Review Article Open Access
Hilar Lymph Node Involvement in Colorectal Cancer Liver Metastases - An Overview
| Renato Micelli Lupinacci*, Fabrício Ferreira Coelho, Jaime Kruger, Marcos Vinícius Perini and Paulo Herman | |
| Liver Surgery Unit, Department of Gastroenterology, University of São Paulo Medical School, Brazil | |
| Corresponding Author : | Renato Micelli Lupinacci Liver Surgery Unit Department of Gastroenterology University of São Paulo Medical School, Brazil E-mail: rmlupinacci@gmail.com |
| Received September 30, 2011; Accepted December 21, 2011; Published December 23, 2011 | |
| Citation: Lupinacci RM, Coelho FF, Kruger J, Perini MV, Herman P (2011) Hilar Lymph Node Involvement in Colorectal Cancer Liver Metastases – An Overview. J Gastroint Dig Syst S6:002. doi:10.4172/2161-069X.S6-002 | |
| Copyright: © 2011 Lupinacci RM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited . | |
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Hepatectomy is the only possible option for cure in any treatment strategy of colorectal liver metastases, and several studies have shown good results, with five-year survival rates ranging from 27 to 56%. Several clinical and pathological predictive factors for survival after liver resection have been studied and the metastatic involvement of the hepatic hilum lymph nodes indicates a poor long-term prognosis. Despite variable results, some authors have reported a not-insignificant improvement in survival rate in liver-metastasis patients with hilar-lymph-node involvement who undergo combined liver resection and lymphadenectomy. Due to the low rates of morbidity and mortality for liverresection surgery, several specialized centers perform liver resections combined with lymphadenectomy in selected cases. It should be noted that the therapeutic value of systemic lymphadenectomy is not yet entirely understood, and only controlled studies comparing groups with and without lymphadenectomy can fully resolve the issue. In any case, hilar lymph node dissection has been shown to be a useful tool for improving the accuracy of extrahepatic disease staging, regardless of its impact on survival. The authors review the incidence and the clinical impact of hilar lymph node metastases, and analyses the possible beneficial role of systematic lymphadenectomy in patients who have undergone liver resection for colorectal-cancer metastases.
| Keywords |
| Hepatectomy; Colorectal carcinoma; Lymphatic metastasis |
| Review |
| For patients with colorectal cancer liver metastases (CRCLM), hepatic resection remains the best treatment option, with reported 5-year survival rates of 27% to 56% [1-3]. Technical improvements and pre-operative chemotherapy has been shown to increase resecability in a substantial number of cases and allowed an expansion of indications to include patients with large and multiple or bilobar lesions [4-6] with acceptable morbidity and mortality [6,7]. |
| Several clinical and pathological predictive factors for survival after hepatic resection have been studied. These factors include primary tumor staging (pT and pN), pre-operative level of carcinoembryonic antigen (CEA) and/or carbohydrate antigen 19.9 (CA 19.9), the interval between the diagnoses of the primary and the metastatic lesions and the presence or absence of extra-hepatic disease and lymph node involvement of the hepatic hilum. Despite some discrepancies in results, most studies have shown that lymph node involvement at the primary site (pN), synchronicity of the lesions, four or more hepatic lesions, and hilar lymph node metastases constitute the major negative prognostic factors, with significant impacts on long-term survival [8-12]. |
| Extra-hepatic disease, especially hepatic lymph node involvement has long been regarded as an exclusion criterion for curative hepatectomy because the prognosis was considered poor, even when adequately resected [13,14]. Recently, however, studies advocate resection for patients with limited extra hepatic disease, especially lung metastases, in highly selected patients providing long-term survival with R0 resection [15-17]. |
| The hepatic lymphatic vessels fall into three categories depending on their locations: portal (the most important), sublobar, and superficial lymphatic vessels [18]. The theoretical mechanisms of intrahepatic spread for CRCLM include metastasis from the primary tumor, but also de novo metastases from the pre-existing liver metastases [19,20]. It is believed that lymph node involvement in patients with CRCLM may actually constitute a tertiary metastasis or ‘‘metastasis of a metastasis’’, a concept proposed by August et al. [19] and subsequently reinforced by other studies [21,22]. |
| The incidence of macroscopic lymph node involvement varies between 2 and 10% in major series [19,23,24], and they indicate a poor long-term prognosis. Based on these results, most authors have considered macroscopic lymph node involvement to be a contraindication to liver resection [12,25,26]. Studies that have employed systematic dissection of suspicious and non-suspicious hilar lymph nodes to diagnose microscopic involvement have found an incidence of 14% to 30% [13,27-29] which proves that assessments based only on palpation and macroscopic inspection are not accurate. A controversial issue is the role of lymph node dissection in majorchain sampling without a complete dissection of the hepatic hilum and the celiac trunk. Kokudo et al. [30] have shown a tendency towards 12B chain involvement in patients with metastases on the right hepatic side and towards 8A chain involvement in lesions located on the left side. Ercolani et al. [27] found that pericholedochal and common hepatic artery stations were the key stations for lymphatic spread from liver tumors, other authors, however, have not found a relationship between the chains involved and the topography of the hepatic lesions [14,23,28,29]. Elias et al. [29] diagnosed microscopic lymph node involvement in several of the lymphatic chains studied without a continuous progression from one chain to another (lymph node jumping metastasis). Therefore, any specific lymph node group will reflect the true state of ganglion involvement in patients with metastatic hepatic lesions and a minimum of 3-4 regional lymph nodes should be dissected to obtain a reliable statement on lymph node status [27,28,31]. |
| The aforementioned studies used hematoxylin and eosin (H&E) stain to evaluate hilar lymph nodes harvested at the time of liver resection, which is not the most sensitive modality for detecting micrometastases. A study with hilar sampling but not routine lymphadenectomy showed the presence of micrometastases in 23% of the patients, and the presence of hilar micrometastases was associated to a shorter recurrence-free survival [32]. We have previously shown our initial results of a prospective study with hilar lymphadenectomy during hepatectomy for colorectal metastasis showing an overall frequency of hilar involvement of 23.6% with 18% of patients presenting micrometastases by H&E or immunohistochemistry analysis (IHC) [31]. Immunohistochemistry is particularly helpful in the detection of micrometastases because it associates a specific immunological reaction with the morphological control of immunoreactive cells. The association of both techniques (IHC and serial sections) as employed in our study seems to be recommended for further evaluation of H&Enegative nodes [33]. Micrometastases research has been routinely used in breast cancer and melanoma cases [14,34-36], and several studies on other malignancies have confirmed that it is able to expand diagnostic sensibility [37-39]. |
| Fault of this lack of consensus, the real incidence of microscopic hilar involvement and/or micrometastases remains dependent of the definition criteria and the techniques employed by each author. |
| One of the most relevant questions is whether hepatic resection is indicated in patients with affected hilar lymph nodes. Studies that have examined the impact of microscopic lymph-node involvement on survival have found it to have a negative effect [13,14,28]. Nevertheless, some authors have reported significant survival rates in liver metastasis patients with associated hilar lymph node disease who undergo combined liver resection and lymphadenectomy [14,23,40-42]. Among these results, Kokudo et al. [30] have shown that the survival rate of patients with lymph node disease who undergo R0 resection and lymphadenectomy is significantly higher than that of residual liver disease patients without lymph node metastases who undergo liver resection (17 months versus 8 months, p < 0.05). Some authors therefore suggest a possible benefit from resection and hilar lymphadenectomy in patients with lymph node involvement [23,43]. Two other studies have found three-year survival rates between 38 and 45% in patients with compromised liver pedicle lymph nodes who undergo liver resection combined with lymphadenectomy [14,40]. However, the location of the compromised lymph nodes seems to be a major issue and resection of positive lymph nodes located in area 1 (hepatoduodenal ligament/ retropancreatic area) may offer long-term survival benefits, on the contrary, when lymph node located in area 2 (along the common hepatic artery/celiac axis) or area 3 (para-aortic) are positive, the longterm survival is significantly worse [14,40]. Recently, Adam et al. [44] described an 18% global five-year survival rate in a selected group of patients with hepatic metastases and compromised hepatic hilar lymph nodes who underwent hepatic resection and lymphadenectomy. |
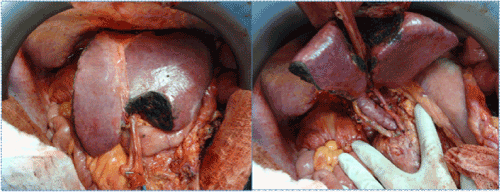
| Comparing the prognosis of patients with IHC or H&E-only detected micrometastases, it seems that the former may be related to a better overall survival [32], although studies in colon and breast cancer patients have not found a relationship between IHC-only metastatic disease in regional lymph nodes and tumor recurrence. [45,46]. Due to the low morbidity and mortality rates in liver-resection surgery, several specialized centers (including ours) perform liver resections combined with extended lymphadenectomy in selected cases (Figure 1). |
| It should be emphasized that the therapeutic value of systemic lymphadenectomy is not yet entirely understood, and that only controlled studies comparing groups with and without lymphadenectomy can fully solve the issue. In any case, hilar lymph node dissection has been shown to be a useful tool for improving the accuracy of hilar nodal involvement and thus disease staging, regardless of its impact on survival, and may potentially influence post-resection chemotherapy recommendations. |
| The search for microscopic hepatic lymph node involvement does not detect all patients that will relapse or those that will not. Moreover it is premature to assume that the adjuvant therapy alone is able to suppress the difference in prognosis that may be related to their presence. Before advocating systemic hepatic hilar dissection, more studies are needed to establish the value of node involvement as an independent prognostic variable. Molecular biology may help us to solve this question and better understand tumor behavior. Meanwhile, the routine search for lymph node involvement should be proposed only as part of clinical trials. |
| From a practical perspective, the clinical importance of lymph node micrometastases for long-term survival is not known, especially in patients undergoing systemic treatment (chemotherapy). There is a shortage of studies evaluating whether detecting micrometastases allows for better staging, is indicative of a poor prognosis, or has limited prognostic value. Despite some authors having shown a correlation between the major prognostic factors and the presence of lymph node metastases [14,29], most studies have not found such associations and have reaffirmed the impossibility of determining risk subgroups for hilar lymph node involvement [28,30-32]. |
| Finally, it is important to highlight the possible role of systemic treatment in patients with macroscopic or microscopic lymph-node disease who undergo resection and lymphadenectomy. It is presumed that modern peri-operative chemotherapy regimens can positively influence the clinical course of these patients, possibly creating a new paradigm for the treatment of colorectal liver metastases and extending the limits of liver resection, even in patients with lymph node involvement. |
| In conclusion, larger studies should be performed to determine the significance of unsuspected perihepatic hilar lymph node metastases, as though the potential benefit of hilar lymphadenectomy, and of changes in chemotherapy strategy by hilar lymph node status. Indeed, subject to a consensus on their definition and standardization of detection methods, it may alter the management of CRCLM treatment in the future. |
References
- Chua TC, Saxena A, Chu F, Zhao J, Morris DL (2011) Predictors of cure after hepatic resection of colorectal liver metastases: An analysis of actual 5- and 10-year survivors. J Surg Oncol 103: 796-800.
- Karanjia ND, Lordan JT, Fawcett WJ, Quiney N, Worthington TR (2009) Survival and recurrence after neo-adjuvant chemotherapy and liver resection for colorectal metastases: a ten year study. Eur J Surg Oncol 35: 838-843.
- Lordan JT, Karanjia ND (2010) 'Close shave' in liver resection for colorectal liver metastases. Eur J Surg Oncol 36: 47-51.
- Hemming AW, Reed AI, Langham MR Jr, Fujita S, Howard RJ (2004) Combined resection of the liver and inferior vena cava for hepatic malignancy. Ann Surg 239: 712-719.
- Ferrero A, Polastri R, Muratore A, Zorzi D, Capussotti L (2004) Extensive resections for colorectal liver metastases. J Hepatobiliary Pancreat Surg 11: 92-96.
- Elias D, Sideris L, Pocard M, Ouellet JF, Boige V, et al. (2004) Results of R0 resection for colorectal liver metastases associated with extra-hepatic disease. Ann Surg Oncol 11: 274-280.
- Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, et al. (2004) Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg 240: 644-657.
- Tanaka K, Nojiri K, Kumamoto T, Takeda K, Endo I (2011) R1 resection for aggressive or advanced colorectal liver metastases is justified in combination with effective prehepatectomy chemotherapy. Eur J Surg Oncol 37: 336-343.
- Adam R, Bismuth H, Castaing D, Waechter F, Navarro F, et al. (1997) Repeat hepatectomy for colorectal liver metastases. Ann Surg 225: 51-62.
- [No authors listed] (1988) Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of indications for resection. Registry of Hepatic Metastases. Surgery 103: 278-288.
- Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, et al. (2005) Analysis of preoperative prognostic factors for long-term survival after hepatic resection of liver metastasis of colorectal carcinoma. J Gastrointest Surg 9: 374-380.
- Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP (1991) Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 110: 13-29.
- Rodgers MS, McCall JL (2000) Surgery for colorectal liver metastases with hepatic lymph node involvement: a systematic review. Br J Surg 87: 1142-1155.
- Jaeck D, Nakano H, Bachellier P, Inoue K, Weber JC, et al. (2002) Significance of hepatic pedicle lymph node involvement in patients with colorectal liver metastases: A prospective study. Ann Surg Oncol 9: 430-438.
- Carpizo DR, Are C, Jarnagin W, Dematteo R, Fong Y, et al. (2009) Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol 16: 2138-2146.
- Adam R, de Haas RJ, Wicherts DA, Vibert E, Salloum C, et al. (2011) Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg 253: 349-359.
- Harms J, Obst T, Thorban S, Busch R, Fink U, et al. (1999) The role of surgery in the treatment of liver metastases for colorectal cancer patients. Hepato-Gastroenterology 46: 2321-2328.
- Ohtani O, Ohtani Y (2008) Lymph circulation in the liver. Anat Rec 291: 643-652.
- August DA, Sugarbaker PH, Schneider PD (1985) Lymphatic dissemination of hepatic metastases. Implications for the follow-up and treatment of patients with colorectal cancer. Cancer 55: 1490-1494.
- Sanchez-Cespedes M, Esteller M, Hibi K, Cope FO, Westra WH, et al. (1999) Molecular detection of neoplastic cells in lymph nodes of metastatic colorectal cancer patients predicts recurrence. Clin Cancer Res 5: 2450-2454.
- Hughes K, Scheele J, Sugarbaker PH (1989) Surgery for colorectal cancer metastatic to the liver. Optimizing the results of treatment. Surg Clin North Am 69: 339-359.
- Dworkin MJ, Earlam S, Fordy C, Allen-Mersh TG (1995) Importance of hepatic artery node involvement in patients with colorectal liver metastases. J Clin Pathol 48: 270-272.
- Beckurts KTE, Hölscher AH, Thorban S, Bollschweiler E, Siewert JR (1997) Significance of lymph node involvement at the hepatic hilum in the resection of colorectal liver metastases. Br J Surg 84: 1081-1084.
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH (1999) Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230: 309-318.
- Gibbs JF, Weber TK, Rodriguez-Bigas MA, Driscoll DL, Petrelli NJ (1998) Intraoperative determinants of unresectability for patients with colorectal hepatic metastases. Cancer 82: 1244-1249.
- Minagawa M, Makuuchi M, Torzilli G, Takayama T, Kawasaki S, et al. (2000) Extension of the frontiers of surgical indications in the treatment of liver metastasis from colorectal cancer: long term results. Ann Surg 231: 487-499.
- Ercolani G, Grazi GL, Ravaioli M, Grigioni WF, Cescon M, et al. (2004) The role of lymphadenectomy for liver tumors: further considerations on the appropriateness of treatment strategy. Ann Surg 239: 202-209.
- Laurent C, Sa Cunha A, Rullier E, Smith D, Rullier A, Saric J (2004) Impact of microscopic hepatic lymph node involvement on survival after resection of colorectal liver metastasis. J Am Coll Surg 198: 884-891.
- Elias D, Saric J, Jaeck D, Arnaud JP, Gayet B, et al. (1996) Prospective study of microscopic lymph node involvement of the hepatic pedicle during curative hepatectomy for colorectal metastases. Br J Surg 83: 942-945.
- Kokudo N, Sato T, Seki M, Ohta H, Azekura K, et al. (1999) Hepatic lymph node involvement in resected cases of liver metastases from colorectal cancer. Dis colon rectum 42: 1285-1290.
- Viana EF, Herman P, Siqueira SC, Taka T, Carvalho P, et al. (2009) Lymphadenectomy in colorectal cancer liver metastases resection: Incidence of hilar limph nodes micrometastasis. J Surg Oncol 100: 534-537.
- Bennett JJ, Schmidt CR, Klimstra DS, Grobmyer SR, Ishill NM, et al. (2008) Perihepatic lymph node micrometastases impact outcome after partial hepatectomy for colorectal metastases. Ann Surg Oncol 15: 1130-1136.
- Mignotte H, Treilleux I, Chassagne-Clément C, Bem C, Lopez R, et al. (2000) Intérêt de l'injection périaréolaire du colorant lymphotrope dans la recherche du ganglion axillaire sentinelle dans le cancer du sein. Bull Cancer 87: 601-604.
- Giuliano AE, Jones RC, Brennan M, Statman R (1997) Sentinel lymphadenectomy in breast cancer. J Clin Oncol 15: 2345-2350.
- Morton DL, Ollila DW (1999) Critical review of sentinel node hypothesis. Surgery 126: 815-819.
- Mullenix PS, Brown TA, Meyers MO, Giles LR, Sigurdson ER, et al. (2005) The association of cytokeratin-only-positive sentinels lymph nodes and subsequent metastases in breast cancer. Am J Surg 189: 606-609.
- Maehara Y, Baba H, Ohno S, Sugimachi K (1995) Cytokeratin staining reveals micrometastases in lymph nodes of early gastric cancer. Surgery 117: 480.
- Noura S, Yamamoto H, Miyake Y, Kim B, Takayama O, et al. (2002) Immunohistochemical assessment of localization and frequency of micrometastases in lymph nodes of colorectal cancer. Clin Cancer Res 8: 759-767.
- Perez RO, Habr-Gama A, Nishida Arazawa ST, Rawet V, Siqueira SA, et al. (2005) Lymph node micrometastasis in stage II distal rectal cancer following neoadjuvant chemoradiation therapy. Int J Colorectal Dis 20: 434–439.
- Ishibashi K, Ohsawa T, Yokohama M, Ishizuka N, Miyazaki T, et al. (2006) Hepatic lymph node involvement in patients with synchronous multiple liver metastases of colorectal cancer. Gan To Kagaku Ryoho 33: 1834-1837.
- Carpizo DR, D´Angelica M (2009) Liver resection for metastatic colorectal cancer in the presence of extrahepatic disease. Ann Surg Oncol 16: 2411-2421.
- Nakamura S, Yokoi Y, Suzuki S, Baba S, Muro H (1992) Results of extensive surgery for liver metastases in colorectal carcinoma. Br J Surg 79: 35-38.
- Wakai T, Shirai Y, Sakata J, Nagahashi M, Kaneko K, et al. (2009) Hepatic lymph node dissection provides a survival benefit for patients with nodal disease of colorectal carcinoma liver metastases. Hepatogastroenterology 56: 186-190.
- Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvant T, et al. (2008) Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol 26: 3672-3680.
- Tschmelittsch J, Klimstra DS, Cohen AM (2000) Lymph node micrometastases do not predict relapse in stage II colon cancer. Ann Surg Oncol 7: 601-608.
- Calhoun KE, Hansen NM, Turner RR, Giuliano AE (2005) Nonsentinel node metastases in breast cancer patients with isolated tumor cells in the sentinel node: implications for completion axillary node dissection. Am J Surg 190: 588-591.
Figures at a glance
 |
| Figure 1 |
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14971
- [From(publication date):
specialissue-2013 - Jul 28, 2024] - Breakdown by view type
- HTML page views : 10512
- PDF downloads : 4459
