Research Article Open Access
Immunoglobulin-binding Bacterial Proteins (IBP) Conjugates and their Reactivity with Immunoglobulin in Enzyme-Linked Immunosorbent Assays (ELISA)
Angel Justiz Vaillant1*, Norma McFarlane-Anderson2 , Brian Wisdom3 , Wayne Mohammed1 , Sehlule Vuma1 , Chalapathi Rao1 , Arvind Kurhade1 , Hellen Asemota2 , Shivnarine Kissoon1 and Geeta Kurhade4
1Department of Para-clinical Sciences, The University of the West Indies, Trinidad and Tobago
2Department of Basic Medical Sciences, The University of the West Indies, Jamaica
3School of Biology and Biochemistry, Medical Biology Centre, The Queen’s University of Belfast, UK
4Department of Pre-clinical Medical Sciences, The University of the West Indies, Trinidad and Tobago
- *Corresponding Author:
- Angel Justiz Vaillant
Department of Para-clinical Sciences
The University of the West Indies, St. Augustine
Trinidad and Tobago
Tel: +868-645-2640/9
E-mail: avail4883@gmail.com
Received date: September 11, 2013; Accepted date: November 25, 2013; Published date: November 27, 2013
Citation: Vaillant AJ, McFarlane-Andersonv N, Wisdom B, Mohammed W, Vuma S, et al. (2013) Immunoglobulin-binding Bacterial Proteins (IBP) Conjugates and their Reactivity with Immunoglobulin in Enzyme-Linked Immunosorbent Assays (ELISA). J Anal Bioanal Tech 4: 175. doi: 10.4172/2155-9872.1000175
Copyright: © 2013 Vaillant AJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
The aim of this study was to create universal chimeric conjugates able to react with both avian and mammalian immunoglobulins to be used as a reagent in Enzyme-linked Immunosorbent Assays (ELISAs). The periodate method was used in the conjugation process of linking horseradish peroxidase to immunoglobulin-binding bacterial proteins. ELISAs were used to prove the efficacy of the conjugates, namely newly synthesize conjugates (NSC) in the detection of immunoglobulins. All NSC bound to mammalian immunoglobulins, but failed to bind avian immunoglobulin Y (IgY), with the exception of the SpLAG-anti-IgY-HRP that was the most versatile binding to the all panel of purified immunoglobulin and sera. In conclusion, the results of these experiments show that SpLAG-anti-IgY-HRP conjugate, a novel reagent, was the most versatile product among NSC and commercially-available conjugates.
Keywords
Immunoglobulins; ELISA; Immunoglobulin-binding bacterial proteins; Protein conjugates
Introduction
Immunoglobulin-binding bacterial proteins (IBBP) are molecules that are widely found in the cell walls of several bacteria and they have the capacity to bind to the Fc or Fab regions of immunoglobulins from different mammalian species. The most well-known IBBP are: Staphylococcal protein A (SpA) [1], Streptococcal protein G (SpG) [2] and Peptostreptococcal protein L (SpL) [3]. They have been used linked to enzymes as immunodetection tools (conjugates) in Enzymelinked Immunosorbent Assays (ELISA) for detection of antigens and antibodies [4].
Peroxidase-(HRP) labelled IBBP are commercially available conjugates. They are considered rather than specific, universal conjugates (bind too many diverse immunoglobulins), including SpA-HRP, SpG-HRP, SpL-HRP, recombinant chimeric SpL-SpA-HRP (SpLA-HRP) [5,6], and some others, such as recombinant chimeric SpA-SpG-HRP (SpAG-HRP) and SpL-SpG-HRP (SpLG-HRP). None of the commercially-available conjugates react universally to both avian and mammalian immunoglobulins. Their binding to avian immunoglobulins is weak or they are non-reactive. In this study, we create a universal chimeric conjugate able to react with both avian and mammalian immunoglobulins to be used as a reagent in Enzymelinked Immunosorbent Assays (ELISAs): the SpLAG-anti-IgY-HRP. In addition, we prepared other chimeric conjugates to compare their affinity to mammalian IgG, including the SpLA-HRP, SpAG-HRP, SpLG-HRP, SpLAG-HRP and SpLA-LG-HRP that were produced by mixing their individual components. All homemade conjugates were named newly synthesized conjugates (NSC). As a part of the study, we compare the sensitivity of the homemade conjugates with that of commercially-prepared products.
Materials and Methods
Conjugate preparation (SpA-HRP, SpG-HRP and SpL-HRP)
All proteins, chemicals and buffers used in these protocols were commercially-available products (Sigma-Aldrich Co. St. Louis, Missouri).
Preparation of chimeric doubled conjugates
The SpAG-HRP conjugate was prepared by mixing 100 μl of both SpA-HRP and SpL-HRP. The SpLA-HRP conjugate was prepared by mixing 100 μl of both SpL-HRP and SpA-HRP. The SpLG-HRP conjugate was prepared by mixing 100 μl of both SpL-HRP and SpGHRP.
Preparation of chimeric tripled conjugates
The SpLAG-HRP conjugate was prepared by mixing 50 μl of each SpL-HRP, SpA-HRP and SpG-HRP. The SpLA-LG-HRP was prepared by mixing 50 μl of each SpLA-HRP, SpL-HRP and SpG-HRP.
Preparation of a chimeric quadrupled conjugate
The SpLAG-anti-IgY-HRP was prepared by mixing 50 μl of antichicken- IgY-HRP with 50 μl each of SpL-HRP, SpA-HRP and SpG-HRP.
Immunoglobulin-Y isolation
The IgY fraction was isolated from the egg yolks of a variety of birds, including chicken, bantam hen, guinea hen, quail, goose, duck, pigeon, parakeet, cattle egret, pheasant and ostrich. The IgY fraction was isolated by the chloroform-polyethylene glycol (PEG) method [7]. The eggs were washed with warm water and the egg yolk was separated from the egg white. The membrane was broken and the egg yolk collected and diluted 1:3 v/v in phosphate buffered saline (PBS), pH 7.4. To 1/3 of the egg yolk mixture, an equal volume of chloroform was added, the mixture was then shaken and centrifuged for 30 min (1000×g, RT). The supernatant was decanted and mixed with PEG 6000 (12%, w/v), stirred and incubated for 30 min at room temperature (RT). The mixture was then centrifuged, as previously described. The precipitate containing IgY was dissolved in PBS (pH 7.4) at a volume equivalent to 1/6 of the original volume of the egg yolk and dialyzed against 1L of PBS (pH: 7.4 for 24 h at 4°C). The IgY was removed from the dialysis tubing. IgY concentration was determined by the Bradford method [8]. IgY samples were stored at -20°C.
ELISAs
Briefly, the microplates were coated, 25 μg/well in 50 μl carbonatebicarbonate buffer pH 9.6 (Sigma) with different avian and mammalian immunoglobulins, 50 μl avian egg whites or 50 μl of sera from different animal species. Plates were washed 4X with 150 μl PBS-Tween 20 buffer (Sigma-Aldrich Co, St. Louis Missouri). Then, 100 μl of each NSC was added, diluted 1:1000 in PBS-non-fat milk to each well and incubated for 1 h at room temperature (RT). The plates were washed 4X with PBSTween. 100 μl of 3 mg/ml o-phenylenediamine solution (OPD) was added and the plates were incubated 15 minutes at RT. The reaction was stopped with 50 μl of 3M H2SO4 solution. The plates were visually assessed for the development of colour (indicating a positive result), and read in a microplate reader at 492 nm. A positive result was taken as equal or above the cut-off point (0.10-0.20 weakly interaction; 0.21- 0.30 moderate interaction and above 0.31 strong interaction; 1+, 2+ and 3+, respectively).
Results and Discussion
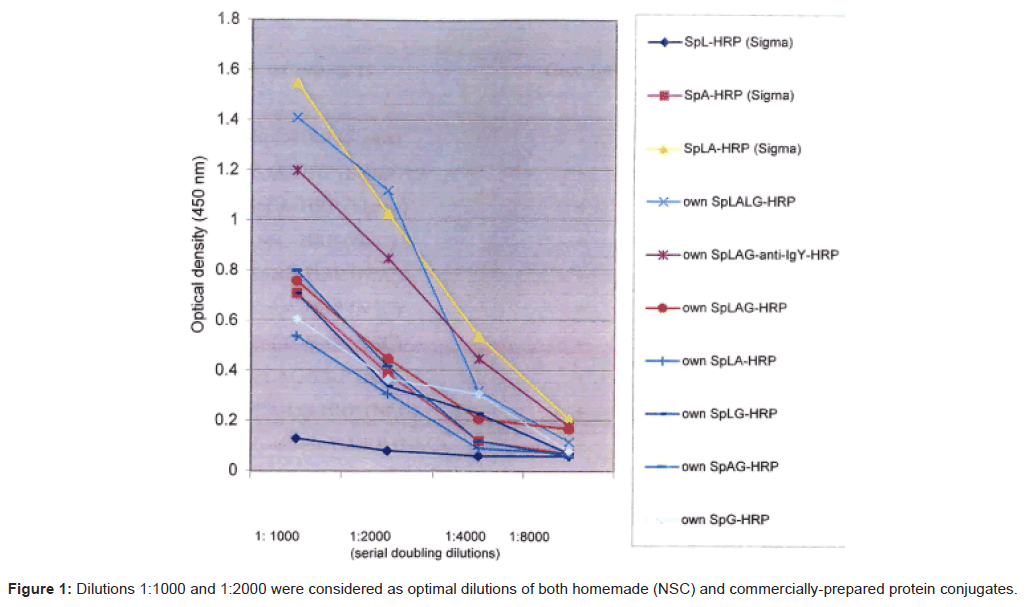
Figure 1 shows the working dilutions of both homemade and commercially-prepared conjugates. They worked well in dilution between 1:1000 and 1:2000. Some of them, such as the SpLA-HRP (Sigma) and the SpLAG-anti-IgY-HRP, gave good signal in dilutions of 1:4000. In addition, as shown in Table 1, the NSC reacted with goat and sheep IgGs. The chicken IgY was detected using the SpLAG-anti-IgYHRP conjugate only.
| Ig samples | SpLAG-HRP (NSC) Reactivity score | SpAG-HRP (NSC) Reactivity score | Anti-chicken IgY-HRP (Sigma) Reactivity score | SpLA-HRP (Sigma) Reactivity score |
|---|---|---|---|---|
| Ostrich IgY | 1+ | 1+ | 1+ | 1+ |
| Pheasant IgY | 0 | 0 | 1+ | 0 |
| Bantam hen IgY | 0 | 2+ | 2+ | 1+ |
| Duck IgY | 0 | 1+ | 1+ | 1+ |
| Guinea hen IgY | 0 | 0 | 1+ | 0 |
| Goose IgY | 0 | 0 | 1+ | 0 |
| Quail IgY | 0 | 0 | 1+ | 0 |
| Chicken IgY | 0 | 0 | 2+ | 0 |
| Pigeon egg white | 0 | 0 | 0 | 1+ |
| Pheasant egg white | 0 | 0 | 0 | 1+ |
| Duck egg white | 0 | 0 | 0 | 1+ |
| Bantam egg white | 0 | 0 | 0 | 1+ |
| Ostrich egg white | 1+ | 1+ | 0 | 1+ |
A positive result was taken as equal or above the cut-off point (0.20-0.30 weakly interaction; 0.31-0.40 oderate interaction and above 0.41 strong interaction; 1+, 2+ and 3+. respectively). Eight replicates of each sample were tested.
Table 1: Comparison of the reactivity of avian IgY with commercial and newly synthesized conjugates.
This paper discusses the interaction between IBBPs, which are proteins with the capacity to interact with immunoglobulins of mammalian species, and few avian IgYs. The importance of these protein interactions is that they can be useful reagents in the detection of specific antibodies. In addition, IBBPs might be useful in affinity chromatography for IgY purification from the blood or egg yolk of birds [9].
The NSC, including SpLA-HRP, SpLG-HRP, SpAG-HRP, SpLAGHRP and SpLA-LG-HRP proved to be efficient binding reagents with the capacities to react with Ig’s from a number of mammalian species. The reactivity of the NSC with avian Ig was more restricted than that of mammalian species. This limitation was not encountered when SpLAG-anti-IgY-HRP conjugate was used. This reacted with a wide range of avian and mammalian immunoglobulins. This is the first documented report of the successful preparation of a SpLAG-anti- IgY-HRP conjugate, which can be considered the most universal of these reagents. The broad reactivity of SpLAG-anti-IgY-HRP across mammalian and avian species would allow for its use in ELISAs and immunoblot analyses. The SpLAG-anti-IgY-HRP conjugate becomes even more important, since there are no commercially available conjugates that can be used for immunodetection in a wide variety of avian and mammalian species, e.g. in the study of zoonotic diseases [4]. This conjugate could be used for immunotesting of infectious diseases in zoo animals, without having the necessity of using species-specific ELISAs, which are expensive and restricted to a particular species.
Potential uses of the NSC include epidemiological surveys of infectious diseases affecting poultry, aiding in the selection of healthy animals for human consumption. For example, NSC could be used in ELISA for the diagnosis of avian diseases, including: infectious bronchitis, Newcastle disease, avian influenza virus infection, reticuloendotheliose virus infection and turkey rhinotrachetis virus infection, chicken anaemia agent, avian encephalomyelitis, avian leucosis/RSV infection and avian retroviruses.
Table 1 showed a direct ELISA that records the cross-reactivity of a commercial anti-chicken IgY-HRP conjugate with avian IgY extracted from the egg yolk. The anti-chicken IgY-HRP conjugate did not react with avian egg whites, because those did contain IgM molecules, but not IgY proteins. Tables 1 and 2 recorded the reactivity of a SpLA-LG-HRP conjugate and a SpLAG-HRP conjugate. These homemade conjugates binding capacity was comparable to that of the commercial SpLA-HRP conjugate. In addition, the comparison of the reactivities between NSCs and commercial conjugates showed that NSCs were more versatile.
| Samples | SpLA-LG-HRP (NSC) Reactivity score | SpLAG-anti-IgY-HRP (NSC) Reactivity score | SpLA-HRP (Sigma) Reactivity score | SpA-HRP (Sigma) Reactivity score | SpL-HRP (Sigma) Reactivity score |
|---|---|---|---|---|---|
| Pig IgG | 3+ | 3+ | 3+ | 2+ | 2+ |
| Rabbit IgG | 3+ | 3+ | 3+ | 2+ | 2+ |
| Goat IgG | 2+ | 2+ | 2+ | 1+ | 0 |
| Sheep IgG | 2+ | 2+ | 2+ | 1+ | 0 |
| Human IgG | 3+ | 3+ | 3+ | 3+ | 3+ |
| Mouse IgG | 3+ | 3+ | 3+ | 3+ | 3+ |
| Cat IgG | 2+ | 2+ | 2+ | 2+ | 0 |
| Skunk sera | 2+ | 2+ | 2+ | 2+ | 0 |
| Coyote sera | 2+ | 2+ | 2+ | 2+ | 0 |
| Raccoon sera | 3+ | 3+ | 2+ | 2+ | 3+ |
| Quail IgY | 0 | 1+ | 0 | 0 | 0 |
| Goose IgY | 0 | 1+ | 0 | 0 | 0 |
| Ostrich IgY | 1+ | 2+ | + | 1+ | 0 |
| Duck IgY | 0 | 1+ | 1+ | 1+ | 0 |
| Pheasant IgY | 0 | 1+ | 0 | 0 | 0 |
| Chicken IgY | 0 | 2+ | 0 | 0 | 0 |
| Cattle egret IgY | 0 | 1+ | 0 | 0 | 0 |
| Guinea hen IgY | 0 | 1+ | 0 | 0 | 0 |
| Bantam hen IgY | 0 | 2+ | + | 1+ | 0 |
| Pigeon IgY | 0 | 1+ | + | 1+ | 1+ |
A positive result was taken as equal or above the cut-off point (0.20-0.30 weakly interaction; 0.31-0.40 moderate interaction and above 0.41 strong interaction; 1+, 2+ and 3+, respectively). Eight replicates of each sample were tested.
Table 2: Comparison of the reactivity (moderate and high reactivities) of the newly synthesized conjugates and commercially available conjugates (Sigma) for the detection of purified or serum immunoglobulins.
Nygren and Hansson [10] had reported that the modified periodate method allowed the preparation of conjugated protein A, which when analyzed by SDS-polyacrylamide gel electrophoresis, showed the presence of polymeric conjugates of large molecular size. Justiz Vaillant et al. [9] reported similar results. Perel’man et al. [11] reported that the periodate method was efficient in the preparation of SpA-peroxidase conjugates, while the preparation of these immunochemicals failed when glutaraldehyde was used as a cross-linker. Yolken and Leister [12] synthesized and used a SpA-peroxidase conjugate in an ELISA. The results of this study suggest that the NSC were effective in their binding to immunoglobulins of different animal species. The development of conjugates, such as SpLAG-anti-IgY-HRP, is of general interest, and could be useful both as a tool in ELISA and western blot, but more importantly for studies of zoonotic diseases.
Conclusion
In conclusion, the results of these experiments show that SpLAGanti- IgY-HRP conjugate, a novel reagent, was the most versatile product among NSC and commercially-available conjugates. Potential uses of this conjugates are immunodiagnosis and Immunoglobulin detection.
Acknowledgement
We are grateful to The Biochemistry Department, Biotechnology Centre, The Microbiology Department University Hospital of the West Indies and the School of Graduate Studies and Research for support and funding.
Competing Interests
The authors declared that no competing interests exist.
References
- Richman DD, Cleveland PH, Oxman MN, Johnson KM (1982) The binding of staphylococcal protein A by the sera of different animal species. J Immunol 128: 2300-2305.
- Higgins DA, Cromie RL, Liu SS, Magor KE, Warr GW (1995) Purification of duck immunoglobulins: an evaluation of protein A and protein G affinity chromatography. Vet Immunol Immunopathol 44: 169-180.
- Björck L (1988) Protein L. A novel bacterial cell wall protein with affinity for Ig L chains. J Immunol 140: 1194-1197.
- Stöbel K, Schönberg A, Staak C (2002) A new non-species dependent ELISA for detection of antibodies to Borrelia burgdorferi s. l. in zoo animals. Int J Med Microbiol 291 Suppl 33: 88-99.
- Svensson HG, Hoogenboom HR, Sjöbring U (1998) Protein LA, a novel hybrid protein with unique single-chain Fv antibody- and Fab-binding properties. Eur J Biochem 258: 890-896.
- Nakane PK, Kawaoi A (1974) Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem 22: 1084-1091.
- Polson A (1990) Isolation of IgY from the yolks of eggs by a chloroform polyethylene glycol procedure. Immunol Invest 19: 253-258.
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248-254.
- Justiz Vaillant AA, Akpaka PE, McFarlane-Anderson N, Smikle MP, Wisdom B (2012) Purification of Immunoglobulin Y (IgY) from the Ostrich (Struthio camelus) by Staphylococcal Protein A (SpA) Affinity Chromatography. J Chromat Separation Techniq 3: 127.
- Nygren H, Hansson HA (1981) Conjugation of horseradish peroxidase to staphylococcal protein A with benzoquinone, glutaraldehyde, or periodate as cross-linking reagents. J Histochem Cytochem 29: 266-270.
- Perel'man EV, Bulk VF, Shchetinina EV, Noskov FS, Zhebrun AB (1986) [Choice of the method for isolating conjugates of staphylococcal protein A with peroxidase for immunoenzyme analysis]. Zh Mikrobiol Epidemiol Immunobiol 68-71.
- Yolken RH, Leister FJ (1981) Staphylococcal protein A-enzyme immunoglobulin conjugates: versatile tools for enzyme immunoassays. J Immunol Methods 43: 209-218.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 14660
- [From(publication date):
November-2013 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10055
- PDF downloads : 4605