Research Article Open Access
Identification and Reduction of Matrix Effects Caused by Solutol Hs15 in Bioanalysis Using Liquid Chromatography/Tandem Mass Spectrometry
Vijaya Bhaskar V1*, Anil Middha2, Sudhir Tiwari1 and Savithiri Shivakumar1
1DMPK Laboratory (Biology Division), GVK BIO, Hyderabad, India
2Department of Pharmacy, Jagadishprasad Jhabermal Tibrewala University, Rajasthan, India
- *Corresponding Author:
- Veeravalli Vijaya Bhaskar
DMPK Laboratory (Biology Division)
GVK BIO, Nacharam, Hyderabad
Andhra Pradesh, India-500076
Tel: +918143853440
E-mail: veeravalli.bhaskar@gmail.com
Received date: March 11, 2013; Accepted date: April 19, 2013; Published date: April 23, 2013
Citation: Vijaya Bhaskar V, Middha A, Tiwari S, Shivakumar S (2013) Identification and Reduction of Matrix Effects Caused by Solutol Hs15 in Bioanalysis Using Liquid Chromatography/Tandem Mass Spectrometry. J Anal Bioanal Tech 4:166. doi: 10.4172/2155-9872.1000166
Copyright: © 2013 Vijaya Bhaskar V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
Ion suppression effect of dosing vehicle excipient Solutol HS15 (SHS15) on the accuracy of liquid chromatography/ tandem mass spectrometry (LC-MS/MS) measurements was studied. Ion supression cause significant errors in accuracy of the measured concentrations of test compounds, thereby invalidating the assessment of pharmacokinetic results. Using SHS15 as a probe compound, the concentration-time profile of the excipient in plasma from rats dosed both orally and intravenously was determined. A total of twelve oligomers were identified for SHS15. The most abundant ions corresponding to SHS15 oligomers at m/z 481, 525, 569, 613, 657, 701, 745, 789, 833, 877, 921, 965 were selected for pseudomultiple reaction monitoring (pMRM) in electrospray mode of ionisation. Analyte peak area of the oligomers was summed up to calculate the plasma concentrations of total SHS15. Plasma concentrations of SHS15 ranging from 1-2 mg/mL in the initial sampling points caused 2-10 fold ion supression on most of the analytes studied. This can result in false rejection of compounds in a drug discovery screen. Several sample preparation methods, enhanced chromatographic selectivity, and alternative ionization methods were investigated as means to avoid or minimize ion suppression effects. The elimination of ion suppression effects was achieved by liquid liquid extraction (LLE) with hexane, tert-butyl-methyl ether (TBME) as sample preparation method. The mechanism of ion supression caused by SHS15 in relation to both liquid and gas phase reactions was proposed.
Keywords
SHS15; LC-MS/MS; MRM; LLE; Matrix effect
Introduction
High throughput pharmacokinetic screening plays an important role in pharmaceutical research to rapidly identify pharmacokinetic profiles of potent and selective compounds [1-3]. Liquid chromatography/tandem mass spectrometry (LC-MS/MS) with either electrospray ionisation (ESI) or atmospheric pressure chemical ionisation (APCI) provides a sensitive and selective detection method for the quantitation of drug candidates in biological matrices [4]. Although LC-MS/MS is extremely sensitive and robust in terms of performance there is potential for ion suppression which leads to incorrect data interpretation. ESI is more prone to ion suppression effects than APCI [5,6]. Ion suppression could originate from endogenous compounds such as phospholipids [7], metabolites, co-administered drugs, internal standards [8], dosing vehicles [9-11], mobile phase additives [12] and plastic tubes [13]. In preclinical pharmacokinetic studies, formulation excipients typically are used at high concentrations to facilitate the dissolution of test compounds in the formulation solution. Solutol HS15 (SHS15) is nowadays the excipient of choice for chemotherapy drugs as lot of toxicity concerns were raised with the use of cremophor EL as formulation excipient [14]. SHS15 has greater solubilising capacity for wide variety of compounds [15]. SHS15 has been proved to be safe in rodents and nowadays an excipient of choice for preclincal pharmacokinetic studies [16]. SHS15 is a nonionic solubiliser and emulsifying agent obtained by reacting 15 moles of ethylene oxide with 1 mole of 12-hydroxystearic acid. SHS15 consists of about 70% of mono and di esters of 12 hydroxystearic acid (lipophilic part) and 30% of free polyethyleneglycol (hydrophilic part) [17]. SHS15, a copolymeric formulation excipient can cause significant signal suppression for certain analytes when minimal sample cleanup is used. The presence of higher concentration of formulation excipient in early time point samples after intravenous or oral administration, can cause significant ion suppression on the analytes [9,10,14]. This effect is more pronounced with the use of ultrafast gradients that causes coelution of many analytes. Ion suppression effects are complicated to handle in a drug discovery environment where hundreds of molecules with differing physicochemical properties (logD7.4, logP, pKa) are handled. These molecules may be differentially ion suppressed depending on their elution on a typical liquid chromatography (LC) gradient, compared with ion suppressing agent and their ability to compete with charge from the suppressing agent. The U.S food and drug administration (FDA) guidance for industry on bioanalytical method validation insists upon the assessment of matrix effects during method validation for quantitative bioanalytical LC-MS/MS methods [18]. Several approaches investigated so far to minimize the ion suppression effects by SHS15 were different HPLC column chemistries, sample dilution [19]. While these strategies are helpful to solve the ion suppression issues on few analytes, a unique solution wasn‘t found for wide range of new chemical entities studied in drug discovery. In this paper, identification of ion suppression due to SHS15 and effective removal of ion suppression are discussed.
The mechanism of ion suppression has been proposed and discussed by several research groups, but is not fully understood. Various mechanisms by which formulation excipients cause ion supression are: charge competition, change in droplet surface tension, preferential ion evaporation, gas phase deprotonation and co-precipitation [20-22]. The mechanism for SHS15 related signal interference has been proposed.
Experimental Section
Materials
Reference compounds (atenolol, caffeine, metaprolol, propranolol, telmisartan, ketoconazole, diltiazem, ranitidine and warfarin) were procured from Sigma Aldrich Co. (St. Louis, MO, USA). Solutol HS15 (SHS15), dimethyl sulfoxide (DMSO), monobasic sodium phosphate, dibasic sodium phosphate and ammonium acetate were procured from sigma Aldrich Co. (St. Louis, MO, USA). Acetonitrile, acetone, water and methanol (HPLC grade) were procured from Merck specialities pvt ltd (Mumbai, India). Formic acid (90% purified) was procured from Merck specialities pvt ltd (Mumbai, India). Sprague dawley rats were procured from Bioneeds ltd (Bangalore, India). Blood collection vacutainers (Lithium Heparin as anticoagulant) were sourced from BD (Franklin lakes, USA).
Plasma concentrations of solutol HS15
Preparation of calibration standards and quality control samples: Master stock solution of SHS15 (40 mg/mL), telmisartan (1 mg/mL; internal standard) was prepared in DMSO. Working standard solutions of SHS15 were prepared by serial diluting master stock with acetonitrile: DMSO: water (2:2:1). Working standard solutions were prepared at 25 fold higher concentration than plasma calibration standards and quality control samples. A total of nine calibration standards and three quality control samples were prepared. Plasma calibration standards (0.40, 0.80, 4.01, 20.04, 57.26, 104.12, 148.74, 167.31, 185.90 μg/mL) and quality control samples (1.64, 117.12, 156.16 μg/mL) of SHS15 were prepared by spiking 2 μL of the working standard solutions into 48 μL of blank rat plasma. Working stock solution of telmisartan (100 ng/mL) was prepared by diluting an aliquot of master stock solution in acetonitrile. Master and working stock solutions were stored at 4°C when not in use.
Animal dosing: SHS15 was administered intravenously (lateral tail vein) at 1.0 g/kg dose and orally (oral gavage needle) at 2.5 g/kg dose to fasted male sprague dawley rats. Dose volume administered was 5 mL/ kg. The composition of dosing vehicle used for the study was ethanol/ SHS15/water at composition of 10:20:70, % v/v/v in intravenous route and 10:50:40, % v/v/v in oral route of administration [23,24]. Serial blood samples were collected into vacutainers containing lithium heparin (anticoagulant) at 0.08, 0.25, 0.50, 1, 2, 4, 8 and 24 h post dose [25] after intravenous administration and 0.25, 0.50, 1, 2, 4, 8 and 24 h post dose [25] after oral administration. Plasma was separated after centrifugation and stored at -80°C until analysis.
Sample preparation: A 50 μL aliquot of plasma (blank control plasma, plasma samples from rats dosed with SHS15, blank plasma spiked with calibration standards and QC samples) was pipetted in to a 96 well polypropylene plate and extracted with 200 μL of acetonitrile containing internal standard. Samples were vortex mixed for 10 min at 1200 rpm and centrifuged at 4000 rpm for 10 min at 4°C. 50 μL of supernatant was pipette transferred in to a fresh analysis plate and diluted with 450 μL of methanol: water (1:1). 10 μL aliquots were injected for LC-MS/MS analysis.
LC-MS/MS analysis: All mass spectrometric estimations were performed on a sciex 3200 QTrap triple quadrupole instrument with turboionspray (Electrospray Ionization) (AB Sciex, Toronto, Canada) using thermo C18 column (50×4.6 mm, 2.5 μm). The HPLC system consisted two of LC20AD UFLC pumps and a SIL HTC autosampler (Shimadzu, Kyoto, Japan). The mobile phase consisted of 0.1% formic acid in water as aqueous component and 100% acetone as organic modifier. A generic gradient LC method (Table 1) with a short run time of 3.5 min was used for the analysis of SHS15 in plasma samples. The column and autosampler were maintained at 40°C and 4°C respectively. The turboionspray source was operated with typical settings as follows: ionization mode, positive; curtain gas, 20 psi; nebulizer gas (GS1), 50 psi; heater gas (GS2), 50 psi; ionspray voltage, 5500 V; temperature, 550°C. The mass spectrometer was set up to perform in MS mode and to run in pseudoMRM mode. The molecular ions of SHS15 and telmisartan were formed using the declustering potentials of 140 V and 40 V respectively. In pseudo MRM mode the most abundant and informative molecular ions were selected at m/z 481.5 (Oligomer 1), 521.5 (Oligomer 2), 569.5 (Oligomer 3), 613.5 (Oligomer 4), 657.5 (Oligomer 5), 701.5 (Oligomer 6), 745.5 (Oligomer 7), 789.5 (Oligomer 8), 833.5 (Oligomer 9), 877.5 (Oligomer 10), 921.5 (Oligomer 11), 965.5 (Oligomer 12) with medium CAD gas setting at a collision energy of 5 V. Molecular ion (m/z, 515.30) of telmisartan was fragmented to m/z, 276.10 at collision energy of 65 V with medium CAD gas setting. Peak areas for all components were automatically integrated using Analyst software version 1.5.
| Time (min) | Flow Rate (mL/min) | %A (Aqueous Modifier) | %B (Organic Modifier) |
|---|---|---|---|
| 0.01 | 1.00 | 95 | 5 |
| 1.00 | 1.00 | 5 | 95 |
| 2.50 | 1.00 | 5 | 95 |
| 2.60 | 1.00 | 95 | 5 |
| 3.50 | 1.00 | 95 | 5 |
Table 1: Generic gradient method used for the analysis of reference compounds and SHS15.
Preparation of plasma samples-SHS15 investigations
Preparation of master and working stock solutions: Master stock solutions of atenolol, caffeine, metaprolol, telmisartan, propranolol, diltiazem, ketoconazole, ranitidine and warfarin (1 mg/ml) were prepared in DMSO. Working standard solutions of SHS15 were prepared by serial dilution from master stock (250 mg/mL) at 25 times higher concentration than plasma concentrations in acetonitrile: water: DMSO (2:2:1). A total of twelve working concentrations of Solutol HS15 were prepared. Plasma concentrations (0.05, 0.125, 0.25, 0.50, 0.75, 1.00, 1.50, 2.00, 2.50, 5.00, 7.50, 10.00 mg/mL) of SHS15 were prepared by spiking 2 μL of working concentrations in 48 μL of blank rat plasma. Pooled working stock solution of reference compounds at 1000 ng/mL concentrations was prepared in acetonitrile: water (1:1). Master stock and working stock solutions were stored at 4°C when not in use.
Sample preparation
Protein precipitation (PPT): A 50 μL aliquot of plasma (blank plasma, plasma samples spiked with SHS15) was pipette transferred in to a 96 well polypropylene plate and extracted with 200 μL of acetonitrile. Samples were vortex mixed for 10 min at 1200 rpm and centrifuged at 4000 rpm for 10 min at 4°C. 150 μL of supernatant was pipette transferred to a fresh analysis plate and diluted with 150 μL of pooled working stock solution of reference compounds. 10 μL were injected for LC-MS/MS analysis.
Liquid Liquid Extraction (LLE): A 50 μL aliquot of plasma (blank plasma, plasma samples spiked with SHS15) was pipette transferred in to a 96 well polypropylene plate and extracted with 1000 μL of ethyl acetate, tert-butyl-methyl ether (TBME) and hexane individually. Samples were vortex mixed for 10 min at 1000 rpm and centrifuged at 4000 rpm for 10 min at 4°C. 800 μL of supernatant was pipette transferred to a fresh evaporation plate and evaporated to dryness under nitrogen at 40°C for 10 min. After evaporation, samples were reconstituted with 300 μL of pooled working stock solution of reference compounds and 10 μL were injected for LC-MS/MS analysis.
LC-MS/MS analysis: All mass spectrometric estimations were performed on a sciex 3200 QTrap triple quadrupole instrument with turboionspray (AB Sciex, Toronto, Canada). The HPLC system consisted two of LC20AD UFLC pumps and a SIL HTC autosampler (Shimadzu, Kyoto, Japan). The stationary phase was XBridge C18 with 3.5 μm particle diameter (Waters, Ireland). The column dimensions were 50×4.6 mm. The mobile phase flow rate was 1.0 mL/min with a split ratio of 1:1 to the ionization source. The mobile phase consisted of the following combinations of aqueous and organic modifiers: 1) 0.1% formic acid in water, 100% acetone (FA-ACET) 2) 0.1% formic acid in water, 100% methanol (FA-MEOH) 3) 10 mM ammonium acetate in water, 100% acetone (AA-ACET) 4) 10 mM ammonium acetate in water, 100% methanol (AA-MEOH). A generic gradient LC method (Table 1) with a short run time of 3.5 min was used for the quantification of reference compounds and SHS15. The column and autosampler were maintained at 40°C and 4°C respectively. The turboionspray source was operated with typical settings as follows: ionization mode, positive; curtain gas, 15 psi; nebulizer gas (GS1), 50 psi; heater gas (GS2), 50 psi (ESI); ionspray voltage (IS), 5500 V (ESI); nebulizer current (NC), 5 A (APCI); temperature, 550°C. Multiple reactions monitoring (MRM) mode was employed for the quantification of reference compounds and pseudo MRM for the quantification of SHS15. List of MRM used for quantification of reference compounds was presented in table 2. Peak areas for all components were automatically integrated using Analyst software version 1.5.
| Compound Name | Q1 Mass (Da) | Q3 Mass (Da) | Dwell Time (m sec) | Declustering Potential (v) | Collision energy (v) |
|---|---|---|---|---|---|
| Propranolol | 260.10 | 116.20 | 100 | 40 | 25 |
| Caffeine | 195.10 | 137.90 | 100 | 45 | 25 |
| Ketoconazole | 531.10 | 82.10 | 100 | 80 | 80 |
| Diltiazem | 415.10 | 178.10 | 100 | 40 | 32 |
| Ranitidine | 315.10 | 176.10 | 100 | 25 | 22 |
| Atenolol | 267.10 | 145.10 | 100 | 40 | 32 |
| Telmisartan | 515.30 | 276.10 | 100 | 65 | 64 |
| Metaprolol | 268.10 | 116.00 | 100 | 50 | 25 |
| Warfarin | 309.20 | 163.00 | 100 | 50 | 21 |
Table 2: List of MRM used for quantifying the reference compounds.
Results and Discussion
Plasma concentrations of SHS15
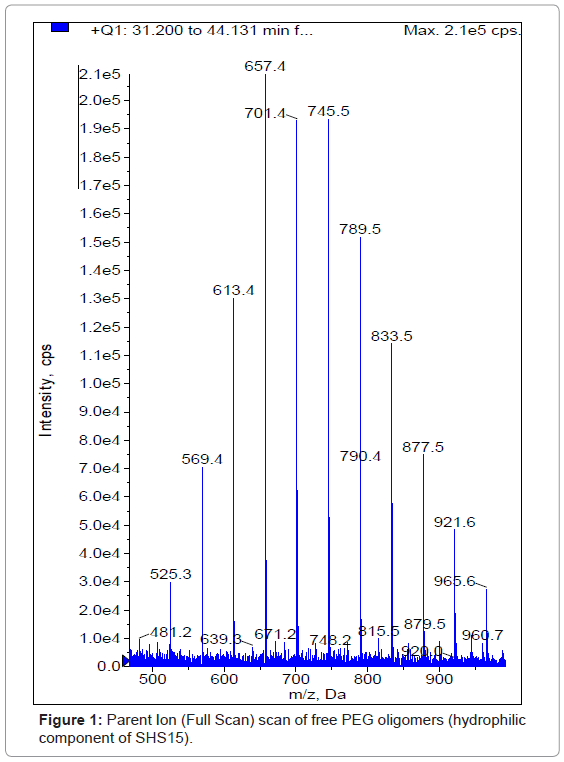
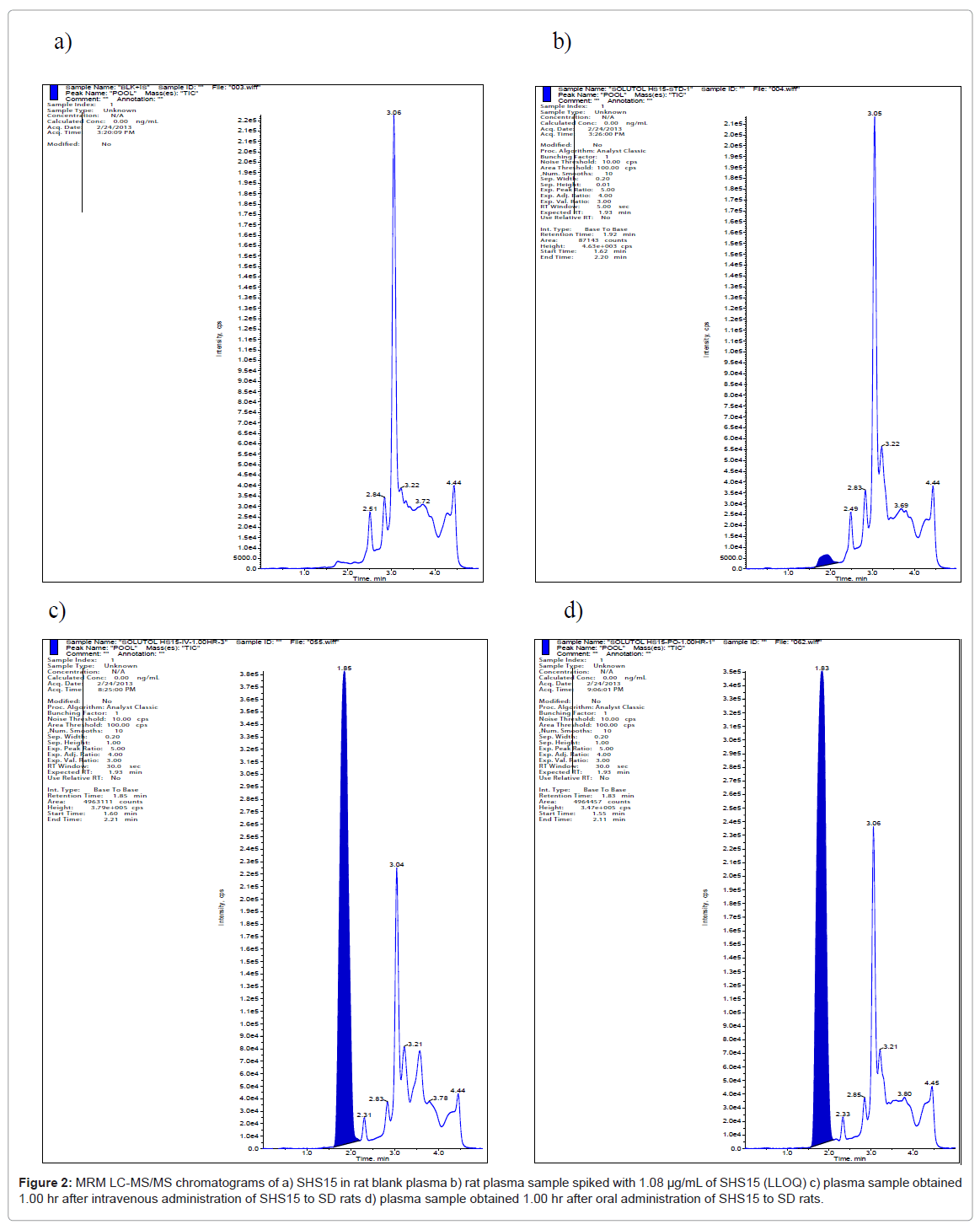
The electrospray ionization of SHS15 produced the abundant molecular ions for free PEG component (hydrophilic) at m/z 481.50, 521.50, 569.50, 613.50, 657.50, 701.50, 745.50, 789.50, 833.50, 877.50, 921.50 and 965.50 (Figure 1) under positive ionization conditions. None of the molecular ions had produced distinct fragments to quantify in MRM mode of analysis. For this reason, molecular ions were monitored in pMRM mode where the molecular ion will be measured as parent and fragment with minimal collision energy of 5V. Plasma samples from both intravenous and oral routes were analyzed with the developed bioanalytical method using LC-MS/MS. For calculating the plasma concentrations of SHS15 as a whole the analyte peak areas of each oligomer was summed up and calibration curve was built. No interference at the retention times of SHS15 (1.92 min) (Figure 2a) was observed in any of the lots screened as shown in representative chromatogram of the extracted blank plasma sample, confirming the selectivity of the present method. Representative chromatogram of SHS15 at LLOQ was shown in figure 2b. Representative chromatograms of SHS15 from intravenous (1.00 hr), oral (1.00 hr) study samples were shown in figures 2c and 2d respectively. SHS15 plasma concentrations following intravenous administration were high (1-5 mg/mL) in the initial sampling points. The oral bioavailability of SHS15 was measured as 13.93%. Mean plasma concentrations of SHS15 after intravenous and oral administration were shown in tables 3 and 4 respectively. Our objective in this study is to measure the maximum physiological concentrations of excipient in plasma after administration to rats.
| Time (hr) | Concentration (μg/mL) | % CV | ||||
|---|---|---|---|---|---|---|
| Rat-1 | Rat-2 | Rat-3 | Mean | STDEV | ||
| 0.08 | 1504.96 | 948.13 | 1205.96 | 1219.68 | 278.67 | 23 |
| 0.25 | 852.98 | 646.15 | 789.48 | 762.87 | 105.95 | 14 |
| 0.50 | 674.73 | 558.44 | 559.33 | 597.50 | 66.88 | 11 |
| 1.00 | 485.58 | 333.70 | 390.31 | 403.20 | 76.76 | 19 |
| 2.00 | 100.29 | 87.19 | 105.66 | 97.71 | 9.50 | 10 |
| 4.00 | 53.16 | 40.38 | 45.76 | 46.43 | 6.42 | 14 |
| 8.00 | 26.45 | 27.60 | 26.55 | 26.87 | 0.64 | 2 |
| 24.00 | 8.72 | 7.52 | 7.98 | 8.07 | 0.60 | 7 |
Table 3: Plasma concentration levels of SHS15 after intravenous administration at 0.37 g/kg dose (hydrophilic component).
| Time (hr) | Concentration (µg/mL) | % CV | ||||
|---|---|---|---|---|---|---|
| Rat-1 | Rat-2 | Rat-3 | Mean | STDEV | ||
| 0.25 | 55.65 | 96.87 | 67.19 | 73.24 | 21.26 | 29 |
| 0.50 | 87.81 | 130.51 | 107.47 | 108.60 | 21.37 | 20 |
| 1.00 | 86.82 | 126.86 | 108.73 | 107.47 | 20.05 | 19 |
| 2.00 | 56.08 | 43.25 | 37.18 | 45.50 | 9.65 | 21 |
| 4.00 | 32.89 | 24.47 | 29.60 | 28.99 | 4.25 | 15 |
| 8.00 | 25.82 | 9.25 | 24.48 | 19.85 | 9.21 | 46 |
| 24.00 | 4.22 | 4.27 | 3.63 | 4.04 | 0.36 | 9 |
Table 4: Plasma concentration levels of SHS15 after oral administration at 0.92 g/ kg dose (hydrophilic component).
Preparation of plasma samples-SHS15 investigations
Structural formulae of reference compounds selected for ion suppression studies were represented in figure 3. Samples extracted by protein precipitation were analyzed with different mobile phase conditions in ESI mode to check if the elution pattern of the excipient behaves differently to that of reference compounds. As it is well known that APCI was less prone to matrix effects compared to ESI, protein precipitated samples were analyzed in this mode. Samples extracted by LLE were analyzed in ESI mode. Analytical conditions used for analysis of LLE samples in ESI mode and protein precipitated samples in APCI mode were similar to the conditions used for the analysis of SHS15 in plasma samples. Solid phase extraction wasn’t tried as alternate extraction technique as this work was done to provide a unique solution for nullifying the ion suppression effects caused by SHS15 in the bioanalysis of new chemical entities. For developing a SPE method, molecular, physico chemical properties of NCEs should be known and a lot of time should be invested on method development which practically is impossible in drug discovery where throughput drives the fate of project.
Peak area of reference compound at each concentration of SHS15 spiked in to plasma was compared against negative control samples to calculate % ion suppression. A total suppression of ± 15% from the control response was considered as acceptable according to US FDA validation guidelines [18]. A detailed discussion on the results obtained with different system conditions for each reference compounds was given below.
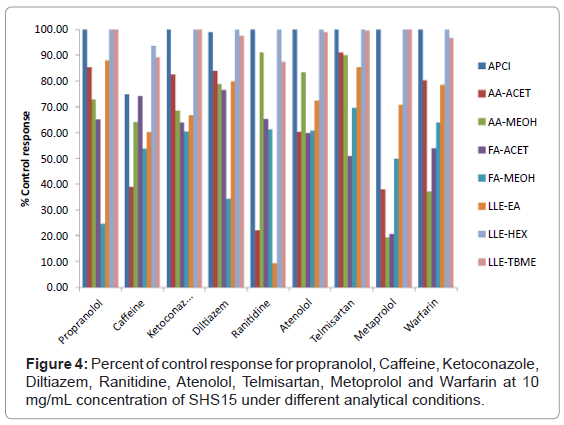
Propranolol: At an excipient concentration of 10 mg/mL, propranolol had >75% ion suppression using mobile phase combination of FA-MEOH (Figure 4). Sample preparation with hexane, TBME and ionization by APCI mode of ionization for protein precipitation samples proved to be the best methodologies for the analysis of propranolol (Figure 4). Mobile phase combination of AA-ACET can be effectively used for protein precipitated samples without suppression effect from the excipient. Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 5a.
Caffeine: At an excipient concentration of 10 mg/mL, caffeine had >60% ion suppression using mobile phase combination of AA-ACET (Figure 4). Suppression effects weren’t observed when samples were extracted using n-hexane and TBME (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 5b.
Ketoconazole: At an excipient concentration of 10 mg/mL, ketoconazole had >30% ion suppression using a) ethyl acetate as extraction solvent b) mobile phase combination of AA-MEOH, FAACET, FA-MEOH (Figure 4). Sample preparation with hexane, TBME and ionization by APCI mode of ionization for protein precipitation samples proved to be the best methodologies for the analysis of ketoconazole (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 5c.
Diltiazem: At an excipient concentration of 10 mg/mL, diltiazem had >65% of ion suppression using mobile phase combination of FA-MEOH for protein precipitated samples (Figure 4). Sample preparation by extraction with hexane, TBME and ionization by APCI mode of ionization for protein precipitation samples proved to be the best methodologies for the analysis of diltiazem (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 5d.
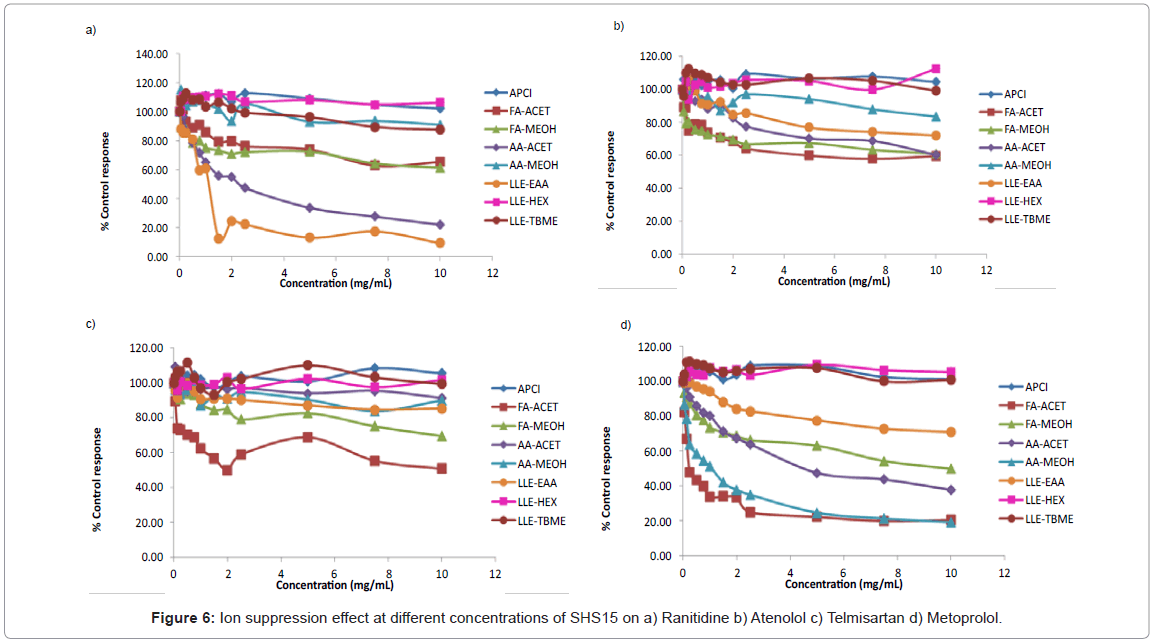
Ranitidine: At an excipient concentration of 10 mg/mL, ranitidine had >75% ion suppression using a) ethyl acetate as extraction b) mobile phase combination of AA-ACET (Figure 4). Suppression effects weren’t observed when samples were extracted using n-hexane, TBME and ionization by APCI mode of ionization for protein precipitated samples (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 6a.
Atenolol: At an excipient concentration of 10 mg/mL, atenolol had >40% ion suppression using mobile phase combination of AA-ACET, FA-ACET, FA-MEOH (Figure 4). Sample preparation by extraction with hexane, TBME and ionization by APCI mode of ionization for protein precipitation samples proved to be the best methodologies for the analysis of atenolol (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 6b.
Telmisartan: At an excipient concentration of 10 mg/mL, telmisartan had >50% ion suppression using mobile phase combination of FA-ACET (Figure 4). Sample preparation by extraction with hexane, TBME and ionization by APCI mode of ionization for protein precipitation samples proved to be the best methodologies for the analysis of telmisartan (Figure 4). Suppression effects weren’t observed using mobile phase combination of AA-ACET, AA-MEOH (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 6c.
Metoprolol: At an excipient concentration of 10 mg/mL, metoprolol had >80% ion suppression using mobile phase combination of AA-MEOH, AA-ACET (Figure 4). Sample preparation by extraction with hexane and ionization by APCI mode of ionization for protein precipitation samples proved to be the best methodologies for the analysis of metoprolol (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 6d.
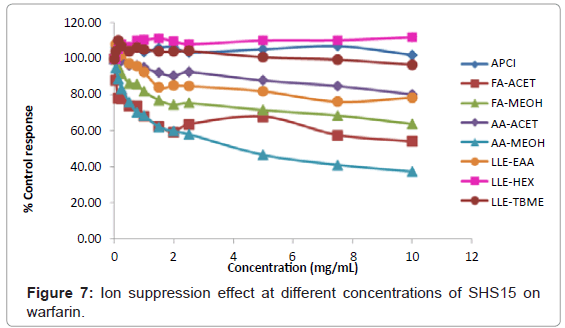
Warfarin: At an excipient concentration of 10 mg/mL, warfarin had >60% ion suppression using mobile phase combination of AA- MEOH and >45% ion suppression using mobile phase combination of FA-ACET (Figure 4). Sample preparation by extraction with hexane, TBME and ionization by APCI mode of ionization for protein precipitation samples proved to be the best methodologies for the analysis of warfarin (Figure 4). Ion suppression at different concentrations of SHS15 under different analytical conditions was shown in figure 7.
Using different mobile phase conditions helped to nullify the ion suppression on propranolol, ranitidine and telmisartan in ESI mode of analysis. No ion suppression effects were observed for propranolol, caffeine, ketoconazole, diltiazem, ranitidine, atenolol, telmisartan, metaprolol and warfarin when samples were extracted using TBME, hexane. Out of solvents tested in LLE, extraction with ethyl acetate was the poor extraction method.
Sample analysis by APCI mode of ionization brought down the ion suppression effects caused by SHS15 on propranolol, ketoconazole, diltiazem, ranitidine, atenolol, metaprolol, telmisartan and warfarin. However, caffeine still had ion suppression in APCI mode of ionization. This shows that SHS15 cause ion suppression both in liquid and gaseous phases. ESI is prone to matrix effects caused by excipients in liquid and gas phase whereas APCI is resistant to liquid phase suppression effects.
We proposed various mechanisms by which Solutol HS15 cause ion suppression effects on different analytes:
1. Increase in surface tension and viscosity of the droplets due to high concentrations of excipients leading to insufficient evaporation (ESI)
2. Charge competition between analyte and ion suppressing agent leading to overall reduced ionization of analyte (ESI/APCI)
3. Co precipitation with non volatile components (ESI/APCI)
4. Gas phase reactions causing charge transfer between analytes and ion suppressing agent (ESI/APCI).
Conclusion
A pMRM based method was developed for the quantification of Solutol HS15 concentration levels in rat plasma samples. Based on the physiological concentration levels of excipient, various approaches such as a) different mobile phase conditions b) different extraction techniques c) different ionization conditions were tested for finding best technique that nullifies ion suppression effects. The approaches for reducing the ion suppression effects in LC-MS/MS are largely analyte dependent. However, sample preparation with hexane, TBME as extraction solvent totally nullified the ion suppression effects caused by Solutol HS15. Mechanism of ion suppression caused by Solutol HS15 was proposed as a) charge competition b) increase in surface tension/ viscosity of droplets c) co-precipitation d) gas phase reactions.
References
- Heath TG, Scott DO (1997) Quantification of a potent 5HT2a antagonist and an active metabolite in rat plasma and brain microdialysate by liquid chromatography-tandem mass spectrometry. J Am Mass Spectrom 8: 371-379.
- Olah TV, McLoughlin DA, Gilbert JD (1997) The simultaneous determination of mixtures of drug candidates by liquid chromatography/atmospheric pressure chemical ionization mass spectrometry as an in vivo drug screening procedure. Rapid Commun Mass Spectrom 11: 17-23.
- Watt AP, Morrison D, Locker KL, Evans DC (2000) Higher throughput bioanalysis by automation of a protein precipitation assay using a 96-well format with detection by LC-MS/MS. Anal Chem 72: 979-984.
- Covey TR, Lee ED, Henion JD (1986) High-speed liquid chromatography/tandem mass spectrometry for the determination of drugs in biological samples. Anal Chem 58: 2453-2460.
- Pommier F, Frigola R (2003) Quantitative determination of rivastigmine and its major metabolite in human plasma by liquid chromatography with atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 784: 301-313.
- Dams R, Huestis MA, Lambert WE, Murphy CM (2003) Matrix effect in bio-analysis of illicit drugs with LC-MS/MS: influence of ionization type, sample preparation, and biofluid. J Am Soc Mass Spectrom 14: 1290-1294.
- Little JL, Wempe MF, Buchanan CM (2006) Liquid chromatography-mass spectrometry/mass spectrometry method development for drug metabolism studies: Examining lipid matrix ionization effects in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 833: 219-230.
- Sojo LE, Lum G, Chee P (2003) Internal standard signal suppression by co-eluting analyte in isotope dilution LC-ESI-MS. Analyst 128: 51-54.
- Shou WZ, Naidong W (2003) Post-column infusion study of the 'dosing vehicle effect' in the liquid chromatography/tandem mass spectrometric analysis of discovery pharmacokinetic samples. Rapid Commun Mass Spectrom 17: 589-597.
- Schuhmacher J, Zimmer D, Tesche F, Pickard V (2003) Matrix effects during analysis of plasma samples by electrospray and atmospheric pressure chemical ionization mass spectrometry: practical approaches to their elimination. Rapid Commun Mass Spectrom 17: 1950-1957.
- Mallet CR, Lu Z, Mazzeo JR (2004) A study of ion suppression effects in electrospray ionization from mobile phase additives and solid-phase extracts. Rapid Commun Mass Spectrom 18: 49-58.
- Mei H, Hsieh Y, Nardo C, Xu X, Wang S, et al. (2003) Investigation of matrix effects in bioanalytical high-performance liquid chromatography/tandem mass spectrometric assays: application to drug discovery. Rapid Commun Mass Spectrom 17: 97-103.
- Weaver R, Riley RJ (2006) Identification and reduction of ion suppression effects on pharmacokinetic parameters by polyethylene glycol 400. Rapid Commun Mass Spectrom 20: 2559-2564.
- Joshi M, Pathak S, Sharma S, Patravale V (2008) Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: Nanoject. Int J Pharm 364: 119-126.
- Bittner B, Mountfield RJ (2002) Intravenous administration of poorly soluble new drug entities in early drug discovery: the potential impact of formulation on pharmacokinetic parameters. Curr Opin Drug Discov Devel 5: 59-71.
- Sherry Ku, Ranga V (2010) Solutol HS15 as a novel excipient. Pharm Technol 108-110.
- Technical information (2012) KolliphorTM HS15. BASF 1-8.
- Food and Drug Administration (2001) Guidance for Industry: Bioanalytical Methods Validation. US Department of Health and Human Services, Center for Drug Evaluation and Research, and Center for Veterinary Medicine.
- Schuhmacher J, Zimmer D, Tesche F, Pickard V (2003) Matrix effects during analysis of plasma samples by electrospray and atmospheric pressure chemical ionization mass spectrometry: practical approaches to their elimination. Rapid Commun Mass Spectrom 17: 1950-1957.
- Chambers E, Wagrowski-Diehl DM, Lu Z, Mazzeo JR (2007) Systematic and comprehensive strategy for reducing matrix effects in LC/MS/MS analyses. J Chromatogr B Analyt Technol Biomed Life Sci 852: 22-34.
- King R, Bonfiglio R, Fernandez-Metzler C, Miller-Stein C, Olah T (2000) Mechanistic investigation of ionization suppression in electrospray ionization. J Am Soc Mass Spectrom 11: 942-950.
- Bonfiglio R, King RC, Olah TV, Merkle K (1999) The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun Mass Spectrom 13: 1175-1185.
- Neervannan S (2006) Preclinical formulations for discovery and toxicology: physicochemical challenges. Expert Opin Drug Metab Toxicol 2: 715-731.
- Sheftel VO (2000) Indirect food additives and polymers: Migration and Toxicology.
- Kwon Y (2002) Pharmacokinetic study design and data interpretation. Handbook of Essential Pharmacokinetics, Pharmacodynamics and Drug Metabolism for Industrial Scientists, Kluwer Academic Publishers, New York, 21-46.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15345
- [From(publication date):
April-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 10647
- PDF downloads : 4698