Research Article Open Access
Immunization with DNA Vaccine Expressing Gn Coupled to C3d Prevents Clinical Symptoms of Infection and Protects Mice against an Aerosol Rift Valley Fever Virus Infection.
Nitin Bhardwaj1,2, Brooke R. Pierce1 and Ted M. Ross1,2*
1Center for Vaccine Research, University of Pittsburgh, Pittsburgh, PA, USA
2Department of Microbiology and Molecular Genetics, University of Pittsburgh, Pittsburgh, PA, USA
- *Corresponding Author:
- Ted M. Ross, Ph D
University of Pittsburgh
School of Medicine Center for Vaccine Research
9047 Biomedical Science Tower 3, 3501
Fifth Avenue Pittsburgh, PA 15261, USA
Tel: +1 412 648.8666
Fax: +1 412 624 4440
E-mail: tmr15@pitt.edu
Received Date: November 02, 2011; Accepted Date: December 20, 2011; Published Date: December 29, 2011
Citation: Bhardwaj N, Pierce BR, Ross TM (2012) Immunization with DNA Vaccine Expressing Gn Coupled to C3d Prevents Clinical Symptoms of Infection and Protects Mice against an Aerosol Rift Valley Fever Virus Infection. J Bioterr Biodef S3:006. doi:10.4172/2157-2526.S3-006
Copyright: © 2012 Bhardwaj N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
Rift Valley fever virus (RVFV) is the causative agent of Rift Valley fever (RVF) and is an emerging infectious disease of zoonotic potential. However, aerosolization of RVFV has been proposed as a potential bioweapon and most vaccines have not been tested against aerosolized RVFV challenge. Previously, two vaccine platforms (DNA plasmids and alphavirus replicons) expressing a soluble form of the RVFV Gn glycoprotein alone or fused to three copies of complement protein, C3d, protected mice against an intraperitoneal (IP) RVFV infection. In this study, both vaccine candidates were used to determine the protective efficacy against an aerosolized RVFV challenge. Each vaccine was administered to mice alone or in a heterologous prime/replicon boost strategy and anti-RVFV immune responses were assessed. DNA plasmids expressing Gn-C3d and alphavirus replicons expressing Gn elicited high titer neutralizing antibodies that were similar to titers elicited by the live-attenuated MP12 virus. However, only Gn-C3d- DNA vaccine completely protected mice against virulent aerosolized RVFV challenge. Most mice receiving replicon based vaccines succumbed to RVFV infection. Surprisingly, even though live-attenuated MP12 vaccine protected mice against IP challenge, MP12 did not provide complete protection against aerosolized RVFV infection. Therefore, vaccine candidates that are effective against peripheral challenge should be tested against aerosolized challenge to determine the complete protection profile, since any bioterrorism attack using RVFV would most likely be in the form of an aerosol.
Keywords
Rift Valley Fever Virus; Aerosol; Bioterrorism; Vaccine; DNA; Alphavirus
Introduction
Rift Valley fever virus (RVFV) is the etiologic agent of Rift Valley fever (RVF) characterized by widespread outbreaks of abortion storms and neonatal mortality in animals and flu like illness with occasional complications in humans [1]. Although mosquito bites have been implicated as the primary source of transmission, outbreaks and laboratory experiments have shown the importance of aerosol infection in human infection [2-6]. Following aerosolization, RVFV can remain viable for more than an hour under optimal conditions (half-life more than 77 min at 25°C and 30% relative humidity) [6]. Humans can be infected by aerosols generated during animal interaction such as slaughtering, handling aborted fetuses, performing necropsies, and conducting laboratory procedures. The potential for aerosolization of RVFV and the high morbidity and mortality associated with infection, even at low doses, has led to RVFV being listed as a potential bioterrorism weapon. In addition, US National Institute for Health has included RVFV in their list of Category A priority agents. (https://www.niaid.nih.gov/topics/BiodefenseRelated/Biodefense/research/Pages/CatA.aspx).
Once confined to the continent of Africa, new RVFV outbreaks have been reported from other countries like Saudi Arabia and Yemen [7]. This reflects a continuous risk for its emergence in newer susceptible areas including countries in the western world. It is noteworthy that prior to the first North American West Nile virus (WNV) outbreak in 1999, this arthropod-borne viral infection was not considered a threat in the USA. However, within a span of few years WNV spread across the country and now it is endemic in USA [8]. With a comparatively broader host and transmission vector range, RVFV is a potentially bigger biological threat to the livestock and human population in countries like the USA and other non-endemic areas of the world [9,10].
To date, there are no commercially available licensed vaccines. An inactivated RVFV vaccine (TSI-GSD-200) developed by the US Army elicits protective immunity in humans, however it requires multiple booster immunizations [11]. A live-attenuated vaccine based on RVFV Smithburn strain is used to vaccinate livestock in Africa [12]. However, this partially attenuated vaccine can cause abortions or teratology in pregnant animals and pathology in newborns [13,14]. Clone 13 is a plaque purified virus from a RVFV 74HB59 strain which was isolated from a RVFV infected person in Central Africa and has a natural deletion in S segment [15]. This deletion makes clone 13 attenuated in virulence and studies are underway to further characterize this strain for possible use as vaccines [16]. Another vaccine candidate, a mutagen-derived strain of the RVFV isolate, MP12, was developed for livestock and humans [17] however, it can cause teratogenic effects when administered to pregnant animals [18].
Only a few studies conducted by United States Army Medical Research Institute of Infectious Diseases (USAMRIID) have tested the ability of anti-RVFV vaccines (inactivated and live-attenuated) to protect against an aerosol RVFV exposure in rats and non-human primates [19-21]. Previous study with laboratory rodents showed that mice vaccinated with formalin inactivated RVFV vaccine protected mice against peripheral virus challenge, however, the elicited immune responses did not completely protect mice against an aerosolized RVFV exposure [22]. Mice vaccinated via an intraperitoneal (IP) route had a ~50% survival rate 14 days post infection [22]. In addition, 68% of vaccinated Wistar Furth rats survived as compared to 3% of nonvaccinated animals [19]. The differences in survival pattern post-aerosol challenge in animals that received similar vaccine could be attributed to the differences in species or immunization schedule.
The ability of RVFV to infect via aerosol route is an important concern whether by accidental or intentional release as a bioterrorism agent [23]. Therefore, candidate RVFV vaccines were tested for their ability to elicit protective immune responses against virulent RVFV administered via aerosol exposure. Recently, our group demonstrated the protective efficacy of two alternative vaccine candidates, an alphavirus replicon expressing RVFV glycoprotein, Gn, and a DNA plasmid expressing RVFV Gn fused to three copies of the C3d complement protein, Gn-C3d, against intraperitoneal virus challenge in mice [24]. In this study, the ability of these same vaccines to protect mice aerosolized RVFV challenge was assessed.
Materials and Methods
Plasmid DNA
pTR600, a eukaryotic expression vector, has been described previously [24]. Briefly, the vector was constructed to contain the cytomegalovirus immediate-early promoter (CMV-IE) plus intron A (IA) for initiating transcription of eukaryotic inserts and the bovine growth hormone polyadenylation signal (BGH poly A) for termination of transcription. The vector contains the Col E1 origin of replication for prokaryotic replication and the kanamycin resistance gene (Kanr) for selection in antibiotic media. A soluble form of Gn from RVFV isolate ZH548 (Genbank DQ380206) without the transmembrane and cytoplasmic tail was PCR amplified and cloned into the pTR600 vaccine vector either alone (Gn) or in frame with three tandem repeats of the mouse homologue of C3d (Gn-C3d) as described previously [24].
Replicons
A soluble form of RVFV Gn lacking the transmembrane and cytoplasmic tail (see above) was introduced behind the 26S subgenomic promoter of the VEE replicon plasmid pVR21 to make Rep-Gn as described previously [24]. VEE replicons expressing influenza hemagglutinin were used as negative controls (Rep-control). VEE replicon plasmids, as well as capsid and glycoprotein plasmids were linearized with Not I, replicon and helper transcripts were generated using mMessage mMachine T7 transcription kits (Ambion), and transcripts electroporated into BHK-21 cells to package replicon particles as described previously [25]. Following packaging, the replicons underwent two rounds of safety testing to ensure that no detectable replication competent virus was present [25,26] at which point the replicons were concentrated by ultracentrifugation through a 20% sucrose cushion and titered using polyclonal antiserum against the VEE nonstructural proteins. Expression of the truncated RVFV Gn protein from the replicon was confirmed by western blot with a Gn specific monoclonal antibody (RV5 3G2-1A) generously provided by Dr. George Ludwig, USAMRIID, Ft. Detrick, Frederick, MD, USA.
In vitro expression of vaccine plasmids
The human embryonic kidney cell line, 293T, was transfected (at 5x105 cells/transfection) with 5μg of DNA by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA.) according to the manufacturer’s guidelines. Supernatants were collected and stored at -20°C. Cell lysates were collected in 500μl of 1% Triton X-100 buffer and stored at -20°C.
To detect specific proteins in the cell supernatant, 1.5% of supernatant was diluted 1:2 in SDS sample buffer (Bio-Rad, Hercules, CA, USA) and loaded onto a 10% polyacrylamide–SDS gel. The resolved proteins were transferred onto a nitrocellulose membrane (Bio-Rad, Hercules, CA, USA) and incubated with a 1:5,000 dilution of anti-RVFV mouse sera in phosphate-buffered saline (PBS) containing 0.05% Tween 20 and 5% skim milk powder. After an extensive washing, bound mouse antibodies were detected by using a 1:5,000 dilution of horseradish peroxidase-conjugated goat anti-mouse antiserum and enhanced chemiluminescence (Amersham, Buckinghamshire, United Kingdom).
Animals and Immunizations
Six-to-eight week old female BALB/c mice (Harlan Sprague- Dawley, Indianapolis, IN, USA) were used for inoculations. Mice, housed with free access to food and water, were cared for under U.S. Department of Agriculture guidelines for laboratory animals. Mice were anesthetized with 0.03 to 0.04ml of a mixture of 5ml of ketamine HCl (100 mg/ml) and 1ml of xylazine (20 mg/ml). Gene gun immunizations were performed on shaved abdominal skin by using the hand-held Bio-Rad gene delivery system as described previously [24,27-30]. For DNA immunizations, mice were immunized three times at three week intervals with 2μg of DNA per 0.5mg of approximately 1-μm gold beads (Bio-Rad, Hercules, CA, USA) at a helium pressure setting of 400 lb/ in2. For replicon immunizations mice were given one dose at week 6 or three doses at weeks 0, 3, and 6 of 1 X 105 infectious unit (IU) of replicons by foot pad route [24]. Blood samples were collected at week 8 post-vaccination. A schematic of the vaccine regimen is listed in Table 1. Use of animals in this study was reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC).
| Vaccine groups | Week 0 | Week 3 | Week 6 | Route |
|---|---|---|---|---|
| Gn | Gn | Gn | Gn | GG |
| Gn-C3d | Gn-C3d | Gn-C3d | Gn-C3d | GG |
| Rep-Gn | Rep-Gn | Rep-Gn | Rep-Gn | FP |
| Gn-C3d/Rep-Gn | Gn-C3d | Gn-C3d | Rep-Gn | GG/FP |
| MP12 | MP12 | - | - | IP |
| DNA control | DNA control | DNA control | DNA control | GG |
| Rep control | Rep control | Rep control | Rep control | FP |
| Naives | - | - | - | - |
Groups of 6-8 week old female Balb/c mice were immunized by the indicated vaccines at weeks 0, 3 and 6. DNA vaccines (Gn, Gn-C3d, DNA control) were administered via gene gun (GG) route. Replicon vaccines (Rep-Gn, Rep control) were administered via foot pad (FP) route. MP12 vaccine was administered via intraperitoneal (IP) route. A group of naïve mice served as unvaccinated controls.
Table 1: Vaccine groups and vaccination regimen.
Live attenuated virus vaccine
The attenuated strain RVFV MP12 (MP12) and ZH501 was propagated and titrated using Vero cells. BALB/c mice (n=5) received a single intraperioteneal injection (i.p.) of MP12 (1 X 105 PFU) 8 weeks prior to infection [24].
Immunological assays
Endpoint ELISA was performed on collected serum samples to assess the anti-Gn immunoglobulin G (IgG) response as previously described [24]. Briefly, plates were coated with 100μl (~10000 pfu equivalent) of inactivated RVFV MP12 overnight at 4°C, blocked with 5% non-fat dry milk in PBS-T (1h) at 25°C, and then extensively washed with PBS-T. 1 X PBS-T contains 3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, 0.05% Tween 20, pH 7.4. Serial dilutions of mouse antisera were allowed to bind (1h) and the plates thoroughly washed with PBS-T. Subsequently, the primary antisera were detected by anti-mouse IgG conjugated to horseradish peroxidase (Bio-Rad, Hercules, CA, USA). The reaction was detected using tetramethybenzidine (TMB) substrate (Sigma, Saint Louis, MO, USA) (1 h) at 25°C. IgG isotypes and serum IgA were also assessed by ELISA as previously described [24]. The secondary antibodies specific for IgA (Southern Biotechnology, Birmingham, AL, USA) was used at varying concentrations determined by optimization.
Neutralizing antibody assays
Antibody-mediated neutralization of RVFV ZH501 was measured using plaque reduction and neutralization test (PRNT) [24]. Briefly, 100 PFU/0.1 ml of RVFV ZH501 was mixed with serial two fold dilutions of heat inactivated (60° C for 30 min) serum samples in 96-well tissue culture plates. Virus-serum mixtures were incubated at 4°C overnight and placed into duplicate 23-mm wells (0.1ml/well) containing confluent monolayers of Vero cells (2 X 105). Cells were incubated for 1h at 37°C and 5% CO2 and overlaid with nutrient medium containing 0.8% agar, 5% fetal bovine serum, 200U penicillin/ml, and 200mg streptomycin/ml. The plates were incubated at 37°C and 5% CO2. After 4 days of incubation, cells were fixed with 10% formalin and stained with 1% crystal violet for visualization of plaques. The neutralizing antibody titer of a serum was considered positive at the highest initial serum dilution that inhibited >50% of the plaques as compared to the virus control titration.
Exposure system and aerosol generation
Mice were exposed to RVFV aerosols in whole-body exposure chambers housed within Class III biological safety cabinets maintained under negative pressure (-1 WC”), as previously described [31]. The animals were exposed inside a whole-body chamber which could contain up to four smaller stainless steel mesh restraint cages holding approximately 10 mice/cage or two guinea pigs/cage. The animal exposures were done with ~1000 PFU targeted dose of RVFV ZH501 that lasted for 30 min. A Collison nebulizer (BGI Inc., Waltham, MA) was used to generate the smaller (1 μm) particles. Exposure concentration, expressed in plaque-forming units (PFU)/ml, was determined by isokinetic sampling of the chamber with an all-glass impinger (AGI; Ace Glass, Vineland, NJ). DMEM medium with 3% sera w/v (Sigma, St. Louis, MO) was used to collect medium in the impinger. The inhaled virus dose was estimated by using Guyton’s formula [32]. Post-challenge, mice were housed in sealed negative-ventilation bio-containment units (Allentown Inc., Allentown, NJ, USA). All manipulations with infected mice and/or samples involving RVFV ZH501 were performed under strict BSL-3 enhanced conditions. The animals were examined twice daily for visual signs of morbidity or mortality, using a lab validated scoring system as previously described [24]. Mice were observed for clinical symptoms that ranged from lethargy, ruffled fur, and weight loss to neurological manifestations, such as hind-limb paralysis or circling. Mice found in a moribund condition were euthanized.
Results
Anti-RVFV antibody responses
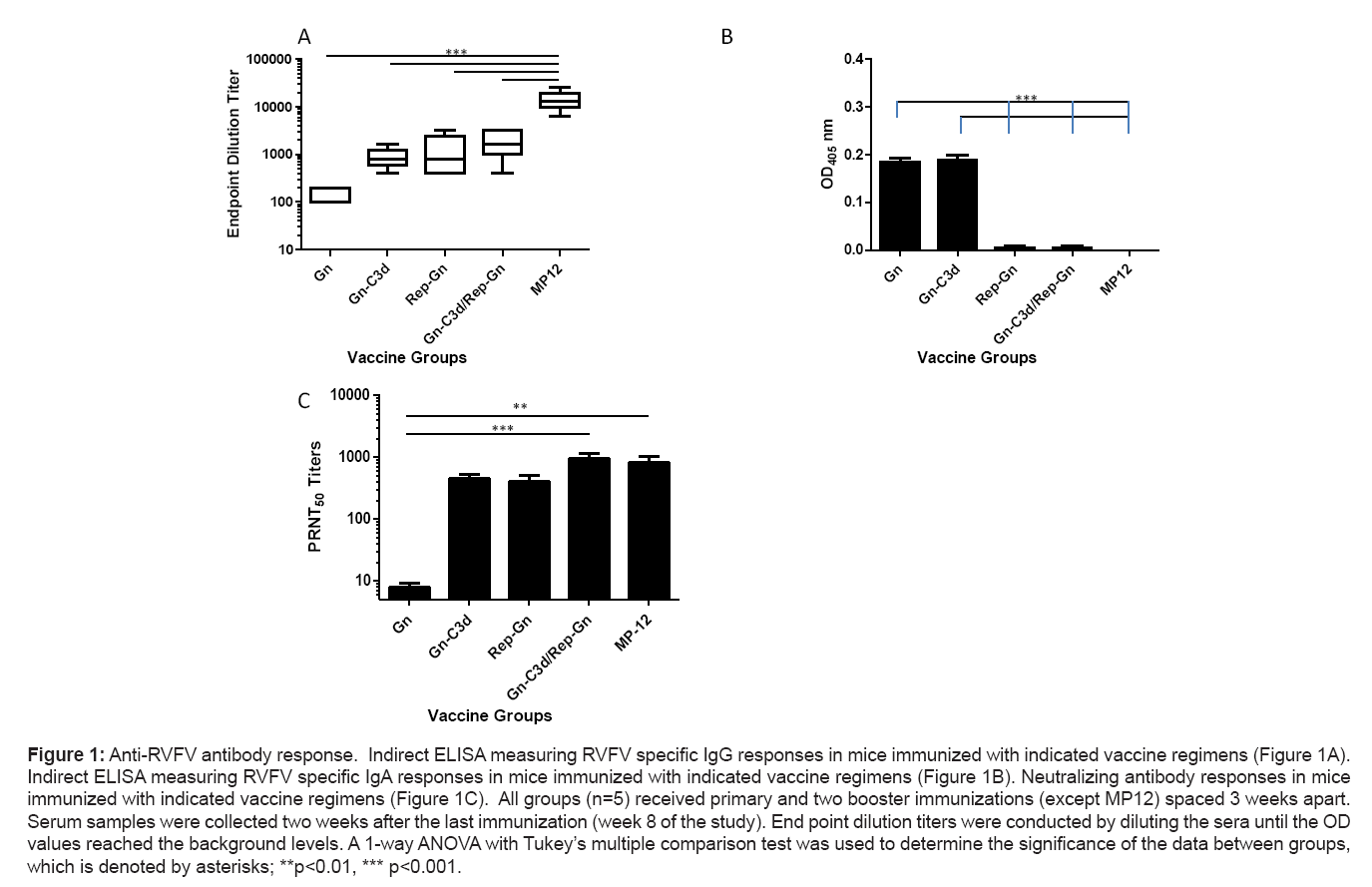
A truncated, soluble form of Gn from the RVFV isolate ZH548 alone or fused to three copies of murine C3d (Gn-C3d) efficiently secreted from cells transfected with DNA plasmid and replicon vector as previously described [24]. After 3 vaccinations, mice vaccinated with DNA plasmid expressing Gn elicited anti-Gn antibodies (1:160), however, the fusion of C3d to Gn enhanced the anti-Gn antibodies (1:880), while mice vaccinated with replicons expressing Gn (Rep-Gn) had an average anti-Gn titer of 1:1280 (Figure 1A). Mice vaccinated twice with Gn-C3d-DNA and then administered a single inoculation of replicon expressing Gn had higher titers than DNA or replicon alone (1:2000). These antibody responses were lower than the responses generated from the sera of mice immunized with live attenuated RVFV MP12 (1:12800). Only mice immunized with Gn or Gn-C3d based DNA vaccines had detectable IgA titers at week 8 of the study (Figure 1B). These titers were specific to the Gn antigen, since controls (DNA plasmid with no insert and replicons expressing the influenza virus hemagglutinin) did not elicit anti-Gn antibodies (data not shown).
Figure 1: Anti-RVFV antibody response. Indirect ELISA measuring RVFV specific IgG responses in mice immunized with indicated vaccine regimens (Figure 1A). Indirect ELISA measuring RVFV specific IgA responses in mice immunized with indicated vaccine regimens (Figure 1B). Neutralizing antibody responses in mice immunized with indicated vaccine regimens (Figure 1C). All groups (n=5) received primary and two booster immunizations (except MP12) spaced 3 weeks apart. Serum samples were collected two weeks after the last immunization (week 8 of the study). End point dilution titers were conducted by diluting the sera until the OD values reached the background levels. A 1-way ANOVA with Tukey’s multiple comparison test was used to determine the significance of the data between groups, which is denoted by asterisks; **p<0.01, *** p<0.001.
To evaluate the presence of virus neutralizing antibodies, week 8 sera from vaccinated or control mice was tested in a standard plaque reduction and neutralization test. Sera from mice immunized with Gn- C3d or Rep-Gn neutralized (PRNT50) RVFV ZH501 generating titers of 1:448 and 1:400 respectively, while priming mice with Gn-C3d- DNA and then boosting with Rep-Gn elicited statistically significant higher titers (1:960) compared to titers achieved in response to Gn immunization (Figure 1c). Mean neutralizing titers of 1:832 were seen with mice vaccinated with the live attenuated MP12 vaccine these titers were not statistically significant from titers achieved with Gn-C3d or Gn-C3d/Rep-Gn vaccination. In contrast, serum samples collected from Gn vaccinated mice had statistically significant lower PRNT50 titers (1:8) when compared with titers in response to MP12 vaccinations (Figure 1c).
Protective efficacy of DNA and replicon vaccines against RVFV aerosol challenge
The mice were challenged two weeks after final vaccination with a lethal dose (1000 PFU) of RVFV ZH501 in a whole body exposure. All the mice vaccinated with Gn-C3d-DNA were protected from virulent virus challenge with no body weight loss or development of clinical symptoms (Figure 3a). All mice that received DNA expressing the Gn only displayed ruffled fur and had enhanced mortality (Figure 2a and Figure 3a). Surprisingly, 40% of mice immunized with MP12 and then challenged with RVFV ZH501 survived lethal challenge and all showed clinical symptoms of infection (Figure 2b and Figure 3b). A similar result was observed with mice immunized with Rep-Gn alone or in Gn-C3d-DNA prime/Rep-Gn boost (Table 2). Unvaccinated naïve mice had severe signs of infection and body weight loss that resulted in all mice succumbing to infection by day 7 post-challenge (Figure 3b and Figure 2b). Mice that received DNA and replicon controls or naïve mice displayed clinical symptoms of infection such as ruffled fur, lethargy, hunched appearance and paralysis (Table 2). In addition to the above clinical symptoms of infection one mouse from the Rep-Gn and MP12 vaccinated group and 3 mice from DNA and replicon control groups exhibited clinical symptoms of CNS involvement characterized by hind limb paralysis (Table 2). None of the mice vaccinated with the Gn-C3d DNA plasmid showed clinical symptoms of CNS involvement.
Figure 2: Post-exposure survival against aerosolized RVFV challenge. Mice vaccinated with indicated test vaccines (Figure 2A) or appropriate controls, live-attenuated MP12 vaccine, DNA plasmid with no insert (DNA control) and replicon expressing influenza HA (replicon control) (Figure 2B) were challenged with 1000 PFU of RVFV ZH501 and monitored for mortality daily postaerosolized RVFV challenge.
Figure 3: Post-exposure sickness score against aerosolized RVFV challenge. Mice immunized with indicated vaccines (Figure 3A) or appropriate controls, live-attenuated MP12 vaccine, DNA plasmid with no insert (DNA control) and replicon expressing influenza HA (replicon control) (Figure 3B) were challenged with 1000 PFU of RVFV ZH501 and monitored for clinical symptoms associated with RVFV infection and mortality daily post-challenge. A two-way ANOVA with Bonferroni’s post-tests was used to determine the significance of sickness score data between different groups, which is denoted by asterisks; **P<0.01, *** P<0.001.
| GROUPS | RUFFLED FUR | LETHARGY | HUNCHED | PARALYTIC SIGNS | DEAD/ EUTHANIZED |
|---|---|---|---|---|---|
| Gn(3) | 5/5 | 3/5 | 2/5 | 0/5 | 3/5 |
| Gn-C3d(3) | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 |
| Rep-Gn(3) | 5/5 | 1/5 | 0/5 | 1/5 | 3/5 |
| Gn-C3d(2)/Rep-Gn(1) | 3/5 | 1/5 | 1/5 | 0/5 | 3/5 |
| MP 12(1) | 5/5 | 2/5 | 1/5 | 1/5 | 3/5 |
| DNA control (3) | 5/5 | 5/5 | 0/5 | 2/5 | 3/5 |
| Rep control (3) | 4/5 | 2/5 | 3/5 | 1/5 | 5/5 |
| Mock vaccinated | 3/5 | 2/5 | 2/5 | 0/5 | 5/5 |
Groups of 6-8 week old female Balb/c mice were immunized by the indicated vaccines or appropriate controls were challenged by aerosolized RVFV ZH501. Postchallenge mice were observed for indicated clinical signs of infection. Numbers in the parentheses indicates the number of vaccinations and the numbers without parentheses indicates the number of mice displaying indicated clinical profile out of a total 5 animals per group.
Table 2: Clinical profile of mice post-aerosol challenge.
Discussion
Rift Valley fever virus is an important biological threat that has the potential to cause widespread damage in terms of animal/human mortality and by imposing international animal trade restrictions thus crippling the economic growth in non-endemic countries [23]. Due to its stability and infectivity, aerosolized RVFV could conveniently be used as a bioterror agent, thus posing a great threat to livestock and human populations. The candidate DNA and replicon vaccines used in this study were previously shown to protect mice against a peripheral RVFV challenge [24]. In this report, these same vaccines were tested for their ability to protect against an aerosolized RVFV challenge.
Even though some of the mice that received candidate vaccines or the live attenuated MP12 vaccine were not protected against aerosol challenge, at least 40% of the mice survived the challenge (Figure 2). Mice that received Gn-C3d-DNA vaccine were completely protected with no clinical signs of infection (Figure 3 and Table 2). The live attenuated MP12 vaccine provides complete protection against IP challenge in mice, but against an aerosol challenge, partial protection was achieved (Figure 2b and Table 2). In contrast, the MP12 vaccine completely protected against RVFV aerosol challenge in macaques [33]. In the mouse model, MP12 may not be able to elicit complete protection at the mucosal surface. In addition variation in animal species, vaccine dose, and challenge virus dose between studies could explain the differences in the outcome. Interestingly, the level of protection conferred by these candidate vaccines post-aerosol challenge seems to differ from the protection achieved post-IP challenge [24].
In unvaccinated mice, the mean time to death was shifted from 3 days post-infection after IP challenge [24] to ~5 days post-infection after aerosol challenge. Infection of the lung mucosa by RVFV aerosols results in hepatitis [1,34-37] and can also cause CNS pathology [2,19,22]. A few mice in the control, MP12, and replicon groups had clinical symptoms of CNS involvement as observed as hind limb paralysis (Table 2).
Why the Gn-C3d DNA vaccine protected against aerosolized RVFV infection and the MP12 and replicon-based vaccines did not is not clear. C3d and its derivative, P28, are well characterized as molecular adjuvants [38-40]. The power of this molecule is that it allows for the effective secretion of protein, but it does reduce the overall expression levels of the conjugated antigen by ~10 fold. However, C3d enhances the immune responses of the antigen over non-conjugated antigens even at reduced protein levels. The serum neutralizing antibody titers against RVFV correlates with prevention of clinical symptoms and protection against parenteral virus challenge [24,26,41,42]. Serum neutralizing antibodies are correlated with the protection from aerosolized RVFV infection in rats [19]. In our study, there was a correlation between neutralizing antibody titers and protection against weight loss. However, it was not predictive of complete protection without development of clinical signs. In spite of higher neutralizing antibody titers, we observed clinical symptoms and mortality in the group of mice that received MP12 vaccine (Table 2). Only mice that received Gn-C3d vaccine had complete protection without development of clinical signs. These results differ from the recently published data on the MP12 induced protection against aerosol RVFV challenge in non-human primates [20,21]. It seems factors other than virus neutralizing antibodies play a significant role in conferring protection against aerosolized infection as was observed in Venezuelan Equine Encephalitis (VEE) vaccine mouse models [43]. Passive transfer of sera from hamsters immunized with the C-84 VEE virus vaccine to naïve hamsters were resistant to VEE virus challenge via subcutaneous route, but they remained susceptible to aerosolized VEE virus infection [43].
Among various antibody isotypes in the serum, IgA is the second most prevalent antibody isotype after IgG [44]. We observed serum IgA titers specific to Gn in mice receiving the DNA vaccines (Gn, Gn-C3d), however, mice vaccinated with MP12 or controls did not have detectable serum IgA titers (Figure 1b). Studies in the past have shown the protection of mice experimentally infected with rotavirus correlates with the production of mucosal and serum IgA [45,46]. Gn- C3d was the only vaccine group that had high neutralizing antibody and serum IgA titers. Antigen presentation plays a significant role in gene-based immunization as they induce MHC class I and II-based antigen presentation of native antigens [47]. Induction of serum IgG and IgA antibody profiles along with cell-mediated immunity has been observed with DNA immunization of cattle with Bovine Herpesvirus 1 (BHV-1) vaccine [48]. Intradermal DNA delivery with the help of gene gun is advantageous as it targets keratinocytes and Langerhans cells; professional antigen presenting cells (APCs) which are capable of secreting cytokines thus stimulating T cell help and induction of antigen specific humoral and cell mediated immune responses [47]. C3d has been shown to stimulate T helper responses [46], as well as stimulating auto-reactive C3d peptide-specific helper T cells which we term ‘co-signal 2’ during antigen presentation [39,49]. However in light of the present experimental results, this could possibly explain the complete protection achieved in Gn-C3d mice group. It however must carefully be interpreted, that various host factors, including innate immunity, might serve as one of the correlates of protection against infection with any pathogen. In summary, our data in the past and data from the present study reflect the potential for this candidate vaccine to elicit protective immune responses against parenteral and mucosal routes of RVFV exposure.
Acknowledgements
The authors would like to acknowledge Doug Reed for assistance with aerosolization of RVFV and we thank Mark Heise for providing the replicon vectors. This research was supported by an award from NIH/NIAID R01AI074946 grant to TMR.
References
- Schmaljohn CS, Hooper JW (2001) Bunyaviridae: The viruses and their replication, in Fields' Virology. Lippincott Williams & Wilkins: Philadelphia.
- Brown JL, Dominik JW, Morrissey RL (1981) Respiratory infectivity of a recently isolated Egyptian strain of Rift Valley fever virus. Infect Immun 33: 848-853.
- Keefer GV, Zebarth GL, Allen WP (1972) Susceptibility of dogs and cats to Rift Valley fever by inhalation or ingestion of virus. J Infect Dis 125: 307-309.
- Miller WS, Demchak P, Rosenberger CR, Dominik JW, Bradshaw JL (1963) Stability and infectivity of airborne yellow fever and Rift Valley fever viruses. Am J Hyg 77: 114-121.
- Francis T, Magill TP (1935) Rift Valley Fever: A Report of Three Cases of Laboratory Infection and the Experimental Transmission of the Disease to Ferrets. J Exp Med 62: 433-448.
- Hoogstraal H, Meegan JM, Khali GM, Adham FK (1979) The Rift Valley fever epizootic in Egypt 1977-78. 2. Ecological and entomological studies. Trans R Soc Trop Med Hyg 73: 624-629.
- Ahmad K (2000) More deaths from Rift Valley fever in Saudi Arabia and Yemen. Lancet 356: 1422.
- Murray KO, Mertens E, Despres P (2010) West Nile virus and its emergence in the United States of America. Vet Res 41: 67.
- Turell MJ, Dohm DJ, Mores CN, Terracina L, Wallette DL Jr, et al. (2008) Potential for North American mosquitoes to transmit Rift Valley fever virus. J Am Mosq Control Assoc 24: 502-507.
- Turell MJ, Linthicum KJ, Patrican LA, Davies FG, Kairo A, et al. (2008) Vector competence of selected African mosquito (Diptera: Culicidae) species for Rift Valley fever virus. J Med Entomol 45: 102-108.
- Pittman PR, Liu CT, Cannon TL, Mangiafico RS, Mangiafico JA, et al. (1999) Immunogenicity of an inactivated Rift Valley fever vaccine in humans: a 12-year experience. Vaccine 18: 181-189.
- Meadors GF 3rd, Gibbs PH, Peters CJ (1986) Evaluation of a new Rift Valley fever vaccine: safety and immunogenicity trials. Vaccine 4: 179-184.
- Kamal SA (2009) Pathological studies on postvaccinal reactions of Rift Valley fever in goats. Virol J 6: 94.
- Botros B, Omar A, Elian K, Mohamed G, Soliman A, et al. (2006) Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J Med Virol 78: 787-791.
- Muller R, Saluzzo JF, Lopez N, Dreier T, Turell M, et al. (1995) Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg 53: 405-411.
- Thiongane Y (2003) Pathogenicity and immunogenicity of the reassortant attenuated strain R566 of Rift Valley fever virus in sheep, in FAO/IAEA International Symposium on Applications of Gene-based Technologies for Improving Animal Production and Health in Developing Countries. Vienna, Austria.
- Caplen H, Peters CJ, Bishop DH (1985) Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol 66: 2271- 2277.
- Hunter P, Erasmus BJ, Vorster JH (2002) Teratogenicity of a mutagenised Rift Valley fever virus (MVP 12) in sheep. Onderstepoort J Vet Res 69: 95-98.
- Anderson GW Jr, Lee JO, Anderson AO, Powell N, Mangiafico JA, et al. (1991) Efficacy of a Rift Valley fever virus vaccine against an aerosol infection in rats. Vaccine 9: 710-714.
- Morrill JC, Peters CJ (2011) Protection of MP-12-Vaccinated rhesus macaques against parenteral and aerosol challenge with Virulent rift valley fever virus. J Infect Dis 204: 229-236.
- Morrill, JC, Peters CJ (2011) Mucosal immunization of rhesus macaques with Rift Valley Fever Mp-12 vaccine. J Infect Dis 204: 617-625.
- Anderson AO, Snyder LF, Pitt ML, Wood OL (1988) Mucosal priming alters pathogenesis of Rift Valley fever. Adv Exp Med Biol 237: 717-723.
- Sidwell RW, Smee DF (2003) Viruses of the Bunya- and Togaviridae families: potential as bioterrorism agents and means of control. Antiviral Res 57: 101- 111.
- Bhardwaj N, Heise MT, Ross TM (2010) Vaccination with DNA plasmids expressing Gn coupled to C3d or alphavirus replicons expressing gn protects mice against Rift Valley fever virus. PLoS Negl Trop Dis 4: e725.
- Balasuriya UB, Heidner HW, Davis NL, Wagner HM, Hullinger PJ, et al. (2002) Alphavirus replicon particles expressing the two major envelope proteins of equine arteritis virus induce high level protection against challenge with virulent virus in vaccinated horses. Vaccine 20: 1609-1617.
- Heise MT (2009) An alphavirus replicon-derived candidate vaccine against Rift Valley fever virus. Epidemiol Infect 137: 1309-1318.
- Pertmer TM (1995) Gene gun-based nucleic acid immunization: elicitation of humoral and cytotoxic T lymphocyte responses following epidermal delivery of nanogram quantities of DNA. Vaccine 13: 1427-1430.
- Green TD, Montefiori DC, Ross TM (2003) Enhancement of antibodies to the human immunodeficiency virus type 1 envelope by using the molecular adjuvant C3d. J Virol 77: 2046-2055.
- Haynes JR (1994) Accell particle-mediated DNA immunization elicits humoral, cytotoxic, and protective immune responses. AIDS Res Hum Retroviruses 10 Suppl 2: S43-45.
- Pertmer TM, Robinson HL (1999) Studies on antibody responses following neonatal immunization with influenza hemagglutinin DNA or protein. Virology 257: 406-414.
- Roy CJ (2003) Impact of inhalation exposure modality and particle size on the respiratory deposition of ricin in BALB/c mice. Inhal Toxicol 15: 619-638.
- Guyton AC (1947) Measurement of the respiratory volumes of laboratory animals. Am J Physiol 150: 70-77.
- Morrill JC, Peters CJ (1947) Protection of MP-12-Vaccinated Rhesus Macaques Against Parenteral and Aerosol Challenge With Virulent Rift Valley Fever Virus. J Infect Dis 204: 229-236.
- Smith DR (2010) The pathogenesis of Rift Valley fever virus in the mouse model. Virology 407: 256-267.
- Flick R, Bouloy M (2005) Rift Valley fever virus. Curr Mol Med 5: 827-834.
- Mims CA (1956) Rift Valley Fever virus in mice. I. General features of the infection. Br J Exp Pathol 37: 99-109.
- Wilson ML (1994) Rift Valley fever in rural northern Senegal: human risk factors and potential vectors. Am J Trop Med Hyg 50: 663-675.
- Bower JF, Ross TM (2006) A minimum CR2 binding domain of C3d enhances immunity following vaccination. Adv Exp Med Biol 586: 249-264.
- Bower JF, Sanders KL, Ross TM (2005) C3d enhances immune responses using low doses of DNA expressing the HIV-1 envelope from codon-optimized gene sequences. Curr HIV Res 3: 191-198.
- Bower JF (2004) Elicitation of neutralizing antibodies with DNA vaccines expressing soluble stabilized human immunodeficiency virus type 1 envelope glycoprotein trimers conjugated to C3d. J Virol 78: 4710-4719.
- Spik K, Shurtleff A, McElroy AK, Guttieri MC, Hooper JW, et al. (2006) Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tickborne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine 24: 4657-4666.
- Gorchakov R, Volkova E, N Yun, Petrakova O, Linde NS, et al. (2007) Comparative analysis of the alphavirus-based vectors expressing Rift Valley fever virus glycoproteins. Virology 366: 212-225.
- Jahrling PB, Stephenson EH (1984) Protective efficacies of live attenuated and formaldehyde-inactivated Venezuelan equine encephalitis virus vaccines against aerosol challenge in hamsters. J Clin Microbiol 19: 429-431.
- Woof JM, Kerr MA (2006) The function of immunoglobulin A in immunity. J Pathol 208: 270-282.
- Burns JW, Krishnaney AA, Vo PT, Rouse RV, Anderson LJ, et al. (1995) Analyses of homologous rotavirus infection in the mouse model. Virology 207: 143-153.
- Feng N, Burns JW, Bracy L, Greenberg HB (1994) Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol 68: 7766-7773.
- Falo LD Jr (1999) Targeting the skin for genetic immunization. Proc Assoc Am Physicians 111: 211-219.
- Loehr BI,Willson P, Babiuk LA, Van Den Hurk VDLS (2000) Gene gun-mediated DNA immunization primes development of mucosal immunity against bovine herpesvirus 1 in cattle. J Virol 74: 6077-6086.
- Knopf PM, Rivera DS, Hai SH, McMurry J, Martin W, et al. (2008) Novel function of complement C3d as an autologous helper T-cell target. Immunol Cell Biol 86: 221-225.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 14039
- [From(publication date):
specialissue-2012 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 9441
- PDF downloads : 4598