Review Article Open Access
Immunostimulants for Aquaculture Health Management
Debtanu Barman1*, Phanna Nen2, Sagar C Mandal3 and Vikash Kumar4
1Center for Aquaculture Research and Development (CARD), St. Xavier’s Vocational Training Center, Don Bosco, Bishramganj, Tripura, India
2Fishery Officer, Freshwater Aquaculture research and Development Center, Cambodia
3College of Fisheries, Central Agricultural University, Lembucherra, Tripura, India
4Central Institute of Fisheries Education, Mumbai, India
- Corresponding Author:
- Debtanu Barman
Center for Aquaculture Research and Development (CARD)
St. Xavier’s Vocational Training Center
Don Bosco, Bishramganj, Tripura, India
Tel: +91-9774624291
E-mail: debtanu08@gmail.com
Received Date: August 08, 2013; Accepted Date: September 24, 2013; Published Date: September 28, 2013
Citation: Barman D, Nen P, Mandal SC,Kumar V (2013) Immunostimulants for Aquaculture Health Management. J Marine Sci Res Dev 3:134. doi: 10.4172/2155-9910.1000134
Copyright: © 2013 Barman D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
Abstract
Aquaculture is gaining momentum in several parts of the world in recent years. Intensification has become a common practice in both finfish and shellfish culture to optimize the returns. High stocking densities, artificial feeding and fertilization have become common husbandry practices in both carp and shrimp culture systems. Due to intensification of culture practices, diseases of microbial etiology of economical significance has surfaced in rearing and grow out ponds and are major threat to the sustainability of the aquaculture industry. Synthetic chemicals and antibiotics have been used to prevent or treat fish and shrimp and have achieved at least partial success. Vaccination against specific pathogens has been developed recently with some success depending on the particular disease. An alternative approach has been the application of various compounds to boost or stimulate the innate immune system of farmed fish and shrimp. These compounds, termed immunostimulants is considered an attractive andpromising agent for the prevention of diseases in fish and shellfish. In recent years, the established beneficial effects of immunostimulants in many livings systems promote their application for disease management in aquaculture practices.
Keywords
Immunostimulants; Immune system; Aquaculture management
Introduction
During the last two decades, the problems of diseases have emerged as the major constraints in aquaculture industry. The increased disease occurrences have resulted in the transfer of pathogenic organisms among countries. Due to this, the shrimp industry of India as well as other Southeast Asian countries has suffered significant economic losses. As there is no effective remedies against these viral diseases, immunostimulants can become powerful tools to control fish and shrimp diseases [1].
An immunostimulant is defined as a chemical, drug, stressor or action that enhances the innate or non-specific immune response by interacting directly with cells of the system activating them. Immunostimulants can be grouped under chemical agents, bacterial preparations, polysaccharides, animal or plant extracts, nutritional factors and cytokines [2]. List of pathogen successfully controlled by using immunostimulants exposure in fish/shrimp like bacteria such as Aeromonas hydrophila, A. salmonicida, Edwardsiella tarda, E. ictaluri, Vibrio anguillarum, V. vulnificus, V. salmonicida, Yersinia ruckeri, Streptococcus spp.; virus such as infectious hematopoietic necrosis, yellow head virus, viral hemorrhagic septicemia and parasite Ichthyopthirius multifiliis.
Immunostimulants are dietary additives that enhance the innate (non-specific) defense mechanisms and increase resistance to specific pathogens [2]. There is no memory component developed and duration of the immune response is very short. Immunostimulants are chemical substances which activate leukocytes [3]. Adjuvant (FCA) is one of the first immunostimulants used in animals to elevate the specific immune response, and it has also been successfully used in conjunction with injection of fish bacterins [4]. So far glucans, which are polymer of glucose found in the cell walls of plants, fungi and bacteria appear to be most promising of all examined in fish and shrimp and oral application found to be the route of choice [5]. Use of these different types of immunostimulants is an effective means to increase the immune competency and disease resistance of fish and shellfish. Research on immune stimulants in aquaculture is under progress and many agents are currently in use in aquaculture industry.
Concept of Immunostimulants
Aquaculture has been grown rapidly for food production in the last few decades. Several commercial fish species have been cultured intensively in narrow or enclosed spaces such as ponds, cages or tanks under overcrowding or high density leading to adversely affect the health of cultured fish with a potentially stressful environment and infectious diseases [2]. The infectious disease-outbreaks have emerged as constraints for the development of aquaculture. The occurrences have spread through the uncontrolled movement of live aquatic animals resulting in the transfer of pathogenic organisms among the countries (Table 3) [1]. Antibiotics and chemotherapeutics have been used to prevent or control bacterial infections in aquaculture for about 20 years [2]. Unfortunately, the use of antibiotics for treatment is not successful and sustainable due to increase in antibiotic-resistant bacteria, negative effect on the indigenous microflora of juveniles or adult fish [6], and the accumulation of antibiotic residues in fish tissue and environment causing human and animal health issues. Vaccination is an effective prophylactic treatment for infectious diseases in fish culture, but it may be very expensive and stressful to fish. A single vaccine is effective against only one specific type of pathogens, but limits the effectiveness for wide range of pathogens due to the complex antigenic structure [7]. Therefore, the needs to look for alternative techniques with eco-friendly disease-prevention have been taken into account.
The alternative technique to prevent the diseases has been proposed that the strengthening of fish immune systems through the application of immunostimulants is one of the most promising methods Immunomodulation by contrast, is a consequence of a change in the number or function of the cells involved in the immune response. The most proven effect of immunostimulants is to facilitate the function of phagocytic cells and increase their bactericidal and fungicidal activities [2]. Immunostimulants can promote recovery from immunosuppressive states caused by any form of stress.
Fish will either survive if they successfully fight against the infection of pathogens, or die if they may not be successful in preventing the spread of the infection. The outcomes of survival or death are largely depended on the efficacy of the immune system to combat the initial infection or the spread of the pathogens. The immune system of fish can be grouped into acquired immunity (specific) and innate immunity (non-specific). Both use physical, cellular and humoral mechanisms to protect against infectious pathogens. The specific immune systems recognize specific antigen on a pathogen, and provide a protection against that specific pathogen. Non-specific immune systems provide a set of protective mechanisms that are inherently available for immediate protection against a wide variety of pathogens [8].
An immunostimulant is a chemical, drug, stressor, or action that elevates the non-specific defense mechanism or the specific immune response [4]. Immunostimulants are practiced in aquaculture as a means to overcome the immunosuppressive effects due to stressors [9,10] or might be used as a prophylactic treatment for expected seasonal outbreaks of known endemic diseases [11] or a suppressive treatment for latent or sub-lethal pathogens. Immunostimulants can also promote recovery from immunosuppression states caused by stress [2]. Various types of immunostimulants evaluated in fish and shrimp are summarized in Table 1.
| Synthetic chemicals | Levamisole, FK-565 (Lactoyltetrapeptide from Streptomyces olvaceogriseus), Quaternary ammonium compounds (QAC). |
| Biological substances | |
| 1) Bacterial derivatives | MDP (Muramyl dipeptide from Mycobacterium species), Lipopolysaccharide (LPS), Freund’s complete adjuvant (FCA), EF203 (fermented egg product), Peptidoglucan (from Brevibacteriumlactofermentumand Vibrio sp.), Clostridium butyricum cells, Achromobacterstenohaliscells, Vibrio anguillarumcells (Vibrio vaccine) |
| 2) Yeast derivatives | b-1, 3 glucan, b-1, 6 glucan |
| 3) Nutritional factors | Vitamin C and E, n-3 fatty acid |
| 4) Hormones | Growth hormone, Prolactin, tri-iodothyronine |
| 4) Cytokines | Interferon, Interleukin |
| 5) Polysaccharides | Chitosan, Chitin, Lentinan, Schizophyllan, Oligosaccharide |
| 6) Animal and plant extracts | Ete (tunicate), Hde (Abalone), Glycyrrhizin, Firefly squid, Quillaja, Saponin (Soap tree) |
| 7) Others | Lactoferrin, Soyabean protein, Quil A, Spirulina, Achyranthesaspera (Herb), Mucorcircinelloides (Fungi) |
Table 1: Immunostimulants evaluated in fish and shrimp.
Vaccines vis-a-vis Immunostimulants
Vaccination is an important tool in preventing infectious disease in humans and animals and both passive and active vaccinations are extensively employed in fish. It is a term that should strictly be applied only when the purpose is long lasting protection through immunological memory. A vaccine targets the specific immune response. It requires primary challenge with antigen and is dependent upon the clonally derived lymphocytes subsets to be implemented [12]. However, most commercial vaccine usually enhances resistance to only one or two specific pathogens and confers only a temporary resistance to disease. Immunostimulants by contrast, can boost immunity to a wide variety of pathogens, thus are nonspecific. Immunostimulations can be achieved in a more general sense by, for instance, targeting complement activation, phagocytosis and cytokines secretion, without necessary or purposefully requiring a specific response to a defined antigen. Examples include zymosan, glucans and lipopolysaccharides and these are best called as true immunostimulants. Comparisons of characteristics of vaccines vis-a-vis immunostimulants are given in Table 2.
| Sl. No. | Vaccine | Immunostimulants |
|---|---|---|
| 1. | Prophylactic for long duration with only one or two treatments. | Prophylactic for short duration, require more treatments. |
| 2. | Efficacy of vaccine is excellent | Efficacy of immunostimulants is good |
| 3. | Limited spectrum of activity. | Wide spectrum of activity |
| 4. | No toxic side effects | No toxic side effects |
| 5. | No accumulation of toxic residues | No accumulation of toxic residues |
| 6. | No environmental impact | No environmental impact |
| 7. | Enhance specific and nonspecific immune response | Mainly enhance nonspecific immune system of larvae before specific immune system matures. |
| 8. | Difficult to vaccinate larvae of fish and shrimp | Easy to vaccinate larvae of fish and shrimp |
| 9. | Costly | Cost effective |
Table 2: A comparison of characteristics of vaccines and immunostimulants.
| Name | Company | Type | Mode of action | Instruction for use | Specific supporting literature | Recommended level of inclusion (kg/ton) |
|---|---|---|---|---|---|---|
| Immustim | Immundyne, USA | B (1,6) branched B (1,3) glucan from yeast |
Activates macrophages | 4 days-yes 3 days-no |
General on similar products | 0.5-12.5 |
| Macroguard | Biotec-Mackzymal, Norway | B (1,6) branched B (1,3) glucan from yeast |
Activates macrophages | -With vaccines in injection - in feed <6-8 weeks cont. |
Yes, on salmonids and tiger shrimp | 1.0 |
| Vitastim | Taitoand Company, Japan | B (1,6) branched B (1,3) glucan from fungi |
Activates macrophages | As feed ingredient | Yes, on shrimp and fish | 1.0 50 mg/kg body weight |
| Aqua-Mune | Park Tonks | B (1,6) branched B (1,3) glucan from baker’s yeast |
Activates macrophages | As feed ingredient | No | 1.0 |
| Penstim | AURUM Aquaculture Ltd, USA | Beta-glucan | Activates macrophages | Immersion for larvae and feed ingredient for larger animals | - | 2.0 to 5.0 in feed |
| Laminarn | Pronova, Noway | B (1,6) branched B (1,3) glucan from brown algal laminariae |
Activates macrophages | As feed ingredient | Yes | - |
| Calcium spirulan | Kelly Moorhead, Hawaii | Sulfated polysaccharide from spirulina | Inhibit viral envelop replication, inhibits virus penetration in host cell | Not known | Yes, on mammals | Not known |
| SP 604 | Alltech Inc, USA | Premix of mannan (Biomos), Cr and Se yeast, probiotics | Multiple: activates macrophages, source of trace minerals | |||
| Agrimos | Agrimerica, USA Santel, France |
Mannan based oligosaccharide from | Good substrate for lactic bacteria, occupies binding sites of pathogens in gut, stimulates immune response | As feed ingredient | Yes, on mammals | 1.0 |
| Elorisan | BUGICO, Switzerland | Organic silicon | Lower lipid permeability, protection of nervous tissues | Intraperitoneal injection | Yes | 0.1 ml of 1% solution |
| DS 1999 | International Aquaculture Biotechnologies Ltd | Bacterin | Activates macrophage | Add directly in culture medium(larval culture) | Yes, field trails | 0.5 in larval diets |
| Levamisole | Janssen Pharmaceutica, Belgium | Tetrahydro-6- phenylimidazolthiazole hydrochloride | Activates macrophages | As feed ingredient in bath | Yes, on mammal and fish | 5-10mg/kg body weight |
| Lysozyme Hydochloride | Belovo, Belgium | Lysozyme from hen’s eggs | Kills/lyses bacterias | As feed ingredient | Yes, mammals (Cheese) |
- |
| Lactoferrin | DMV international, the Netherlands | Lactoferrin from bovine milk | Binds Iron, makes it unavailable to pathogens | As feed ingredient | Yes, on mammals | - |
| Selenium yeast | Alko, Finland | Selenium Yeast | Acts a yeast (activates macrophages) and source of selenium | As feed ingredient | - | 1.0 |
| Polypeptides fish hydrolysates | Tepual, Chile Sopropeche, France |
Short peptides | Activates macrophages | As feed ingredient | - | - |
| Blood plasma | Harimex, The Netherlands | Blood serum (immunoglobulins, glycoproteins) | Activates macrophages, Binds on gut bacterial receptors | As feed ingredient | Yes, on mammals | 50-100 |
| Fish oils | Fish oil producer | Omega-3 fatty acids | Lower prostaglandin E-2 production (Immunodepressor), increase membrane fluidity | As feed ingredient | Yes, on mammals | 10-100 |
(Source: Devresse et al. 1997. Nutrition and Health. Aquaculture Division, INVE scientific department, Belgium)
Table 3: Some commercial immunostimulants currently available on the market in different countries.
Application of Immunostimulants used in Fish and Shrimp
Synthetic chemicals
Levamisole is an antihelminthic chemical compounds used to treat the nematodes infection in human and animals as well (Table 1). It can stimulate immune response in vitro. Levamisole enhanced phagocytic activity, the NBT reaction and increase antibody producing cells oral administration of levamisole increased the number of leucocytes, lysozyme activities in serum and the stimulated NBT reduction and phagocytic index of phagocytic cells [13]. However, no differences were found in the levels of hematocrit, leucocrit or immunoglobulin using levamisole in rainbow trout [14]. Findlay et al. [15] have recommended application of levamisole as immunostimulant in fish. It has been observed that rainbow trout exposed to a bath treatment containing 5, 10, 25 μg/ml levamisole for 2 hrs period showed resistant to Y. ruckeri [16].
Biological substances
Bacterial derivatives: MDP (Muramyl dipeptide): MDP (N-acetylmuramyl- L-alanyl-D-isoglutamine), obtained from Mycobacterium. Kodama et al. [17] revealed that intra peritoneal injection of rainbow trout with MDP-Lys increased the phagocytic activities, respiratory burst and migration activities of kidney leucocytes as well as resistance of the fish to A. salmonicida.
LPS (lipopolysaccharide): LPS is a cell wall component of Gramnegative bacteria. It was reported that LPS effective in preventing A. hydrophilla disease and stimulating innate immune response of rainbow trout [18]. Salati et al. [19] reported that LPS can stimulate phagocytosis and the production of superoxide anions in Atlantic salmon macrophages and LPS can stimulate B-cell proliferation and enhance macrophage phagocytic activity in red sea bream Pagrus major. Also, LPS stimulates the production of macrophage activating factor in goldfish lymphocytes [20]. These substances are very potent even in very low doses and may occur as contaminants in bacterin preparations and used in fish immunizing programmes. LPS stimulate hemocytes proliferation; enhance phagocytic activity as well as the microbicidal activity of shrimp [21].
FCA (Freund’s complete adjuvant): FCA is a mineral oil adjuvant containing killed Mycobacterium butyricum, increase the immune response in fish. FCA can increase respiratory burst, phagocytic and NK cell activity of leucocytes in rainbow trout protect against V. anguillarm infection [22]. In contrast, yellowtail injected with FCA did not show increased resistance to P. piscicida infection, although the adjuvant effect of FCA on a P. piscicida vaccine was observed in fish [23].
Vibrio bacterin: Vibrio anguillarum bacterin (inactivated whole cell vaccine) is the most successful vaccine for salmonid fish, administered through injection,oral and immersion methods [2]. Immunostimulation of V. anguillarum bacterin was seen in fish and shellfish. In black tiger shrimp the migration of hemocytes treated with vibrio bacterin can be increased [24]. Further, Norqvist et al. [25] also reported that vaccination of rainbow trout with attenuated V. anguillarum stimulates protection against A. salmonicida challenge.
Clostridium butyricum cells: C. butyrium bacterin can enhance theresistance to vibriosis in rainbow trout by oral administration by leucocyte activation, including phagocytosis and increased superoxide anion production [26]. Young et al. [27] revealed that C. butyricum shows immunostimulatory effects like stimulation of macrophages and NK cells and improves further protection against Candida infection.
Achromobacter stenohalis cells: A. stenohalis is a gram-negative aerobic organism which has been isolated from sea water. Inactivated A. stenohalis can enhance immune responses of kidney cells, complement activity and increase protection against A. salmonicida challenge. The LPS of this bacterium activates mouse macrophages and B-lymphocytes [28].
EF203: EF203 is a fermented product of chicken eggs and oral administration of it to rainbow trout stimulates the activity of leucocytes such as phagocytosis and chemiluminescence and increases protection against Streptococcus infection [29].
Yeast derivatives: Glucans, long chain polysaccharide extracted from yeast, are good stimulators of non-specific defence mechanism in animals including fish and shellfish like phagocytic activity and protection against bacterial pathogens. Several types of glucan has been investigated in fish such as yeast glucan, peptide-glucan β -1,3, glucan (VST). Yeast glucan (β 1-3- and β1-6-linked glucan) and β-1,3glucan (VST) is derived from cell walls of baker’s yeast like Saccharomyces cerevisiaeand Schizophyllum commune, respectively [30]. β-glucans comprised diverse group of polysaccharides of D-glucose monomers linked with β-glycosidic bonds. Cellular and non-cellular defense mechanisms are increased in activity after treatment with β-glucan like lysozyme activity, phagocyte activity, complement activity and bactericidal activity of macrophages. A number of reports reveal that dietary β-glucan administration increases resistance to infection like Selvaraj et al. [31] reported that highest antibody titre against A. hydrophila injected with β-glucan (100-1000 μg glucans/fish). In addition, Robertsen et al. [32] recorded that intraperitoneal injection of β-glucan prepared from cell walls of Saccharomyces cerelisiae injected to Atlantic salmon showed increased resistance to V. anguilllarm, V. salmonicidia and Y. ruckeri.
Polysaccharides
Chitin and Chitosan: Chitin is a polysaccharide which constitutes the principal component of exoskeletons of crustacean and insect and cell walls of few fungi [2]. It can stimulant macrophage activity and give resistance from certain bacteria. Kawakami et al. [23] Chitosan, de-N-acetylated chitin can increased protection against A.salmonicida infection when injected with or immersed in chitosan solution in brook trout, Salvelinus fontinalis [33].
Lentinan, Schizophyllan and Oligosaccharide: Lentinan, Schizophyllan and Oligosaccharide can increase cellular and noncellular defense mechanisms like lysozyme activity, phagocyte activity and complement activity in fish.
Animal and plant extracts
Ete (Tunicate) and Hde (Abalone): An extract from the marine tunicate, Ecteinascida turbinate (Ete) and a glucoprotein fraction of water extract (Hde) from abalone, Haliotis discus hannai. It can enhance the killing of tumor cells in vitro and inhibits tumor growth in vivo. Ete (Tunicate) can enhance the phagocytosis and increased survival of Eel when injected against A.hydrophila [34]. In addition, when rainbow trout injected with Hde against V. anguillarum infection showed increased survival along with enhanced phagocytic activites [35].
Firefly squid: Firefly squid, Watasenia scintillans, can stimulate the immune system of rainbow trout such as the production of superoxide anion, potential killing activities by macrophages and the lymphoblastic transformation of lymphocytes in vitro.
Chinese medicinal herbs (Astragalus membranaceus and Lonicera japonica) extracts can beused as immunostimulants to enhance immune response and disease resistance of cultured fish species [7]. The herbal immunostimulants such as Emblica officinalis, Cynodon dactylon and Adathoda vasica improved the immune system and reduced microbial infection in the goldfish Carassius auratus [36] and similar work was also carried out on another ornamental fish Poecilia sphenops using herbal immunostimulants. Nile tilapia shows enhanced phagocytic activity after treated with Astragalus extract for one week [7,37]. Dugenci et al. [38] documented that ginger extract to be very effective in enhanced phagocytic and extracellular burst activity of white blood cells in rainbow trout.
Nutritional factors
Vitamin C: Vitamin C is involved in several physiological functions including growth, devel¬opment, reproduction, wound healing, response to stressors and possibly lipid metabolism through its action on carnitine synthesis while administering in feed. Vitamin C (Ascorbic acid) is a co-factor in many biological processes including collagen synthesis and cellular functions related to neuromodulation, hormone and immune systems. It has been observed by Tewary and Patra [39] that higher levels of dietary vitamin C significantly increased the protection against A. hydrophila.
Vitamin E: Vitamin E can enhance specific and cell-mediated immunity against infection in Japanese Flounder Paralichthys olivaceus [40] and macrophage phagocytosis in fish such as channel catfish Ictalurus punctatus [41] and turbot Scopthalamus maximus [42]. Vitamin E deficiencies in trout result in reduced protection against Y. ruckeri [43].
Hormones
Growth hormone (GH): GH directly affects immune competent cells like macrophages, lymphocytes and NK cells. In fish, exogenous growth hormone (GH) has mitogenic activity on lymphocytes and activates NK cells and production of superoxide anions of leucocytes.
Prolactin: Prolactin also directly affects immune competent cells like macrophages, lymphocytes and NK cells. It can enhance the production of superoxide anions of leucocytes. Sakai et al. [44] documented that prolactin helps in increased level of production of superoxide anion in rainbow trout by leucocytes.
Lactoferrin, consist of a single peptide chain with molecular wt. ~ 87,000 Da and posses 2 Fe-binding sites per molecule, most popular physiological fluids of mammals [2].
Cytokines: Cytokines are polypeptides or glyco-proteins which act as modulators in the immune System. Cytokines may be useful as powerful immunostimulants if their structures can be identified and recombinant molecules prepared.
Algal derivatives
Laminaran is a β (1, 6)-branched β (1, 3)-D-glucan, a major component in sub-littoral brown algae, e.g. Phaeophyceae. Almost all B-(1, 3) D-glucan display poor water solubility which makes them less easy to handle than aqueous soluble laminaria. Laminaran obtained from Laminaria hyperhorea has immune modulatory effect on immune system as well.
Immunological system in fish
The immune system is the system which continuously fights against the pathogen and give proper protection to our body. The two types of immune system are innate immune system or non specific immune system and acquired immune system or adaptive immune system. The essence of the immunological system of the vertebrates is to react and protect against the infections. Proper work of the immunological system of a fish involves different cells and organs. There are different factors that affect the immunological response of the fish. Inherent factors like health and age, extrinsic like temperature or changes in abiotic parameters them all together affect the health condition, and thus the response. Those changes cause in some cases stress, which if achieves high level generates an immunological system collapse [45].
Cells involving on the immune system are leucocytes or white cells. Those can be found in the blood stream or on tissues. Lymphoid tissues on fishes are thymus, spleen, anterior kidney and lymphoid tissues associated to mucus and intestine [46]. The classification of leucocytes as in the vertebrates has been done following morphological criteria whereby various groups can be distinguished such as lymphocytes, granulocytes and macrophages [47]. A short explanation of each group is done below in order to know the main characteristic and functions.
Lymphocytes
High differentiated cells with capability to respond on stimuli. The most common are mature lymphocytes with an irregular surface or border [46]. Previous studies have defined lymphocytes as a high metabolically potential due to its high number of organelles in the cytoplasm as golgi apparatus, mitochondria, ribosome, and endoplasmic reticule. They are found in all over the body circulating on the blood stream and gathered on lymphoid organs and the quantity is very variable. The main function is to produce antibodies, immunological memory, and regulatory factors as lymphokines in response of the humoral and cell specific immune [48]. Lymphocytes B are bone marrow derived while T is thymus derived. T cells are responsible for cell mediated immunity as well as providing assist to B lymphocytes; those last are responsible to produce antibodies against antigen [49].
Granulocytes
Occurrence and functions varies within species of fish, the origin is focus on the kidney tissues. In teleost, there are describe three based on morphology [46,50] including Neutrophil, eosinophil and basophil, the first being the most common. Granulocytes react responding in the presence of foreign material going into the body but without recognizing specific antigens. This kind of defending is called in non-specific defense mechanism, explained little forward. When the invasion is occurred those cells migrate and destroy the estrange particles by phagocytosis or just by killing by a cytotoxic response.
Macrophages
Based on Literature many test has been done in several species of fish. After several tests it seems that macrophages can be use to evaluate the health of the fish as a kind of indicators. Those cells play an important role on killing pathogens as immune response. As exposed in the work of Fernández et al. [46], based also in many previous studies, macrophages are the main phagocyte cells on fishes. The pathogens are killed by two ways, releasing toxic substances or by ingestion, known as phagocytosis. It involves producing ROS-reactive oxygen species or microbiocidal oxygen radicals. This generating activity is known as respiratory burst, and is not only produce by phagocytosis. Lymphokines can regulate macrophage functions like MAF, macrophage activating factor [51]. The immunological system of fish can be divided in two branches depending on the functionality such as natural or nonspecific and acquired or specific. The non-specific immune system is considered to be the most important ones for immunonisation.
Immunostimulation of non-specific defense mechanisms
Most immune-stimulatory compounds examined in fish and shellfish have been shown to have immune enhancing potential through heightening of nonspecific immune responses of the organisms. The nonspecific immune responses in contrast to the specific response does not require prior exposure to an antigen and consists of barriers such as skin, scales, lytic enzymes and phagocytic cells. The nonspecific immune response is also considered to be the first line of defence against invading pathogenic microorganisms and is the sole immunological mechanism by which invertebrates protect themselves from disease. In contrast to specific immunity, which only recognizes a particular antigen or pathogen, each component of the nonspecific response can recognize a broad array of foreign agents. It has been hypothesized that fish and shellfish are more reliant on nonspecific immune response. For these reasons, a large portion of the research on immunostimulation has focused on up-regulating the nonspecific immune response of the organisms. It is well established that mononuclear phagocytes or macrophages plays a central role in the cellular part of the nonspecific defence mechanism of fish [52]. Haemocytes and the prophenoloxidase (proPO) system are the primary defence mechanisms of shrimp. Both semi-granular and granular cells carry out the functions of the proPO system [53]. Phenoloxidase is the terminal enzyme in the proPO activation system and is activated by lipopolysaccharides or peptidoglycans from bacteria and β-1, 3 glucan from fungi through the pattern recognition molecules [54] Phenoloxidase activity has been detected in many species of penaeid shrimp such as Sao Paulo shrimp Farfantepenaeus paulensis, yellow leg shrimp Fafantepenaeus californiensis, tiger shrimp Penaeus monodon, blue shrimp Litopenaeus stylirostris and white shrimp L. vannamei [55-59]. The activation state of these cells and enzyme systems are often used as measures of non-specific immunostimulation. Other measures employed for this purpose include cell migration, phagocytosis and bactericidal activity as well as changes in numbers of leucocytes and the activation potential of cells upon stimulation, as measured by oxidative radicals and enzymes [52]. The nonspecific immune responses such as phagocytosis and the production of oxidative radicals are quickly activated by the immunostimulants and help to protect the host against a broad spectrum of pathogens [4].
Method of administration
Immunostimulants potentiate the immunity of the host itself, enabling it to defend more strongly against pathogens. Several immunostimulants also stimulate the natural killer cells, complement, and lysozyme and antibody response of fish [53]. There are mainly 3 ways to deliver immunostimulants including injection, immersion and oral uptake. Injection of immunostimulants can produce strong non-specific response but its costly affairs with lots of time and labour intensive as well, applicable only for large size of fish more than 10-15g in body weight in intensive aquaculture system. It has been reported that injection has wide protection against a range of pathogens like intra-peritoneal injection with glucan injected to channel catfish shows increased in phagocytic activity reducing fish mortality challenge with Edwardsiella ictaluri [54]. For small fish vaccination is impractical. Immersion produces less non-specific immune response, but more cost effective than injection, increase more stress to fish while handling, applicable in intensive culture system. Immersion method is very effective during acclimation of juveniles to ponds in field condition. Using immersion of levamisole showed increase in circulating leukocytes, phagocytic rate and increase protection against P. damselae sub sP. piscicida in European Seabass [55].
Oral ingestion produces good non specific immune response and can be the most cost effective method with economically viable. It is mostly suited for extensive aquaculture system. Immunostimulants powders are mixed with feed using a fish oil coating. Now a day, bioencapsulation method is also followed to immunize the fish larvae during their early larval stages with live fed organisms.
Timing of administration
It is necessary to apply immunostimulants at the right time. Anderson [4] proposed that the application of immunostimulants should be implemented before the outbreak of disease to reduce diseaserelated losses. Effective dosage and exposure time will be further more complicated based on different culture systems with feeding regime [56]. In Atlantic salmon injection with high dose of glucans @ 100 mg/ kg led to absence of protection for 1 week, but maximum benefits only occurs after 3-4 weeks. Also, at low dose of injection @ 2-10 mg/kg, give protection only 1 week [32]. Similarly, it has been noticed that increase in the number of NBT positive cells in African Catfish fed with glucan or oligosaccharide over 30 days, but not over 45 days [57].
Mode of action
The mode of action of immunostimulants is to activate the immune systems of organisms, to enhance the immunity level against invading pathogens. The approach is very diverse in nature or may be poorly understood and also depends on the type of immunostimulants, dose, route of administration, time and length of exposure.
Following are some of the mechanism of actions:
• Stimulators of T-lymphocytes- Levamisole, Freund’s Complete Adjuvant (FCA), Glucans, Muramyl dipeptide, FK-565 (Lactoyl tetrapeptide from Streptomyces olivaceogriseus).
• Stimulates of B-cells- Bacterial endotoxions, Lipopolysaccharides.
• Macrophage activator- Glucans, Chitin and Chitosan
• Inflammatory agents including chemotoxins
• Cell membrane modifiers- Detergents and Sodium dodecyl sulphate, Quaternary ammonium compounds (QAC), Saponins
• Nutritional factors- Vitamin C and E, n-3 fatty acids
• Cytokines- Leukotriene, Interferon
• Heavy metals- Cadmium
• Animal and fish extracts- Mitogens
In general immunostimulants enhance the phagocytosis and bacterial killing ability of macrophage, complements, lymphocytes and nonspecific cytotoxic cells, resulting in resistance and protection to various diseases and invading microorganisms.
Detection of immunostimulation
An increase in any characteristics such as phagocytosis, production of superoxide anions etc in treated fish and shellfish over controls is evidence of immunostimulation. Following are some of the methods of detection of immunostimulation.
1. Haematocrit and leucocyte count: Leucocytes mediate nonspecific immunity. So raised leucocytes count with an essentially unchanged haematocrit is an indication of immunostimulation.
2. Phagocytic activity: Phagocytosis is a common reaction of cellular defence and generally recognized as a central and important way to eliminate microorganisms or foreign particles. Phagocytosis can be assayed by incubating blood with a killed bacterial culture and examining stained smears for phagocytes containing bacteria [58].
The phagocytic activity are defined as phagocytic ratio (PR) and phagocytic index and are expressed as
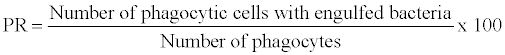
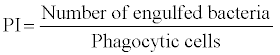
Bactericidal activity: Bactericidal activity can be assayed by incubating macrophages with a live bacterial culture and then washing off the supernatant liquid, lying the macrophages and examine the numbers of live bacteria [59].
3. Oxidative radical production: A major way in, which neutrophil granulocytes contribute to nonspecific immunity, is by the production of oxidative radicals. Nitro-blue tetrazolium (NBT) reacts with oxidative radicals producing a dark blue color and is used to identify neutrophils actively producing them.
4. Myelo-peroxidase production: Activated neutrophils also produce myelo-peroxidase. The level of activation can be determined by incubating blood smears in an indicator reagent and examining cells under the microscope for degree of staining.
5. Immunoglobulin concentration: Some serum immunoglobulins are humoral antibodies and therefore heighten specific immunity, many others regulate nonspecific immunity.
6. In vitro measurement: Jeney and Anderson [10] have described an in vitro method for screening substances for immunostimulation. In essence finally divided pieces of rainbow trout spleen are maintained in a tissue culture medium with a test substance and after 4 days cell suspension are prepared. For neutrophils the cell suspensions are treated with NBT and examined by spectrophotometry; for phagocytes aliquots of the cell suspension are shaken for 15 min. with a suspension of fixed sheep erythrocytes and then smears are made for microscopy.
7. In vivo measurement: In fish, specific immunity develops slowly and thus it is possible to assess immunostimulation by a challenge test with virulent bacteria, which rapidly kills large number of fish at a time. Any delay or reduction in mortality in treated fish compared to untreated group may be attributed nonspecific immunity systems.
Attributes of immunostimulants
• Safe for the environment and human health, biocompatible and biodegradable
• Promote a healthy body status by triggering the immune system of the host
• Nontoxic to finfish and shellfish with no known side effects
• Enhance disease resistance against broad spectrum of pathogens
• Reduce mortality due to opportunistic pathogens
• Prevent viral diseases
• Enhance the efficacy of antimicrobial substances
• Enhance the efficacy of vaccines and antibiotics
• Cheap, ecofriendly and easily available.
Efficacy and limitation of immunostimulants
The use of immunostimulants can protect fish from several infectious diseases and decrease mortality rates by increasing fish resistance against infectious bacteria such as Vibrio anguillarum, V. salmonicida, Aeromonas salmonicida and Streptococcus sp., viral infections such as IHN (Infectious Hematopoetic Necrosis) and yellowhead (YHV) disease and parasitic infections such as white spot disease and sea lice; and immunostimulants do not increase resistance against Renibacterium salmoninarum, Pseudomonas piscicida or Edwardsiella ictaluri infections due to their resistances to phagocytosis and abilities to survive within macrophages [60]. Use of immunostimulants in cultured fish result in macrophage activation, increased phagocytosis by neutrophils and monocytes, increased lymphocyte numbers, increased serum immunoglobulins, and increased lysozyme [2]. Different immunostimulants have effectiveness for different life stages based on solubility and give a different degree of protection against the pathogens [56]. An algal extract, laminaran, more soluble than the fungal and yeast glucans, has also proven to activate macrophages [61] and to increase respiratory burst activity in anterior kidney leucocytes of Atlantic salmon [62]. The laminaran has been promoted for its superior solubility compared with other β-1, 3-glucans, considering as Candidate substances for diet application [56]. Absorption of laminaran from water by yolk-sac larvae of Atlantic halibut, Hippoglossus hippoglosus, through the skin and intestinal epithelium [63] suggests that it may have the potential to enhance immunity in early life stages before the development of acquired immunity.
However, effect of immune stimulants on nonspecific immune mechanism is normally of short duration [64]. It has been shown in salmon gave maximum leucocyte responses just 2 days after injection with M-glucan [59], and that yeast β -glucan gave an increase in respiratory burst activity 4-7 days after treatment [65]. The protection in trout using glucans and chitosan was greatly reduced after 14 days of immune stimulation [33]. The use of several immunostimulants for prolonged periods does not appear to provide additional advantages with respect to a single dose [64]. Tseng et al. [66] observed that after 29 days, the protection induced in blue gourami by injection of 20 mg/kg laminaran for 22 days, was not significantly different from the effect of a single injection of 20 mg/kg laminaran. But, [6] Misra et al. (2006) reported that most of the immune parameters such as leucocyte count, phagocytic ratio, phagocytic index, lysozyme activity, complement activity, serum bactericidal activity were significantly enhanced on 42 days after three i.p. injection of 10 mg of β-glucan/kg body weight of Labeo rohita fingerlings, which would lead to long-term protection in fishes. The effects of immunostimulants are not directly dose dependent, and high dose or overdosage may not enhance and may inhibit the immune responses [64], reported that the increase in respiratory burst activity of glucans-treated macrophages was maximal at glucan concentrations of 0.1-1 μg/ml, whereas at10 μg/ml no effect was seen and at 50 μg/ml glucan was inhibitory [2]. Vitamin E is immunostimulatory in concentration of 50-300 IE/kg feed, but very high vitamin E levels (>1000-5000 IE/kg diet) fed for a prolonged time have an immunosuppressive effect [64]. Galeotti et al. [56] concluded that genetics of the species, life history stage, and the culture environment all interact with the type and dosage of immunostimulants to contribute to the efficacy of the immunostimulatory substance.
Immunostimulants in aquaculture health management
Immunostimulats have been extensively studied in fish and shellfish both at whole animal and on a cellular level. It has been used as prophylactics to control infections disease of animals and also playing the role of alarm molecules that activate the immune system [67]. Fish and shrimp depends more heavily on nonspecific defence mechanisms than mammals and therefore immunostimulats play a vital role in health management strategies of aquatic organisms. There are at least 20 different compounds, including levamisole, lipopolysaccharides, glucan, vitamin C and E etc. that are used as immunostimulats, adjuvants and vaccine carries in fish [4]. Among these compounds, glucan is one of the most promising stimulants for nonspecific defence mechanism and also the most studied immunostimulant in aquatic species. Glucan has been reported to enhance resistance against bacterial pathogens such as Vibrio anguillarum, Aeromonas salmonicida, A. hydrophila and Yersinia ruckeri in several species in fish such as the carp Cyprinus carpio, Atlantic salmon Salmo salar, rainbow trout Onchorhynchus mykiss, yellow tail Seriola quinquradiata and African catfish Clarias gariepinus [10,68]
Applications of β-1,3 glucan enhances the nonspecific cellular defence mechanisms of animals by increasing the number of phagocytes and the bacterial killing activity of macrophages in rainbow trout, Atlantic salmon, catfish, and carp [69] and through production of superoxide anions by macrophages [70]. In an Indian major carps, Labeo rohita, yeast glucan have been observed to enhance the phagocytic activity of leucocytes and stimulate generation of reactive oxygen species (ROS) in phagocytes [71].
In recent years, nucleotides and their metabolites have received heightened attention as potential immunomudulators [72]. They play key roles in numerous essential physiologic functions, including encoding genetic information, mediating energy metabolism and signal transduction [73]. The beneficial influences of oral administration of nucleotides on immune functions, vaccine efficiency or disease resistance has also been demonstrated in fish such as Atlantic salmon, coho salmon, rainbow trout, common carp, hybrid tilapia and hybrid striped bass [74,75]. Burrells et al. [76] reported beneficial effects of dietary nucleotides when challenging salmonids with infectious salmon anaemia virus, Vibrio anguillarum, Piscirickettsia salmonis and sea lice. They hypothesized that dietary nucleotides are capable of enhancing the potential of the immune system in general to mount greater and more rapid specific responses, as compared to the primarily nonspecific capacity of phagocytes induced by glucan. Choudhury et al. [77] reported that dietary yeast ribonucleic acid at 0.4% enhances phagocyte respiratory burst and protection of Labeo rohita juveniles by Aeromonas hydrophila. In addition, dietary nucleotides have also been observed to modulate gene expression [78], a phenomenon also confirmed in fish by Low et al. [79]. However, the way in which dietary nucleotides modulate gene expression during development of adaptive immunity is still not clear.
The immunostimulatory potential of levamisole in fish is of considerable interest in the USA and elsewhere, because it has approval by the U.S Food and Drug Administration for treatment of helminthes infections in ruminants. Levamisole a synthetic phenylimidazolthiazole has also been found to be possible modulator of the immune responses of carp and rainbow trout [13,80]. After treatment with levamisole both fish species showed enhanced nonspecific immune activity and resistance to an experimental challenge with pathogenic bacteria [58]. The increased protection may be correlated with increased phagocytosis, cytokine expression by macrophages, lymphocyte proliferation following exposure to mitogens and antibody response.
The immunostimulants properties of whole microorganisms, chitin particles, lactoferrin, sodium alginates, vitamin C and E, dietary carbohydrates also received considerable attention in fish and shellfish health management strategies. Little information exists regarding the in vitro effect of chitin on fish immune system. Some studies do exists on the in vivo administration of chitin and it has been found that injection or dietary administration of chitin may enhance the innate immune system of several fish species [81]. Esteban [82] reported that dietary administration of lactoferrin, a glycoprotein enhances the nonspecific immune responses of gilthead seabream Sparus auratus. Sodium alginate extracted from brown algae has been reported to enhance the resistance of common carp Cyprinus carpio against Edwadsiella tarda infection and increase the nonspecific defence system of C. carpio [83]. Bagni et al. [84] reported that alginic acid (Ergosan) and yeast β-glucan (Macrogard) activate innate immune response in sea bass (Dicentrarchus labrax), particularly under conditions of immunodepression related to environmental stress.
In recent years, whole microorganisms have been tested for their possible immunostimulant properties in fish. The oral administration or injection of yeasts Saccharomyces cerevisiae or Candida utilis and fungus Mucor circinelloides have been shown to increase both humoral and cellular immune responses and to increase or confer resistance against pathogenic bacteria in channel catfish, rainbow trout and gilthead sea bream [85,86]. Rao and Chakrabarti [87] reported that Achyaranthes aspera, a herb that stimulates both specific and nonspecific immunity in Indian major carp, Catla catla.
Dietary supplementation of certain vitamins may be effective means of increasing immunocompetency and disease resistance of fish [88]. Elevated doses of vitamin C have been shown to enhance immune responses such as macrophage activities, cell proliferation, natural killer cell activity, complement and lysozyme levels in fish [89]. A positive effect of vitamin C together with vitamin E on the immune response of fish has also been found [90,91]. Feeding high levels of ascorbic acid has been reported to enhance protection against bacterial infections viz. Edwardsiella tarda, E. ictaluri, V. anguillarum, A. salmonicida and against parasitic infection (Ichthyopthirius multifilis). Sahoo and Mukherjee [92] reported that high level of dietary vitamin C has been used to counteract immunosuppression caused by aflatoxin B1 contaminated feed in immunocompromised rohu (Labeo rohita). Recently, Kumar et al. [93] reported that non-gelatinized carbohydrates (46%) along with supplementation with 50 mg kg-1 amylase stimulated the immune system in L. rohita juveniles. Therefore, immunostimulants have an immense importance in disease management in aquaculture.
Studies on the effect of immunostimulants on shrimp and prawn are still at an infant stage. ß-glucan have received attention in recent years for their ability to increase disease resistance in shrimp and prawn because the limitation of the specific immune response of these animals and the nature of the disease agents make the development of effective vaccine impractical. ß-glucan have been successfully used to increase the resistance of Penaeus japonicus against vibriosis [94], further studies using Penaeus monodon showed protection against vibriosis, white spot syndrome virus and Vibrio damsela and V. harveyi [95] and also enhancement of survival and immunity during brood stock rearing [96]. All these effects were caused by direct impact on haemocytes via stimulation of phagocytosis, cell adhesion and superoxide anion [97]. Surprisingly the glucan-induced resistance is maternally transmitted [98]. Surprising data has been obtained in the fresh water crayfish Pacifastatus lenieusculus. The injection of glucan caused a shortterm severe loss of haemocytes, followed by a rapid recovery due to accelerated release of cells from the haematopoietic organs.
Macrobrachium rosenbergii post larvae showed enhanced growth and resistance to V. alginolyticus by dietary administration of ß-glucan. Cheng et al. [99] reported that dietary administration of sodium alginate enhances the immune ability of white shrimp L. vannamei and increase its resistance against V. alginolyticus infection. Vitamin C has also plays a key role in animal health as an antioxidant by inactivating damaging free radicals producing through normal cellular activity and diverse stressors. Lightner et al. [100] reported that inadequate dietary levels of vitamin C in juvenile shrimp may result in black dead syndrome, reduced growth rates, poor feed conversion ratios, decreased resistance to stress and reduced capability to heal wounds. According to Merchie et al. [101], vitamin C plays a role as immunostimulants, as evidenced by the ability of P. monodon postlarvae and juveniles to avoid baculovirus and to resist disease caused by V. harveyi and saline shock. However, the mode of action of vitamin C as an immunostimulant is not clear, although its antioxidant role and in consequence cell protection could be a mechanism to preserve haemocytes, improving the general immunological system of shrimp. Therefore results suggest that although the shrimp immune system is nonspecific, it would be possible to enhance disease resistance against pathogens in shrimp by careful and regular use of immunostimulants.
Conclusion
Immunostimulants appear to be most promising and useful tools for prophylactic treatment of farmed fish and shrimp. It is safer than chemotherapeutics and their range of efficacy is wider than vaccination. However, these compounds will not replace vaccines proper nutrition or good management techniques. The strength of these compounds appear to lie in their ability to enhance larval culture before the specific immune system matures and the animals can be vaccinated and able to improve nonspecific immune function against a broad spectrum of pathogens.
Thus, application of immunostimulants to aquatic health management has immense potential, but in order to capitalize on this issue a lot of scientific research is necessary to understand the mode of action. Many of the in vitro tests are expensive to conduct, limiting the ability to rapidly screen potential immunostimulant regimens for efficacy. Additional research is needed to define the specific dosage rates and efficacy of various compounds for a variety of aquatic species and their pathogens and to decrease costs of the immunostimulants. It is expected that during coming years immunostimulants will find more application to make aquaculture sustainable. Therefore, immunostimulants may be an effective tool for controlling infectious diseases in aquaculture.
References
- Jadhav VS,Khan SI, Girkar MM, Gitte MJ (2006) The role of immunostimulants in fish and shrimp aquaculture. Aquaculture Asia3: 24-27.
- Sakai M (1999) Current research status of fish immunostimulants. Aquaculture172: 63-92
- Lunden T, Bylund G (2000) The influence of in vitro and in vivo exposure to antibiotics on mitogeninduced proliferation of lymphoid cells in rainbow trout (Oncorhynchusmykiss). Fish Shellfish Immunol10: 395-404.
- Anderson DP (1992) Immunostimulants, adjuvant, and vaccine carriers in fish: applications to aquaculture. Ann Rev Fish Dis2: 281-307.
- Philip R,Sreekumar K, Anas A, Bright Singh IS (2001)Immunostimulants –Source, diversity, commercial preparations and mode of application. NatlWorkshop on Aquaculture Medicine 74.
- Misra CK, Das BK, Mukherjee SC, Pattnaik P (2006) Effect of multiple injections of Beta-glucan on non-specific immune response and disease resistance in Labeorohita fingerlings. Fish Shellfish Immunol20: 305-319.
- Ardo L, Yin G, Xu P, Váradi L, Szigeti G, et al. (2008) Chinese herbs (AstragalusmembranaceusandLonicerajaponica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromisniloticus) and resistance against Aeromonashydrophila. Aquaculture275: 26-33.
- Balfry SK, Higgs DA (2001)Influence of dietary lipid composition on the Immune system and disease resistance of finfish. Nutrition and Fish health book 213-234.
- Thompson I, WhiteA, Fletcher TC, Houlihan DF, Secombes CJ (1993) The effect of stress on the immune response of Atlantic salmon (Salmosalar L.) fed diets containing different amounts of vitamin C. Aquaculture114, 1-18.
- JeneyG, Anderson DP (1993)Glucan injection or bath exposure given alone or in combination with bacterin enhances the nonspecific defence mechanism in rainbow trout Oncorhynchusmykiss.Aquaculture116: 315-329.
- Nikl L, Evelyn TPT, Albright LJ (1993) Trials with an orally and immersion-administered beta-1, 3 glucan as an immunoprophylactic against Aeromonassalmonicida in juvenile chinook salmon Oncorhynchustshawytscha. Diseases of Aquatic Organisms17: 191-196.
- Smith VJ, Brown JH, Hauton C (2003) Immunostimulation in crustaceans: does it really protect against infection.Fish Shellfish Immunol15: 71-90.
- Siwicki AK (1989) Immunostimulating influence of levamisole on non-spacific immunity in carp (Cyprinuscarpio). Dev Comp Immunol13: 87-91.
- Ispir U, Dorucu M (2005) A Study on the Effects of Levamisole on the Immune System of Rainbow Trout (Oncorhynchusmykiss, Walbaum). Turk J Vet AnimSci29: 1169-1176.
- Findlay VL, Zilberg D, Munday BL (2000) Evaluation of levamisole as a treatment for amoebic gill disease of Atlantic salmon, SalmosalarL. Journal of Fish Diseases23: 193-198.
- Ispir U (2009) Prophylactic effect of levamisole on rainbow trout ( Oncorhynchusmykiss) against Yersiniaruckeri.Pesq Vet Bras29: 700-702.
- Kodama H, Hirota Y, Mukamoto N, Baba T, Azuma I (1993) Activation of rainbow trout (Oncorhynchusmykiss) phagocytes by muramyl dipeptide. Dev Comp Immunol17: 129-140.
- Nya EJ, Austin B (2010) Use of bacterial lipopolysaccharide (LPS) as an immunostimulant for the control of Aeromonashydrophilla infections in rainbow trout, Oncorhynchusmykiss (Walbaum).J applMicrobiol108: 686-694.
- Salati F, Hamaguchi M, Kusuda R (1987) Immune response of red sea bream to Edwardsiellatardaantigens. Fish Pathol22: 93-98.
- Neumann NF,Fagan D, Belosevic M (1995) Macrophage activating factors. secreted by mitogen stimulated goldfish kidney leucocytes synergies with bacterial lipopolysaccharide to induce nitric oxideproduction in teleost macrophages. Dev Comp Immunol19: 475-482.
- Karunasagar I, Otta SK, Karunasagar I, Joshna K (1996) Application of Vibrio vaccine in shrimp culture. Fishing Chimes16: 49-50.
- Kajita Y, Sakai M, Atsuta S, Kobayashi M (1992) Immunopotentiantion activity of Freund’s complete adjuvant in rainbow trout Oncorhynchusmykiss. Nippon Suisan Gakkaishi58: 433-437.
- Kawakami H, Shinohara N, Sakai M (1998) The Non-specific Immunostimulation and Adjuvant Effects of Vibrio anguillarumBacterin, M-glucan, Chitin and Freund's Complete Adjuvant against Pasteurellapiscicida Infection in Yellowtail. Fish Pathol33: 287-292.
- Horne MT, Poy M, PranthanpipatP(1995) Control of vibriosis in black tiger shrimp, Penaeusmonodon, by vaccination. In: The Third Asian Fisheries Forum (Eds. Chou, L. M. et al.). Asian Fisheries Society Manila, Philippines, 459-467.
- Norqvist A, Hagstrom A, Wolf-Watz H (1989) Protection of rainbow trout against vibriosis and furunculosis by the use of attenuated strains of Vibrio anguillarum. Appl Environ Microbiol55: 1400-1405.
- Sakai M, Yoshida T, Atsuta S, Kobayashi M (1995) Enhancement of resistance to vibriosis in rainbow trout, Oncorhynchusmykiss(Walbaum.), by oral administration Clostridium butyricumbacterin. J Fish Dis 18: 187-190.
- Young CH, Kaneda S, Mikami Y, Arai T, Igarashi K, et al. (1987) Protection activity induced by the bacterial vaccine, heat-killed ClosteridiumbutyricumagainstCandida albicansinfections in mice. JpnJMedMycol28: 262-269.
- Isogai H, Isogai E, Fujii N, Oguma K, Chang KL, et al. (1989) Biological effects of lipopolysaccharide from Acromobacterstenohalison lymphocytes and macrophages.Nihon JuigakuZasshi51: 1003-1010.
- Yoshida T, Sakai M, Kitao T, Khlil SM, Araki S, et al. (1993) Immunodulatory effect of the feremented products of chicken egg, EF203, on rainbow trout, Oncorhynchusmykiss.Aquaculture 109: 207-214.
- Pais R, Khushiramani R, Karunasagar I, Karunasagar I (2008) Effect of immunostimulants on the haemolymphhaemagglutinins of tiger shrimp Penaeusmonodon. Aquaculture Research39: 1339-1345.
- Selvaraj V, Sampath K, Sekar V (2005) Administration of yeast glucan enhances survival and some non-specific and specific immune parameters in carp (Cyprinuscarpio) infected with Aeromonashydrophila. Fish Shellfish Immunol19: 293-306.
- RobertsenB, RØrstad G, Engstad R, Raa J (1990) Enhancement of non-specific disease resistance in Atlantic salmon, Salmosalar L., by a glucan from Saccharomyces cerevisiae cell walls. J Fish Dis 13: 391-400.
- Anderson DP, Siwicki AK (1994) Duration of protection against aeromonasalmonicida in brook trout immunostimulated with glucan or chitosan by injection or immersion. ProgFish Culturist56: 258-261.
- Davis JF, Hayasaka SS (1984)The enhancement of resistance of the American eel, Anguilla rostrata Le Sueur, to a pathogenic bacterium Aeromonashydrophila, by an extract of the tunicate Ecteinascidiaturbinate.Journal of Fish Diseases7: 311-316.
- Sakai M, Kamiya H, Atusta S, Kobayashi M (1991) Immunodulatory effects on rainbow trout, Oncorhynchusmykiss, injected with the extract of abalone, Haliotisdiscushannai. JApplIchthyol7: 54-59.
- Minomol M (2005) Culture of Gold fish Carassiusauratus using medicinal plants having immunostimulant characteristics. M. Phil Dissertation, M. Sundaranar University, India.
- Yin G, Jency G, Racz T, XuP,Jun X, et al. (2006) Effect of two Chinese herbs (AstragalusradixandScutellaria radix) on non specific immune response of tilapia, Oreochromisniloticus. Aquaculture253: 39-47.
- Dugenci SK, Arda N, Candan A (2003) Some medicinal plants as immunostimulant for fish. JEthnopharmacol88: 99-106.
- Tewary A, Patra BC (2008) Use of Vitamin C as an immunostimulant-Effect on growth, nutritional quality, and immune response of Labeorohita (Ham.). Fish PhysiolBiochem34: 251-259.
- Villegas JG, Fukada H, Masumoto T, Hosokawa H (2006) Effect of Dietary Immunostimulants on Some Innate Immune Responses and Disease Resistance against Edwardsiellatarda Infection in Japanese Flounder (Paralichthysolivaceus). Aquaculture Science54: 153-162.
- Wise DJ, Tomasso JR, Gatlin DM, Bai SC, Blazer VS (1993) Effects of dietary selenium and vitamin E on red blood cell peroxidation, glutathione peroxidase activity, and macrophage superoxideanion production in channel catfish. J AquatAnimHealth5: 177-182.
- Pulsford AL, Crampe M, Langston A, Glynn PJ (1995) Modulatory effects of disease, stress, copper, TBT and vitamin E on the immune system of flatfish. Fish &Shellfish Immunol5: 631-643.
- Blazer VS, Wolke RE (1984) The effects of a-tocopherol on the immune response and non-specific resistance factors of rainbow trout (Salmogairdneri Richardson). Aquaculture37: 1-9.
- Sakai M, Kobayashi M, Kawauchi H (1996) In vitro activation of fish phagocytic cells by GH, prolactin and somatolactin. J Endocrinol151: 113-118.
- Olabuenaga SE (2000) Fish immune system. Gayana (Concepc.)64: 205-215.
- Fernández AB, de Blas I, Ruiz I (2002) Immunological system in Teleost. Cells and organs. Aquatic magazine, 16, Spanish.
- Ellis AE (1977) The leucocytes of fish: A review. Journal of Fish Biology 11:453-491.
- Campbell T, Murru F (1990) An introduction to fish hematology. The compendium-Small Animal12: 525-533.
- Ronald R (2001) Fish Pathology. 4thedition, Wiley 590.
- Hine PM (1992) The granulocytes of fish. Fish &Shellfish Immunology2: 79-88.
- Valentino S (2008) Basic fish immunology. Basic Science Review of Literature. Web of B-13DGlucan. Beta13DGlucan.org.
- Sealey WM (2000) Probiotics and immunostimulants. In: Encyclopedia of Aquaculture, (eds. R. R. Stickney ). John Wiley and Sons, Inc. New York, 676-680.
- Johansson MW, Soderhall K (1989) Cellular immunity in crustaceans and the proPO system. ParasitolToday5: 171-176.
- Smith VJ, Söderhäll K, Hamilton M (1984) ß-1.3-glucan induced cellular defense reaction in the shore crab, Carcinusmaenas. Comp BiochemPhysiol77: 636-639.
- Chen D, Ainsworth AJ (1992) Glucan administration potentiates immune defense mechanisms of channel catfish,IctaluruspunctatusRafinesque.J Fish Dis15: 295-304.
- Galeotti M, Volpatti D, Jeney G (1995) The nature of non-specific immune response of sea bass (Dicentrarchuslabrax) to Pasteurellapiscicida following bath exposure to levamisole. European Assoc Fish Pathologist Seventh IntConfPalma de Mallorca, Spain.
- Sung HH, Kou GH, Song YL (1994)Vibriosis resistance induced by glucan treatment in tiger shrimp (Penaeusmonodon). Fish Pathol29: 11-17.
- Gannam AL, Schrock RM (1999) Immunostimulants in fish diets.J ApplAqua9: 53-89.
- Yoshida T, Kruger R, Inglis V (1995) Augmentation of non-specific protection in African catfish, Clariasgariepinus (Burchell), by the long term oral administration of immunostimulants. J Fish Dis 18: 195-198.
- Perazzolo LM, Barracco MA (1997) Theprophenoloxidase activating system of the shrimp Penaeuspaulensis and associated factors. Dev Comp Immunol21:385-395.
- Dalmo RA, Seldjelid R (1995) The immunomodulatory effect of LPS, laminaran and sulphatedlaminaran [beta-(1,3)-D glucan] on Atlantic salmon, Salmosalar L., macrophages in vitro. Journal of Fish Diseases 18: 175-185.
- Dalmo RA, Bogwald J, Ingebrigsten K, Seljelid R (1996) The immunomodulatory effect of laminaran [beta-(1,3)-D-glucan] on Atlantic salmon, Salmosalar L. anterior kidney leucocytes after intraperitoneal, peroral, and peranal administration. Journal of Fish Diseases 19: 449-457.
- Strand HK, DalmoRA(1997) Absorption of immunomodulating beta-(1,3)-glucan in yolk-sac larvae of Atlantic halibut, Hippoglossushippoglosus (L.) fed diets containing different amounts of vitamin C. Aquaculture14: 1-17.
- Galeotti M (1998) Some aspects of the application of immunostimulants and a critical review of methods for their evaluation. J ApplIchthyol14: 189-199.
- Jorgensen JB, Robertson B (1995) Yeast beta-glucan stimulates respiratory burst activity of Atlantic salmon (Salmosalar L.) macrophages. Dev Comp Immunol19: 43-57.
- Tseng IT, Chen JC (2004) The immune response of white shrimp Litopenaeusvannamei and its susceptibility to Vibrio alginolyticus under nitrite stress. Fish Shellfish Immunol17: 325-333.
- Lopez N, Cuzon G, Gaxiola G, Taboada G, Valenzuela M, et al. (2003) Physiological, nutritional and immunological role of dietary ß 1-3 glucan and ascorbic acid 2-monophosphate in Litopenaeusvannameijuveniles. Aquaculture224: 223-243.
- Matsuyama H, Mangindaan REP, Yano T (1992) Protective effect of schizophyllan and scleroglucan against Streptococcus sp. infection in yellowtail (Seriolaquinqueradiata). Aquaculture101: 197-203.
- Rao YV, Das BK, Jyotrymayee P, Chakrabarti R (2006) Effect of Achyranthesaspera on the immunity and survival of Labeorohita infected with Aeromonashydrophila.Fish Shellfish Immunol20: 263-273
- Jorgensen JB, Lunde H, Robertsen B (1993a) Peritoneal and head kidney cell response to intraperitioneally injected yeast glucan in Atlantic salmon, (Salmosalar L.). J Fish Dis 16: 313-325.
- Ali A, Karunasagar I, Pais R, Tauro P (1996) Effect of yeast glucans on the immune response of Indian major carp Labeorohita. Abst37thAnn ConfAssocMicrobolIndia.
- Li P, Lewis DH, Galtin DM (2004) Dietary oligonucleotides from yeast RNA influences immune responses and resistance of hybrid striped bass (Moronechrysops x Moronesaxatilis) to Streptococcus iniae infection. Fish Shellfish Immunol16: 561-569.
- Aggett R, Leach JL, Rueda R, MacLean WC (2002) Innovation in infant formula development: a reassessment of ribonucleotides in 2002. Nutrition19: 375-384.
- Ramadan A, Afifi NA, Moustafa M, Samy AM (1994) The effect of ascogen on the immune response of tilapia fish to Aeromonashydrophilavaccine. Fish Shellfish Immunol4: 159-165.
- Sakai M, Taniguchi K, Mamoto K, Ogawa H, Tabata M (2001)Immunostimulant effects of nucleotide isolated from yeast RNA on carp CyprinuscarpioL. J Fish Dis24: 433-438.
- BurrellsC, William PD, Forno PE (2001) Dietary nucleotides: a novel supplement in fish feeds. 1. Effects on resistance to disease in salmonids. Aquaculture199: 159-169.
- Choudhury D, Pal AK, Sahu NP, Kumar S, Das SS, et al. (2005) Dietary yeast RNA supplementation reduces mortality by Aeromonas hydrophila in rohu (Labeo rohita L.) juveniles. Fish Shellfish Immunol19: 281-291.
- Sánchez-Pozo A, Gil A (2002) Nucleotides as semiessential nutritional components. Br J Nutr87: 135-137.
- Low C, Wadsworth S, Burrells C, Secombes CJ (2003) Expression of immune genes in turbot (Scophthalmusmaximus) fed a nucleotide-supplemented diet. Aquaculture221: 23-40.
- Kajita Y, Sakai M, Atsuta S, Kobayashi M (1990) The immunomodulatory effects of levamisole on rainbow trout, Oncorhynchusmykiss. Fish Pathol25: 93-98.
- Cuesta A, Esteban MA, Meseguer J (2003) In vitro effect of chitin particles as the innate cellular immune system of gilthead seabream(Sparusaurata L.). Fish Shellfish Immunol15: 1-11.
- Esteban MA, Rodriguez A, Cuesta A, Meseguer J (2005) Effects of lactoferrin on non-specific immune responses of gilthead seabream (Sparusauratus L.). Fish Shellfish Immunol7: 109-124.
- Fujiki K, Yano T (1997) Effects of sodium alginate on the non-specific defence system of the common carp (Cyprinuscarpio L.). Fish Shellfish Immunol7: 417-427.
- Bagni M, Romano N, Finoia MG, Abelli L, Scapigliati G, et al. (2005) Short- and long-term effects of dietary yeast ß-glucan (Macrogard) and alginic acid (Ergosan) preparation on immune response in sea bass (Dicentrarchuslabrax). Fish Shellfish Immunol18: 311-325.
- Ortuno J, Cuesta A, Rodriquez A, Esteban MA, Meseguer J (2002) Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response to gilthead seabream (Sparusaurata L.). Vet ImmunolImmunopathol85: 41-50.
- Rodriguez A, Cuesta A, Esteban MA, Meseguer J (2004) The effect of dietary administration of the fungus Mucorcircinelloides on non-specific immune responses of gilthead seabream. Fish Shellfish Immunol16: 241-249.
- Rao YV, Chakrabarti R (2005) Stimulation of immunity in Indian major carp Catlacatlawith herbal feed ingredients. Fish Shellfish Immunol18: 327-334.
- Sahoo PK, Mukherjee SC (2002) The effect of dietary immunomodulation upon Edwardsiellatardavaccination in healthy and immunocompromised Indian major carp (Labeorohita). Fish Shellfish Immunol12: 1-16.
- Verlhac V, Gabaudan J, Obach A, Schuep W, Hole R (1996) Influence of dietary glucan and vitamin C on non-specific and specific immune responses of rainbow trout (Oncorhynchusmykiss). Aquaculture143: 123-133.
- Wahli T, Verlhac V, Gabaudan J, Schuep W, Meier W (1998) Influence of combined vitamins C and E on non-specific immunity and disease resistance of rainbow trout, Oncorhynchusmykiss (Walbaum). J Fish Dis21: 127-137.
- Mulero V, Esteban MA, Meseguer J (1998) Effect of in vitro addition of exogenous vitamin C and E on gilthead seabream (Sparusaurata L.) Phagocytes. Vet ImmunolImmunopathol66: 185-199.
- Sahoo PK, Mukherjee SC (2003) Immunomodulation by dietary vitamin C in healthy and aflatoxinB1-induced immunocompromisedrohu (Labeorohita). Comp Immunol Micro Inf Dis 26: 65-76.
- KumarS, Sahu NP, Pal AK, Choudhury D, Yengkokpam S, et al. (2005) Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in L. rohita juveniles. Fish ShellfishImmunol19 : 331-344.
- Itami T, Takahashi Y, Tsuchihira, Igusa H, Kondo H (1994) Enhancement of disease resistance of kuruma prawn Penaeusjaponicus and increase in phagocytic activity of prawn haemocytes after oral administration of ß-1, 3-glucan (Schizophyllan). In: L. M. Chou, A. D. Munro, T. J. Lam, T. W. Chen, L. K. K. Cheong, J. K. Ding, K. K. Hooi, H. W. Khoo, V. P. E. Phang, K. F. Shim and C. H. Tan, (eds.), Third Asian fisheries forum, Asian Fisheries Society, Manila, Philippines, 375-378.
- Su MS, Liu KF, Chang CF, Liao IC (1995) Enhancement of grass prawn Penaeusmonodonpostlarvae viability by beta-1, 3-glucan from Schizophyllum commune. J Taiwan Fish Res 3: 125-132.
- Huang CC, Song YL (1999) Maternal transmission of immunity to white spot syndrome associated virus (WSSV) in shrimp (Penaeusmonodon). Dev Comp Immunol23: 545-552.
- Soderhall I, Bangyeekhun E, Mayo S, Soderhall K (2003) Hemocyte production and maturation in an invertebrate animal proliferation and gene expression in hematopoietic stem cells of Pacifastatuslenieusculus. Dev Comp Immunol27: 661-672.
- Misra CK, Das BK, Pradhan J, Pattnaik P, Sethi S, et al. (2004) Changes in lysosomal enzyme activity and protection against Vibrio infection in Macrobrachiumrosenbergii (De Man) post larvae after bath immunostimulation with ß-glucan. Fish Shellfish Immunol17: 389-395.
- Cheng W,Liu CH, Kuo CM, Chen JC (2005) Dietary administration of sodium alginate enhances the immune ability of white shrimp Litopenaeusvannamei and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol18: 1-12.
- Lightner DV, Hunter B, Magarelli PC, Colvin LB (1979) Ascorbic acid: nutritional requirement and role in wound repair in penaeid shrimp. Proc World MaricSoc10: 513-528.
- Merchie G, Kontara EKM, Lavens P, Robles R, Kurmaly K, et al. (1998) Effect of vitamin C and astaxanthin on stress and disease resistance of postlarval tiger shrimp Penaeusmonodon (Fabricius). Aquac Res 29: 579-585.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 37040
- [From(publication date):
November-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 29155
- PDF downloads : 7885
