence indicates that testosterone drives food intake for males and females, but has no effect on cocaine intake; while estrogen attenuates motivation for food in males and females, and increases cocaine intake in females only. Given that ovarian hormones play a powerful, and opposing, role in food and cocaine reinforcement for females, concurrent reinforcement paradigms that allow animals to choose between food and cocaine are likely to produce significant sex and ovarian hormone effects.
Role of value, price, and sex on concurrent reinforcement
The majority of studies in rodent models of addiction, particularly those examining sex differences, have focused largely on rate of responding for a single reinforcer when no other reinforcer is available. However, these models fail to address a significant aspect of addiction as defined by the DSM-IV, which includes the choice of drug over other previously important activities (DSM-IV-TR, 2000). Therefore, experimental choice models are of great interest because they may model the maladaptive choice for cocaine over other rewards as observed in cocaine-dependent humans [30]. Currently, two experimental models of operant choice have been utilized in the literature. The first is “concurrent reinforcement”; this procedure allows the subject to respond for two reinforcers that are both freely available during the entire self-administration session. The second choice procedure employs a “discrete trial” procedure which is defined by opportunity to respond for two reinforcers that are presented at the same time for a choice, with a limited number of trials available per self-administration session. Discrete trials allow the experimenter to examine how the subject will respond when forced to make a decision between two appetitive stimuli, and this model is used in the majority of studies discussed here. Importantly, during discrete trials, when the subject makes a cocaine choice, they sacrifice a food choice, and the number of cocaine versus food choices can be used as a measure of the relative incentive value for cocaine over food. This method is distinct from the concurrent reinforcement method that has food or water (or other appetitive reinforcer) freely available during cocaine self-administration. In those studies, rats may spend less time self-administering cocaine due to another reinforcer being present. However this method fails to examine choice behavior in an explicit manner because responding is unlimited and does not always lead to reinforcement or a specific choice between reinforcers (i.e. responding during an imposed time-out after delivery of a reinforcer) which may lead to perseverative responding on an operandum. Further, in some versions of concurrent reinforcement experiments, no operant is imposed to access the alternative (non-drug) “reinforcer” whereas access to the drug requires performance of an operant. Additionally, reinforcers can be preferred at different intervals, such that a small reinforcer, of which an animal can consume many, may be taken more frequently than a larger reinforcer, of which an animal can only consume a few. This is particularly common in the drug reinforcement literature where response rates and numbers of infusions for low doses of drug are much higher than those for a high dose of the drug when examined under a low value fixed ratio of responding schedule [28], conversely, when the same doses are examined under progressive ratio of responding schedule the higher dose will elicit a higher level of responding for a single additional reinforcer relative to the lower dose [52]. In addition, it has been reported that drug dose and lever responding increase proportionally under a seeking–taking chain schedule of reinforcement in which seeking and taking drugs are measured on separate levers [53]. Accordingly, in order to most appropriately study choice between reinforcers, it is ideal to only allow responding that leads directly to reinforcement, such that a direct consequence of making a lever response is to make a choice to receive one reinforcer, which also sacrifices gaining the other reinforcer. This can be achieved by allowing the opportunity to engage in operant responding for two reinforcers under a discrete trial regimen in which following a choice of one reinforcer, further responding is not allowed for a period of time (to minimize response perseverance) and there is a finite number of reinforcers available in each session.
Data from cocaine-dependent humans in discrete trial choice experiments (mostly male) indicate that when they have the option to receive money or an intravenous injection of cocaine, the vast majority will choose cocaine [54-56]. However, other reports show that monetary reward can suppress cocaine choice, especially when the monetary value is increased from $.00-$2.00 [57,58]. Interestingly, when food alternatives are matched to monetary value they fail to suppress cocaine choice, indicating that food relative to money is devalued in cocaine-dependent subjects [58]. Furthermore, when subjects are given the option of viewing cocaine-associated pictures or pleasant, neutral, or unpleasant pictures, cocaine-dependent subjects will choose cocaine-related pictures over pleasant pictures more often than control subjects [59]. Another report indicated that gambling for money (alternative reinforcer: chance to win $0-20) was able to significantly decrease cocaine choice as a function of number of chances to earn money in cocaine dependent humans [60]. Therefore, the choice to take cocaine under the discrete trial procedure is dependent on the incentive value of the alternative reinforcer in humans. In addition, cocaine choice is also dependent upon the “price” (price = number of responses required to receive a dose of cocaine) of cocaine, it has been reported that increasing the price of cocaine (400-1000 responses) will shift choice to a monetary alternative ($0.25) [61]. These effects of value and price have also been detected in concurrent reinforcement experiments in alcohol [62] nicotine [63] dependent subjects when the subjects must choose between the drug and another reinforcer.
Similarly, studies employing discrete trials in (mostly male) non-human primates have demonstrated that drug choice varies as a function of both the drug dose and the amount of food given as an alternative. Specifically, male rhesus monkeys will display ~ 25% cocaine choice when the cocaine dose (0.03 mg/kg/infusion) and food value are low (1 g); however, when the cocaine dose is increased (0.3 mg/kg/infusion) the percent cocaine choice increased significantly (> 75%); in addition, when the food value was high (16 g), the cocaine choice was < 50% even for a high dose of cocaine (0.3 mg/kg/infusion) [64]. These effects of drug dose have also been displayed in rhesus monkeys when they are given the choice between food and 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) [65]. Further, numerous other studies have reported similar findings indicating that cocaine choice is a function of the relative value of the reinforcers [66-77].
In addition to the value of the reinforcers, price can also alter the percentage of drug choices in non-human primates in experiments employing the discrete trials. For example, rhesus monkeys trained to choose between cocaine and food pellets on a fixed-ratio 30 schedule (FR30) have a cocaine preference; however, when the number of responses required to receive intravenous cocaine (1.0 mg/kg/infusion) was increased from 30 to 480, cocaine preference was abolished and a preference for food was exhibited [66]. The same holds true for food price, when the FR is increased for food; cocaine choice increases [66]. Furthermore, the impact of price upon cocaine choice has been demonstrated in male cynomolgus monkeys, when a progressive-ratio procedure was used to increase response requirements for the preferred reinforcer (cocaine or food, depending on the monkey), choice of that reinforcer decreased [74]. The effect of reinforcement price has also been shown to impact reinforcer selection when baboons are given the choice between amphetamine and a food alternative, such that as the price for food reinforcement increases baboons make more amphetamine selections [75]. Together this evidence indicates that parameters (value and price) of choice paradigms can significantly alter the selection between the available reinforcers.
In rodent models (male subjects), choice procedures have found results that appear to be divergent from those observed in non-human primate and human studies, as most studies have failed to find that rodents exhibit a cocaine preference over other reinforcers. In studies using the concurrent reinforcement (free access) procedure, rats trained on food self-administration do not decrease their responding for food when cocaine became available; further, non-drug reinforcers significantly reduce methamphetamine and cocaine reinforced responding [78,79]. In addition, in choice studies using a discrete trial procedure, male rats given the choice between cocaine and sucrose reinforcement almost exclusively prefer sucrose [80], even when the cocaine dose is increased (0.25-1.5 mg/kg/inf) and even following an extensive history of cocaine intake [81].
However, Thomsen, et al. [82] found high cocaine choice at both low and high cocaine doses (0.18 – 1.0 mg/kg/inf) and required multiple responses to receive reinforcement, which may constitute a critical factor for observing high cocaine choice in male rats. However, Thomsen et al. [82] conducted liquid food self-administration training prior to catheter surgery, and conducted cocaine self-administration training (1.0 mg/kg/inf) immediately prior to the start of discrete trials with no additional food training. This may have created a cocaine bias to result in almost 100% cocaine choice in their male rats. In addition, Lenoir et al. [81] employed 0.2% sodium saccharin as their alternative to cocaine, whereas Thomsen et al. [82] employed 32% Ensure® protein drink in water; sweetened water may be a more powerful reinforcer than the dilute liquid food, which may contribute to the contrasting results.
Recent studies in our laboratory have examined choice to take cocaine employing food as an alternative reinforcer using the discrete trials procedure. We assessed sex, estrous cycle, and hormone effects in choice between food (45 mg grain pellets) and cocaine (0.4 or 1.0 mg/kg/infusion i.v.) reinforcement in male, intact (freely cycling) female, and ovariectomized (OVX) female rats treated with either vehicle (peanut oil) or estrogen benzoate (EB; 5 μg/ day). Rats were maintained under food-restricted (20 g for females and 25 g for males of rat chow per day) conditions or ad libitum conditions during separate experiments. Rats were trained to lever press on the right lever for food and on the left lever for cocaine on alternating days for a minimum of 10 days. For training sessions, only 1 lever was extended (i.e. only cocaine or food available) and rats were trained to press each lever for a specific reinforcer under a Fixed Interval: 20s (FI 20-sec) schedule of reinforcement. After training was completed, 5 concurrent reinforcement sessions (FI 20-sec) were conducted during which both levers were extended allowing the rat to select either food or cocaine. It was noted that rats responded during the TO period, so after the completion of concurrent reinforcement sessions, 5 discrete trial sessions (FI 20-sec) were conducted under the same conditions as the concurrent reinforcement sessions with the exception that during the 20-s interval, both levers were retracted to prevent non-reinforced responding, thus, minimizing perseveration of response patterns. An additional set of male and female rats was tested under a Fixed- Interval 10 minute schedule (FI 10-min) during the discrete trial sessions; due to the interval being extended, the immediate effects of cocaine are presumed to be diminished prior to the next discrete trial, allowing the assessment of choice with minimal contribution of anorexic effects.
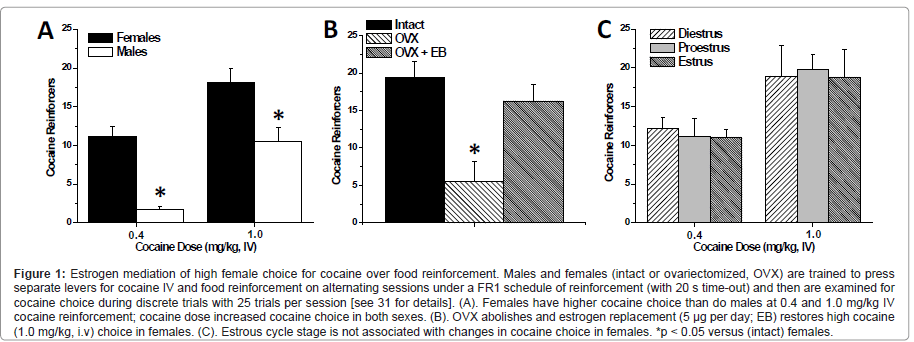
Consistent with human and primate studies, the choice to select cocaine over food is highly dependent on the dose of cocaine (Figure 1A) and on the duration of the inter-trial interval. Interestingly, the observed level of cocaine choice in males was not highly sensitive to food restriction (25 g per day versus ad libitum rat chow) [31], however, the impact of larger magnitude changes in daily restriction on cocaine choice have not been assessed. During discrete trials conducted under a FI 10-min schedule, both female and male rats reduced the number of cocaine choices relative to the FI 20-sec schedule, however female rats still choose cocaine more often than males. The observed level of cocaine choice in males tested with 10 min inter-trial intervals is comparable to the findings of Ahmed and colleagues [80,81], who report 25% or less cocaine choice (days 6-15) in male rats during discrete trials with 10-minute intervals and employed a forced choice between a 0.25 mg i.v. cocaine reinforcer (in rats weighing between 221-276 g which is approximately a 1.0 mg/ kg i.v. dose) versus a sweetened water reinforcer. This suggests that grain pellets (2 or 3 x 45 mg) and sweetened water (20 μl of 0.2% saccharin) are of approximately equal relative reinforcement value compared to cocaine in male rats. Moreover, this indicates that even though increasing the delay between selections can reduce cocaine choice, sex differences in cocaine choice are not likely to be explained by differences in the anorexic, or other immediate, effects of cocaine. Together, these findings suggest that food pellets are a highly palatable reinforcer (equivalent to sucrose) and further parametric studies (e.g. additional cocaine dose, different schedules of reinforcement, higher food restriction levels, etc) are warranted to characterize this model of drug choice. The male choice behavior our study [31] and those of the Ahmed group [80,81] contrasts to the report showing males with extremely high cocaine choice [82], but may imply that male rats require a higher response requirement and differential cocaine-self administration training in order to display a high cocaine preference. For instance, the self-administration training conducted for Kerstetter et al. [31] allowed rats to acquire cocaine and food self-administration on alternating days prior to concurrent reinforcement and discrete trial choice behavior. Such that on the first day a rat conducts cocaine self-administration and the following day they conduct food Self administration (first day is counter-balanced). This was carried out until the rat’s behavior stabilized, at which point they would begin concurrent reinforcement. This may explain why male rats did not achieve the same level of cocaine choice as in the Thomsen, et al. [82] study, which conducted only cocaine self-administration training immediately prior to choice sessions. It is likely that the novel finding reported by our laboratory that females choose cocaine over food more often than males will extend to other drugs of abuse such as amphetamine and methamphetamine. Therefore, this study should be replicated with other drugs of abuse in order to discover if females value a variety of pharmacological reinforcers more highly than males when provided with food as an alternative.
Figure 1:Estrogen mediation of high female choice for cocaine over food reinforcement. Males and females (intact or ovariectomized, OVX) are trained to press separate levers for cocaine IV and food reinforcement on alternating sessions under a FR1 schedule of reinforcement (with 20 s time-out) and then are examined for cocaine choice during discrete trials with 25 trials per session [see 31 for details]. (A). Females have higher cocaine choice than do males at 0.4 and 1.0 mg/kg IV cocaine reinforcement; cocaine dose increased cocaine choice in both sexes. (B). OVX abolishes and estrogen replacement (5 �?µg per day; EB) restores high cocaine (1.0 mg/kg, i.v) choice in females. (C). Estrous cycle stage is not associated with changes in cocaine choice in females. *p < 0.05 versus (intact) females.
The role of reproductive cycle and on gonadal hormones on cocaine choice
Although the majority of these studies examine choice behavior in males only, some studies have examined sex differences in cocaine reinforcement under concurrent access conditions. Female rats reduce cocaine intake (FR1, i.v. 0.2 mg/kg/inf) significantly more than male rats when a running wheel is made available during cocaine self-administration sessions [83]. In addition, female primates will reduce their intake of PCP (FR 4-32) more significantly than males when they are given sucrose access concurrently [84]. However, these studies did not employ discrete trials, imposed equal operants on access to reinforcers, and/or have limited quantity of reinforcers, but rather introduced an alternative reinforcer with non-limited access for a defined time period which may serve as a distracter to drug reinforcement. This is in contrast to a study from our laboratory that examined sex differences utilizing both concurrent reinforcement under discrete trial choice procedures [31] which found that female rats will choose cocaine over food significantly more than male rats during concurrent reinforcement and discrete trials at both moderate (0.4 mg/kg/inf) and high (1.0 mg/kg/inf) cocaine doses. At the high dose, intact females display a cocaine preference (> 70% cocaine choice), whereas males display a mild food preference (< 50% cocaine choice) during discrete trials (Figure 1A). Accordingly, under these conditions, females place a higher relative value on cocaine than do males, which is in concordance with studies that show female rats having higher response rate for and intake of cocaine reinforcement than male rats [22,24,27].
The high cocaine choice exhibited by females is dependent on the presence of estrogen. Kerstetter et al. [31] demonstrated that OVX female rats choose cocaine significantly less than intact females during discrete trials. Females that underwent ovariectomy displayed approximately 20% cocaine choice, whereas intact females display ~ 80% cocaine choice (Figure 1B). It is important to note that these OVX females began self-administration sessions at least three weeks after the OVX surgery in order to allow for the long-term effects of gonadal hormones to dissipate. Further, OVX females given estrogen replacement (5 mg of estrogen benzoate daily) exhibited almost identical choice as intact females (Figure 1B). This finding is in general agreement with reports from both cocaine and food self-administration studies which show that cocaine intake can be decreased via OVX, and increased via estrogen administration [26,85]; and OVX may increase motivation for food, and estrogen may be decreasing the value of food [46-50].
However, despite the effects of ovariectomy and estrogen on cocaine choice, estrous cycle does influence this behavior in female rats (Figure 1C). The lack of estrous cycle effect on cocaine choice contrasts with the ability of estrous cycle phase to modulate locomotor response to cocaine [86], progressive ratio reinforcement schedules [32,33], and the magnitude of cocaine-primed reinstatement of cocaine-seeking behavior [24,34]. This indicates that the levels of ovarian hormones at the time of testing are not sufficient to elicit high cocaine choice and suggests that the impact of ovarian hormones on cocaine choice is not due to fast-acting (i.e. neurosteroid) effects. Rather, the data to date suggest that the long-term effects of estrogen (due to administration across the experiment) are necessary to support high cocaine choice in intact females. The impact of estrogen to augment the value of other drugs of abuse over food in females is an area that has not yet been examined, and it would be valuable to determine if these effects would extend to other psychostimulants and other drug classes.
Taken together, the impact of sex and ovarian hormones upon the incentive value of cocaine relative to food is significant and may explain some of the clinical sex differences in the profile of cocaine dependence. Further research is needed to fully explore the relationship between sex hormones and the relative reinforcing properties of cocaine; for instance, understanding how estrogen (and perhaps other gonadal hormones) increases the relative incentive value of cocaine may be critical to improve cocaine dependence treatment in women. Additionally, it is important to determine if the observed sex differences in choosing cocaine over food in an animal generalizes to other drugs of abuse, including abused prescription drugs, in order to determine if this appears to be a common feature to the addiction profile of women abusing different substances [9].
Acknowledgements
This work was supported by DA-027525 (TEK), as well as a NARSAD Young Investigator Award to TEK.
References
- Westermeyer J, Kopka S, Nugent S (1997) Course and severity of substance abuse among patients with comorbid major depression. Am J Addict 6: 284- 292.
- McCance-Katz EF, Carroll KM, Rounsaville BJ (1999) Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict 8: 300-311.
- Kosten TA, Gawin FH, Kosten, TR, Rounsaville BJ (1993) Gender differences in cocaine use and treatment response. J Subst Abuse Treat 10: 63-66.
- Wong CJ, Badger GJ, Sigmon, SC, Higgins ST (2002) Examining possible gender differences among cocaine-dependent outpatients. Exp Clin Psychopharmacol 10: 316-323.
- Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H (2005) Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology (Berl) 180: 169-176.
- McKay JR, Rutherford MJ, Cacciola JS, Kabasakalian-McKay R, Alterman AI (1996) Gender differences in the relapse experiences of cocaine patients. J Nerv Ment Dis 184: 616-622.
- Roy A (2001) Characteristics of cocaine-dependent patients who attempt suicide. Am J Psychiatry 158: 1215-1219.
- Zilberman ML, Hochgraf PB, Andrade AG (2003) Gender differences in treatment-seeking Brazilian drug-dependent individuals. Subst Abus 24: 17-25.
- Santolaria-Fernández FJ, Gómez-Sirvent JL, González-Reimers CE, Batista-López JN, Jorge-Hernández JA, et al. (1995) Nutritional assessment of drug addicts. Drug Alcohol Depend 38: 11-8.
- Back SE, Lawson KM, Singleton LM, Brady KT (2011) Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav 36: 829-834.
- Mahoney JJ 3rd, Hawkins RY, De La Garza R 2nd, Kalechstein AD, Newton TF (2010) Relationship between gender and psychotic symptoms in cocainedependent and methamphetamine-dependent participants. Gend Med 7: 414-421.
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A (2009) Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol 31: 706-719.
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK (1999) Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol 7: 274-283.
- Evans SM, Haney M, Foltin RW (2002) The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 159: 397-406.
- Sofuoglu M, Babb DA, Hatsukami DK (2002) Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav 72: 431-435.
- Evans SM, Foltin RW (2006) Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology 31: 659-674.
- Vansickel AR, Stoops WW, Rush CR (2010) Human sex differences in d-amphetamine self-administration. Addiction 105: 727-731.
- Lynch WJ & Carroll ME. (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology 144: 77-82.
- Lynch WJ, Roth ME, Mickelberg JL, Carroll ME (2001) Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav 68: 641-646.
- Campbell UC, Morgan AD, Carroll ME (2002) Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend 66: 61-69.
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 161: 304-313.
- Lynch WJ (2008) Acquisition and maintenance of cocaine selfadministration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197: 237-246.
- Lynch WJ, Taylor JR (2004) Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology 29: 943-951.
- Kerstetter KA, Aguilar VR, Parrish AB, Kippin TE (2008) Protracted timedependent increases in cocaine-seeking behavior during cocaine withdrawal in female relative to male rats. Psychopharmacology (Berl) 198: 63-75.
- Lynch WJ, Mangini LD, Taylor JR (2005) Neonatal isolation stress potentiates cocaine seeking behavior in adult male and female rats. Neuropsychopharmacology 30: 322-329.
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME (2007) Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol 15: 461- 471.
- Roth ME, Carroll ME (2004) Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav 78: 199-207.
- Anker JJ, Gliddon LA, Carroll ME (2008) Impulsivity on a Go/No-go task for intravenous cocaine or food in male and female rats selectively bred for high and low saccharin intake. Behav Pharmacol 19: 615-629.
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME (2011) Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend
- Van Etten ML, Higgins ST, Budney AJ, Badger GJ (1998) Comparison of the frequency and enjoyability of pleasant events in cocaine abusers vs. non-abusers using a standardized behavioral inventory. Addiction 93: 1669- 1680.
- Kerstetter KA, Ballis MA, Duffin-Lutgen S, Carr AE, Kippin TE (2011) Sex differences in selection between food and cocaine. Neuropsychopharmacology Submitted
- Roberts DC, Bennett SA, Vickers GJ (1989) The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 98: 408-411
- Hecht GS, Spear NE, Spear LP (1999) Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol 35: 136-145.
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, et al. (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 182: 245-252.
- Lynch WJ (2008) Acquisition and maintenance of cocaine selfadministration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology (Berl) 197: 237-246.
- Kerstetter KA, Su ZI, Ettenberg A, Kippin TE (2011) Sex and estrous cycle differences in cocaine-induced approach-avoidance conflict. Addict Biol.
- Ettenberg A (2004) The opponent-process properties of self-administered cocaine. Neurosci Biobehav Rev 8: 721-728.
- Jackson LR, Robinson TE, Becker JB (2006) Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology 31: 129-138.
- Kucerova J, Vrskova D, Sulcova A (2009) Impact of repeated methamphetamine pretreatment on intravenous self-administration of the drug in males and estrogenized or non- estrogenized ovariectomized female rats. Neuro Endocrinol Lett 30: 663-670.
- Feltenstein MW, Byrd EA, Henderson AR, See RE (2009) Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology 34: 343-352.
- Larson EB, Anker JJ, Gliddon LA, Fons KS, Carroll ME (2007) Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol 15: 461-471.
- Anker JJ, Holtz NA, Zlebnik N, Carroll ME (2009) Effects of allopregnanolone on the reinstatement of cocaine-seeking behavior in male and female rats. Psychopharmacology (Berl) 203: 63-72.
- Anker JJ, Carroll ME (2010) Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend 107: 264-267.
- Anker JJ, Zlebnik NE, Carroll ME (2010) Differential effects of allopregnanolone on the escalation of cocaine self-administration and sucrose intake in female rats. Psychopharmacology (Berl) 212: 419-429.
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, et al. (2004) Effect of gonadectomy and gonadal hormone replacement on cocaine selfadministration in female and male rats. Neuropsychopharmacology 29: 929- 942.
- van Hest A, van Haaren F, van de Poll NE (1989) Perseverative responding in male and female Wistar rats: effects of gonadal hormones. Horm Behav 23: 57-67.
- Wallen WJ, Belanger MP, Wittnich C (2001) Sex hormones and the selective estrogen receptor modulator tamoxifen modulate weekly body weights and food intakes in adolescent and adult rats. J Nutr 131: 2351-2357.
- Asarian L, Geary N (2006) Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci 361: 1251-1263.
- Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L (2009) Divergent effects of estradiol and the estrogen receptor-alpha agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res 1268: 88-96.
- Santollo J, Eckel LA (2009) Effect of a putative ERalpha antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav 97: 193-198.
- Fudge MA, Kavaliers M, Baird JP, Ossenkopp KP (2009) Tamoxifen produces conditioned taste avoidance in male rats: an analysis of microstructural licking patterns and taste reactivity. Horm Behav 56: 322-331.
- Martelle JL, Czoty PW, Nader MA (2008) Effect of time-out duration on the reinforcing strength of cocaine assessed under a progressive-ratio schedule in rhesus monkeys. Behav Pharmacol 19: 743-746.
- Veeneman MM, van Ast M, Broekhoven MH, Limpens JH, Vanderschuren LJ (2011) Seeking-taking chain schedules of cocaine and sucrose selfadministration: effects of reward size, reward omission, and a-flupenthixol. Psychopharmacology (Berl).
- Donny EC, Bigelow GE, Walsh SL (2003) Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug & Alcohol Dependence 69: 289-301.
- Haney M, Hart CL, Foltin RW (2006) Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology 31: 1814-1821.
- Collins ED, Vosberg SK, Ward AS, Haney M, Foltin RW (2007) The effects of acute pretreatment with high-dose memantine on the cardiovascular and behavioral effects of cocaine in humans. Exp Clin Psychopharmacol 15: 228-237.
- Higgins ST, Bickel WK, Hughes JR (1994) Influence of an alternative reinforcer on human cocaine self-administration. Life Sciences 55: 179-187.
- Stoops WW, Lile JA, Rush CR (2010) Monetary alternative reinforcers more effectively decrease intranasal cocaine choice than food alternative reinforcers. Pharmacol Biochem Behav 95: 187-191.
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, et al. (2009) Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry 66: 169-176.
- Vosburg SK, Haney M, Rubin E, Foltin RW (2010) Using a novel alternative to drug choice in a human laboratory model of a cocaine binge: a game of chance. Drug Alcohol Depend 110: 144-150.
- Stoops WW, Lile JA, Glaser PE, Hays LR, Rush CR (2010) Intranasal cocaine functions as reinforcer on a progressive ratio schedule in humans. Eur J Pharmacol 644: 101-105.
- Fillmore MI, Rush CR (2001) Alcohol effects on inhibitory and activational response strategies in the acquisition of alcohol and other reinforcers: priming the motivation to drink. J Stud Alcohol 62: 646-656.
- Spiga R, Martinetti MP, Meisch RA, Cowan K, Hursh S (2005) Methadone and nicotine self-administration in humans: a behavioral economic analysis. Psychopharmacology (Berl) 178: 223-231.
- Nader MA, Woolverton WL (1991) Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology (Berl) 105: 169-174.
- Banks ML, Gould RW, Czoty PW, Nader MA (2008) Relationship between response rates and measures of reinforcing strength using a choice procedure in monkeys. Behav Pharmacol 19: 365-369.
- Nader MA, Woolverton WL (1992) Choice between cocaine and food by rhesus monkeys: effects of conditions of food availability. Behav Pharmacol 3: 635-638.
- Woolverton WL, English JA, Weed MR (1997) Choice between cocaine and food in a discrete-trials procedure in monkeys: a unit price analysis. Psychopharmacology (Berl) 133: 269-274.
- Anderson KG, Woolverton WL (2003) Effects of dose and infusion delay on cocaine self-administration choice in rhesus monkeys. Psychopharmacology (Berl) 167: 424-430.
- Paronis CA, Gasior M, Bergman J (2002) Effects of cocaine under concurrent fixed ratio schedules of food and IV drug availability: a novel choice procedure in monkeys. Psychopharmacology (Berl) 163: 283-291.
- Anderson KG, Velkey AJ, Woolverton WL (2002) The generalized matching law as a predictor of choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 163: 319-326.
- Gasior M, Paronis CA, Bergman J (2004) Modification by dopaminergic drugs of choice behavior under concurrent schedules of intravenous saline and food delivery in monkeys. J Pharmacol Exp Ther 308: 249-259.
- Negus SS (2004) Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology (Berl) 176: 204-213.
- Negus SS, Mello NK (2004) Effects of chronic methadone treatment on cocaine- and food-maintained responding under second-order, progressiveratio and concurrent-choice schedules in rhesus monkeys. Drug Alcohol Depend 74: 297-309.
- Czoty PW, McCabe C, Nader MA (2005) Effects of the 5-HT(1A) agonist (+/-)-8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on cocaine choice in cynomolgus monkeys. Behav Pharmacol 16: 187-191.
- Foltin RW (1997) Food and amphetamine self-administration by baboons: effects of alternatives. J Exp Anal Behav 68: 47-66.
- Negus SS (2005) Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a doseaddition analysis. Psychopharmacology (Berl) 180: 115-124.
- PW, McCabe C, Nader MA (2004) Assessment of the relative reinforcing strength of cocaine in socially housed monkeys using a choice procedure. J Pharmacol Exp Ther 312: 96-102.
- Dworkin SI, Mirkis S, Smith JE (1990) Reinforcer interactions under concurrent schedules of food, water, and intravenous cocaine. Behavioral Pharmacology 1: 327-338.
- Ping A & Kruzich PJ (2008) Concurrent access to sucrose pellets decreases methamphetamine-seeking behavior in Lewis rats. Pharmacol Biochem Behav 90: 492-496.
- Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, et al. (2010) Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One 5: e11592.
- Lenoir M, Serre F, Cantin L, Ahmed SH (2007) Intense sweetness surpasses cocaine reward. PLoS One 2: e698.
- Thomsen M, Fink-Jensen A, Woldbye DP, Wörtwein G, Sager TN, et al. (2008) Effects of acute and chronic aripiprazole treatment on choice between cocaine self-administration and food under a concurrent schedule of reinforcement in rats. Psychopharmacology (Berl) 201: 43-53.
- Cosgrove KP, Hunter RG, Carroll ME (2002) Wheel-running attenuates intravenous cocaine self-administration in rats: sex differences. Pharmacol Biochem Behav 73: 663-671.
- Cosgrove KP, Carroll ME (2003) Effects of a non-drug reinforcer, saccharin, on oral self-administration of phencyclidine in male and female rhesus monkeys. Psychopharmacology (Berl) 170: 9-16.
- Zhao W, Becker JB (2010) Sensitization enhances acquisition of cocaine self-administration in female rats: estradiol further enhances cocaine intake after acquisition. Horm Behav 58: 8-12.
- Quiñones-Jenab V, Ho A, Schlussman SD, Franck J, Kreek MJ (1999) Estrous cycle differences in cocaine-induced stereotypic and locomotor behaviors in Fischer rats. Behav Brain Res 101: 15-20.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 14871
- [From(publication date):
specialissue-2011 - Jul 17, 2024] - Breakdown by view type
- HTML page views : 10463
- PDF downloads : 4408