Review Article Open Access
Hereditary Gastrointestinal Polyposis Syndromes: A Review Including Newly Identified Syndromes
Aaron Ryan Huber1*, Jennifer J Findeis-Hosey2 and Christa L Whitney-Miller2
1University of Rochester Medical Center, Surgical Pathology Unit, USA
2University of Rochester Medical Center, School of Medicine and Dentistry, Rochester, USA
- Corresponding Author:
- Aaron Ryan Huber
University of Rochester Medical Center
Surgical Pathology Unit
601 Elmwood Avenue
Rochester, NY 14618, USA
Tel: (585) 275-1702
Fax: (585) 276-2802
E-mail: aaron_huber@urmc.rochester.edu
Received Date: November 06, 2013; Accepted Date: November 21, 2013; Published Date: November 29, 2013
Citation: Huber AR, Findeis-Hosey JJ, Whitney-Miller CL (2013) Hereditary Gastrointestinal Polyposis Syndromes: A Review Including Newly Identified Syndromes J Gastroint Dig Syst 3:155. doi:10.4172/2161-069X.1000155
Copyright: © 2013 Huber AR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
There are multiple hereditary and non-hereditary polyposis syndromes that were originally categorized as adenomatous or hamartomatous. More recently, serrated polyps and their syndromes have been defined. Nearly all of these syndromes have a risk of colorectal cancer in the individuals and affected family members. Most of these syndromes are associated with extracolonic manifestations, including extracolonic tumors. The major clinical features, genetic mechanisms, and clinical management of Familial Adenomatous Polyposis (FAP), Peutz-Jeghers Syndrome (PJS), Juvenile Polyposis Syndrome (JPS), PTEN Hamartoma Syndrome (PTHS), and the more recently described syndromes MUTYH-Associated Polyposis, Hereditary Mixed Polyposis Syndrome, Serrated Polyposis Syndrome, and Polymerase Proofreading Associated Polyposis are summarized in this article.
Keywords
Polyposis; Colorectal cancer; Adenocarcinoma; Tumors
Introduction
Hereditary gastrointestinal polyposis syndromes were traditionally separated into hamartomatous and adenomatous types [1]. Recently, a new type of polyposis syndrome has been defined and is associated with various types of serrated polyps [2]. Almost all of these syndromes have an increased risk of colorectal cancer and most are associated with an increased risk of a variety of extracolonic tumors, both benign and malignant [1,3-8]. This is a rapidly evolving field with some entities being redefined, some entities having their genetic basis unraveled, and new entities emerging among the polyposis syndromes. We will subsequently review the major hereditary polyposis syndromes and provide an update on both the current nomenclature and the current concepts in pathogenesis.
Familial Adenomatous Polyposis (FAP) and Variants
Familial adenomatous polyposis (FAP) and its variants are the prototypical adenomatous polyposis syndromes and are all phenotypic variants of a single autosomal dominant disorder due to germline mutations in the adenomatous polyposis coli (APC) gene [3]. FAP accounts for less than 1% of all colorectal cancers and has an incidence of 1/8,000 to 1/10,000 live births [4-7]. FAP is associated with an essentially 100% lifetime risk of colorectal carcinoma at an average age of 39 years [1,5,6]. The age of polyposis diagnosis is variable but adenomas are usually present by the second decade of life and the majority of patients have developed polyps by the fourth decade of life [1,3]. FAP and the variants have a range of different extracolonic manifestations but all have adenomatous polyps within the gastrointestinal tract [1,3].
Clinical features
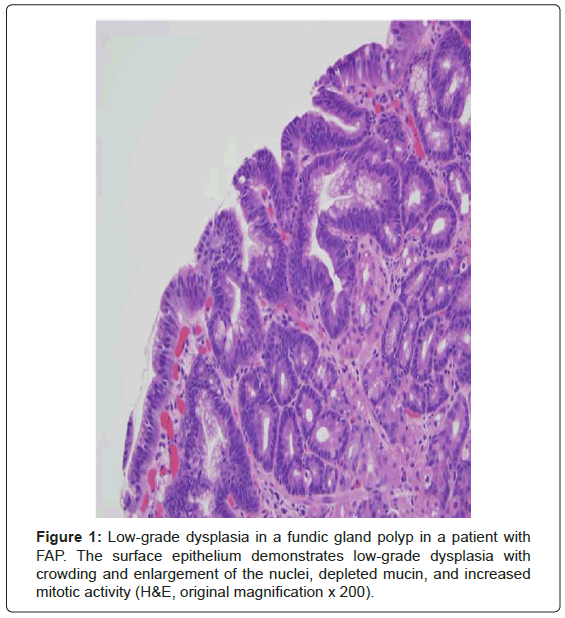
By definition, the classic form of FAP has greater than 100 colonic adenomatous polyps and these polyps usually number in the thousands [1,3-7]. This defining number was originally selected because all patients with FAP in the St. Mark’s Hospital Polyposis Registry had more than 100 polyps [1]. This number was obtained from carefully examined surgically resected colectomy specimens and was not endoscopically derived [1]. Besides colorectal polyps, these patients commonly develop upper gastrointestinal polyps. The duodenum is the second most common location and the periampullary region in particular is prone to the development of adenomas [1,3]. Small bowel adenocarcinoma is the second most common malignancy after colorectal adenocarcinoma with a 4-12% lifetime risk in FAP patients and is the leading cause of morbidity and mortality after colectomy is performed [1,5,6]. Fundic gland polyps are very common in FAP patients and often harbor lowgrade dysplasia, a feature that is strongly associated with FAP (Figure 1) [3]. There is also a low cumulative lifetime risk (less than 1-2%) of extracolonic malignancies including pancreatic cancer, thyroid cancer (particularly papillary), pancreatoblastoma, and medulloblastoma [5,6]. Congenital hypertrophy of the retinal pigment epithelium (CHRPE) is present in over 90% of FAP patients; this characteristic can be used clinically as a diagnostic marker of the syndrome [6].
The attenuated form of FAP (AFAP), as the name implies, is associated with fewer polyps, averaging approximately 30 colorectal adenomas [3]. This is a less severe form of the disease with a later onset of colorectal carcinoma, averaging 12 years later than the classic form, and a lower risk of developing colorectal carcinoma [3,5]. Gardner syndrome refers to the polyposis syndrome plus other extracolonic manifestations including fibromatosis (desmoid tumors), osteomas of the skull and mandible, epidermal inclusion cysts, nasopharyngeal angiofibroma, dentigerous cysts, and abnormal dentition [1,3]. Fibromatosis occurs in approximately 10% of patients with FAP and is most frequently seen within the abdomen and small bowel mesentery but also in the abdominal wall or extremities. FAP patients have an 852 times higher risk of developing fibromatosis than the general population [3,6]. Turcot syndrome refers to two groups with different germline cancer causing mutations and central nervous system tumors [3]. The first group is comprised of FAP/Turcot patients and they account for 75% of all Turcot patients [3]. These patients tend to have adenomatous polyposis and cerebellar medulloblastomas and this variant has been also referred to as Crails syndrome [3,6]. The second group comprises 25% of Turcot patients; these patients have Lynch syndrome and not FAP and have a tendency to develop glioblastoma multiforme [3].
Genetics
FAP and the variant phenotypes are all due to germline mutations in the APC gene which has been mapped to chromosome 5q21-22 [3-7]. The APC gene is a large tumor suppressor gene involved in the Wnt signaling pathway which has many functions including repression of apoptosis, induction of cell cycle progression and proliferation, and control of cell growth [6,7]. Approximately 75% of mutations are inherited and the remaining 25% are new mutations in patients with no family history of the disease [3,4,6]. The APC gene is large and there are over 300 recognized disease causing mutations [3,4,7]. The most common mutations are either nonsense or truncating frameshift mutations which collectively account for approximately 95% of APC mutations [4,6,7]. Recently, certain genotype-phenotype correlations have been noted for FAP; however the correlation is not exact as even within the same family identical mutations may have phenotypic heterogeneity [3]. Mutations in the central portion of the APC gene are associated with classic FAP (between codons 169-1393) and severe polyposis (codons 1290-1400) [3,4,6,7]. Mutations at the 3’ and 5’ ends and in exon 9 of the gene are associated with the attenuated FAP phenotype [3,4,6,7].
Clinical management
First degree relatives of patients with FAP should be screened with APC gene testing at the age of 10-years-old [3,6]. If no mutation is identified, periodic flexible sigmoidoscopy from age 12-50 with decreasing frequency each decade and then routine colorectal cancer screening after the age of 50 is recommended [3,6]. Upper endoscopy should be performed initially at the age of 25-years-old and the every 6 months to 4 years depending on the severity of the upper tract polyposis burden [3,6].
Surgery remains the treatment of choice to diminish colorectal cancer risk in FAP and colectomy should be performed at diagnosis or shortly thereafter [3,5,6]. For duodenal lesions, surveillance and endoscopic removal of polyps is the management of choice for small lesions although the recurrence rate is high [5,6]. Surgery is usually reserved for patients with high upper gastrointestinal tract polyp burden and/or high grade dysplasia [6].
Chemoprevention using non-steroidal anti-inflammatory drugs has been extensively studied. Overall, selective (celecoxib and rofecoxib) and non-selective (sulindac and aspirin) COX-2 inhibitors do reduce polyp count and reduce adenomas in the retained rectal pouch in the short-term [3,5,6]. These medications have no real benefit and no significant effect on duodenal adenomas [3,5,6].
Peutz-Jeghers Syndrome
Clinical features
Peutz-Jeghers syndrome (PJS) is an autosomal dominant hamartomatous polyposis syndrome [1,3-10]. This is the second most common hamartomatous polyposis syndrome with an estimated incidence of 1/120,000-1/200,000 live births [3,6,7]. The syndrome is characterized and defined by hamartomatous polyps of the gastrointestinal tract, mucocutaneous pigmentation, and an elevated risk for a wide variety of malignancies [1,3-10].
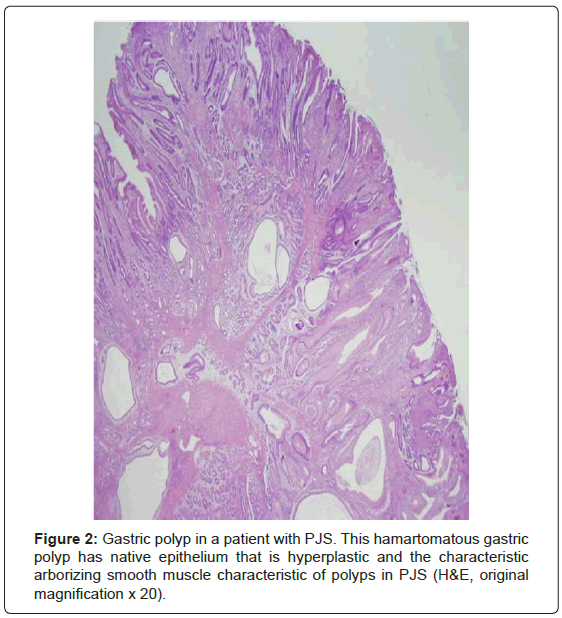
The hamartomatous polyps are most common in the small bowel (64-96% of cases) and only slightly less frequent in the stomach (24- 49%) and colon (27-53%) [1,3,4,6-10]. Rarely, polyps occur in other mucosal surfaces including the gallbladder, respiratory tract, and urinary tract [1,6,9,10]. The polyps, like any hamartoma, are composed of elements indigenous to the organ from which they arose arranged in a disorderly fashion [3]. The polyps typically have an arborizing pattern caused by a prominent central smooth muscle component with papillary fronds covered by normal epithelium (Figure 2) [1,3,4,6,9,10]. Dysplasia is uncommon; however, a potential histopathologic pitfall is the presence of misplaced mucinous epithelium (pseudoinvasion) in the submucosa, the muscularis propria, or even through the wall of the small intestine. This phenomenon occurs in approximately 10% of small bowel polyps and should not be misinterpreted as invasive welldifferentiated carcinoma [1,3,4,6,10]. The polyps frequently present with intussusception or obstruction, bleeding, or abdominal pain in the second decade of life [2,6,7-10].
The mucocutaneous pigmentation or melanin spots are a hallmark clinical feature and are present in over 95% of patients with PJS and are most likely to be present on the lips (95%) or buccal mucosa (66- 83%); however, they also can occur on the skin around the eyes, mouth and/or nose, on the palms and soles, and less commonly on the digits [1,3,6,9,10]. Notably, the pigmented spots on the skin may fade with age, though they tend to persist on the buccal mucosa [1,3,6,9,10]. These spots may be present prior to the gastrointestinal manifestations of the disease [6,8].
PJS patients are at risk for a wide variety of benign and malignant tumors. Carcinoma of the gastrointestinal tract is a frequent complication and the reported prevalence in PJS ranges from 2-12% [1,3,11]. Well-documented cases of carcinoma, some with associated dysplasia, have been seen in the esophagus, stomach, small bowel, ovary, uterus, testis, and colon, with the stomach and small bowel as the two most frequent sites [1,3,4-7,9,10]. The risk of non-gastrointestinal carcinomas is 15 times the rate in the general population and includes tumors of the breast (bilateral), pancreaticobiliary tree, and lung [3,4-10]. In 1970 Scully first described an unusual ovarian tumor, the sex cord tumor with annular tubules (SCTAT) tumor, in patients with PJS [1,3]. This tumor can be identified in almost all females with PJS and tends to be bilateral, multifocal, and small [1,3]. When SCTAT occurs in women without PJS the tumors are unilateral, larger, and usually do not have foci of calcification [1]. Calcifying Sertoli cell tumors occur in males beginning at a young age and may have malignant potential [1,3,4,6,9,10]. Well-differentiated adenocarcinoma of the uterine cervix (adenoma malignum, minimal deviation adenocarcinoma) also occurs more commonly in females with PJS [1,3,4,6,9,10].
Genetics
Peutz-Jeghers syndrome is caused by truncating mutations in the PJS gene which was shown to be a novel serine threonine kinase gene, named STK11 (also known as LKB1), on the short arm of chromosome 19 [12]. STK11 has multiple functions including cell cycle regulation, mediation of apoptosis, and cellular polarity [5]. Approximately 50% of cases of PJS are familial and approximately 70% of familial cases have a mutation in the STK11 gene [3]. Approximately 50% of cases are new onset and 30-70% of these cases have the mutation in STK11 [3]. The remaining cases do not have an identifiable germline mutation; it has been suggested that short comings in mutational analyses and patient selection account for a large percentage of these cases. Other possible reasons include genetic heterogeneity in PJS [6].
Clinical management
Genetic testing is advised for at-risk offspring of PJS patients when symptoms occur or in their late teens if they remain asymptomatic [6]. If there is no family history but patients have the hallmark cutaneous signs genetic testing is also indicated [6].
The main goals of treatment for PJS are preventing polyp-related complications and the early detection of cancer [5]. Colonoscopy, endoscopy, and video capsule endoscopy should begin around 8 years of age and are repeated every 3 years if polyps are identified or at 18 years of age if no polyps are identified [5]. After age 18, exams should be performed every 3 years until age 50 when the interval should be reduced to annually or biennially because of the increased rate of cancer [5]. To prevent polyp complications capsule endoscopy or MR enterography starting at age 8-10 years should be performed with removal of any polyp greater than 1 cm in size [5]. For extraintestinal cancers, it is suggested that women undergo annual breast MRI or ultrasound beginning at the age of 25-30 years of age with conversion to annual conventional mammography at the age of 50 years [3,5,10]. Males should have an annual testicular exam from birth until age 12 and follow-up of any abnormality with ultrasound [5]. Some groups advocate pancreatic endoscopic ultrasound and/or MRCP beginning at age 30 and done at an interval of every 1-2 years [3,6,10]. Additionally, an annual pelvic exam and ultrasound by age 20 and Pap smear by the age of 18 are recommended for women [3,10].
Juvenile Polyposis Syndrome
Clinical features
Juvenile polyposis syndrome (JPS) is the most common hamartomatous polyposis syndrome occurring in approximately 1/100,000 live births [3,6,7,10]. This syndrome is inherited as an autosomal dominant trait with variable penetrance and typically presents in the first or second decade of life with rectal bleeding, a prolapsed rectal polyp, abdominal pain, diarrhea, protein-losing enteropathy, or anemia [1,3,5,6,7,8,10]. Approximately, 20-50% of patients have a family history while approximately 25-50% of patients have new mutations with no family history [3,6,7,9,10]. The polyps are most commonly seen in the colon and rectum, particularly the rectosigmoid, but may diffusely involve the gastrointestinal tract [1,3,5-10]. The syndrome is defined by the World Health Organization (WHO) as: (1) more than five juvenile polyps in the colon or rectum, (2) juvenile polyps throughout the intestinal tract, or (3) any number of juvenile polyps in a patient with a family history of JPS [5,6,8,10]. In addition to cases fitting the WHO classification, there are other forms of juvenile polyposis. A rare form occurs in infancy that is a severe, life-threatening form of the disease with diarrhea, hemorrhage, malnutrition, protein-losing enteropathy, and death at an early age [4,5,6,10]. This particular form is caused by microdeletions of the 10q23.2-10q23.3 region containing BMPR1A and PTEN genes [9]. Notably, sporadic solitary juvenile polyps also occur in approximately 2% of the pediatric population and are non-syndromic and have no increased risk of malignancy [4,6,9,10]. Extraintestinal features or anomalies occur in 30% of patients with JPS and cardiovascular and cranial/skeletal anomalies are the most common, each occurring in approximately 12% [4,13].
It was noted early on and it is now well established that there is a definite increased risk of gastrointestinal cancer, primarily colorectal carcinoma, in patients with JPS [1,3,4,6-10,13]. This risk ranges from 20-70%, increases with age, and appears to arise in adenomatous epithelium within the polyps [1,3,4,6-10]. It is, therefore, imperative for the pathologist to carefully examine all juvenile polyps to exclude a component of dysplastic epithelium since it is thought that carcinomas arise from these dysplastic changes [1,3,6,13], while being cautious not to over- interpret reactive epithelial changes as dysplasia [1,3].
Morphologically, juvenile polyps have a fairly characteristic appearance. Grossly, the polyps are usually 1-1.5 cm in diameter with a smooth surface [1]. Approximately 20% of polyps are “atypical” and these polyps may be larger (up to 5 cm in diameter) and have a multilobated or papillary surface [3,8,9]. Histologically, the polyps demonstrate cystically dilated mucin-filled glands within an abundant edematous and inflamed lamina propria, which is the most characteristic feature [1,3,4,6,9]. The surface of the polyp is often eroded [1,3,4]. The polyps in JPS patients tend to be larger, are more often papillary or multilobulated, are less often eroded, have less abundant stroma, and have more proliferative smaller glands [3,4,6,8,9]. It is worth reiterating that dysplasia can be present in juvenile polyps and is more common in those polyps that are described as the “atypical” variant [1,3,4,6,8,9].
Genetics
JPS is caused by a germline mutation in either the SMAD4/DPC4 gene on the long arm of chromosome 18 or the bone morphogenetic protein receptor 1A (BMPR1A) gene on the long arm of chromosome 10 [3,4,5-10,13,14]. Both of these genes are involved in the transforming growth factor-β (TGF-β) signaling pathway [3,5-10,14]. Mutations in these two genes are found in approximately 60-70% of JPS patients [3,8]. The PTEN gene was believed to be involved in JPS but these patients likely have Cowden syndrome instead [3,4,6,10].
Approximately 20% of patients with a SMAD4 mutation have a combined syndrome of hereditary hemorrhagic telangiectasia (HHT) and JPS [5,6,8,13]. Additionally, a few patients with ENG gene missense mutations have been identified; however, it is unclear at this time how this contributes to JPS [8].
Clinical management
There are two goals of gastrointestinal surveillance in JPS: (1) preventing complications and morbidity related to polyps (i.e. GI bleeding, anemia) and (2) detection and prevention of cancer [13]. The data are still not entirely clear whether endoscopic surveillance and polypectomy actually prevent cancer partially because the true cancer risk is still not clearly defined [13]. Upper and lower gastrointestinal endoscopy should begin at age 12 years, or earlier if symptomatic, and be repeated every 1-3 years according to the severity of the disease and polyp burden [13]. A complete blood count and full cardiovascular exam should be performed annually [13]. In SMAD4 mutation carriers an electrocardiogram, chest radiograph, and echocardiogram should be performed before general anesthesia and consideration should be made for HHT screening protocols [13]. For familial cases, genetic testing should be considered in at-risk offspring at age 4 years [13]. Surgical management consisting of total colectomy is indicated for those with an extensive polyp burden, uncontrolled bleeding or diarrhea, dysplastic polyps, or families with a high incidence of colorectal cancer [3,6]. Some recommend colectomy by the age of 20 years for all JPS patients [3].
PTEN Hamartoma Tumor Syndrome (PTHS)
Clinical features
There are two rare polyposis syndromes that both show autosomal dominant inheritance with a relatively high penetrance (approximately 80%) that are caused by germline mutations in the phosphatase and tensin homologue (PTEN) gene: Cowden syndrome (CS) with a prevalence of approximately 1/200, 000 to 1/250, 000 and Bannayan- Riley-Ruvalcaba syndrome (BRRS) (also known as Ruvalcaba- Myrhe-Smith syndrome) [1,3,4-10]. Hamartomatous gastrointestinal polyps occur in both syndromes [1,3,6]. The polyps in CS may occur throughout the gastrointestinal tract from the esophagus to the rectum with the most frequent sites being the stomach (75%), colon (66%), esophagus (66%), and duodenum (37%) [10]. In BRRS the polyps are most common in the colon and tongue [3]. Most polyps are inflammatory-type polyps that are indistinguishable from juvenile polyps; however, a more myofibroblastic than edematous stroma is usually present [3,4]. A mixture of polyps with different histologies is common and has been found to be associated with PTEN mutation [8]. The types of polyps include ganglioneuromas, lipomas, adenomas, nodular lymphoid hyperplasia, juvenile, inflammatory, and glycogenic acanthosis in the esophagus [1,8,10].
Both syndromes have different extraintestinal manifestations. Approximately 83% of CS patients have characteristic cutaneous facial lesions that are histologically trichilemmomas [1,3]. Other skin lesions include acral keratoses in 63% [1,3,9,10]. Oral papillomas that are histologically fibromas are also common and occur in approximately 83% of patients [1,3]. CS patients also have a variety of soft tissue tumors including lipomas (40%), hemangiomas (22%), and neuromas (11%) [1,3]. There are additional extraintestinal manifestations in CS including ovarian cysts (22%), benign thyroid lesions (60%), and a variety of craniofacial anomalies [1,9,10]. Although CS patients originally were believed to have a negligible risk for colorectal cancer recent data suggest that among PTEN mutation carriers 13% develop colorectal carcinoma before the age of 50 [8]. These patients also have an increased risk of breast cancer (25-50%), thyroid cancer (10%), and endometrial cancer (10%) [3,6,7,10]. Finally, Lhermitte-Duclos disease is a pathognomonic feature of CS and consists of mental retardation and cerebellar dysplastic gangliocytomas [3,9,10].
BRRS patients have, in addition to their hamartomatous intestinal polyposis, macrocephaly, lipomas, hemangiomas, lipid myopathy, mental deficiency, craniofacial anomalies, and pigmented macules on the shaft and glans of the penis [1,3,6,7,10]. Since CS and BRRS are essentially phenotypic variants of the same disease they have similar risks and distributions of malignancies [3].
Genetics
Both CS and BRRS are due to germline mutations in the phosphatase and tensin homologue (PTEN) gene on the long arm of chromosome 10 [3,4-10]. PTEN plays an important role in apoptosis, in preventing uncontrolled cell growth, and is possibly involved in cell migration and adhesion [9]. Mutations in the PTEN gene are found in approximately 80% of those with CS and approximately 60% of those with BRRS [3,7,10]. Approximately 10-50% of CS cases are familial and the exact frequency of de novo mutations is not entirely clear [7,10].
Clinical management
Cancer screening is the current focus of management with particular emphasis on earlier and more frequent breast cancer screening in women [10]. This includes an annual mammogram and breast MRI beginning at age 30-35 years and a clinical breast exam every 6 months beginning at age 25 years [10]. For both men and women, an annual thyroid ultrasound and annual thyroid exam commencing at the age of 18 are recommended [10]. Annual dermatologic exams should be considered [10].
More Recently Described Polyposes
(MUTYH-associated polyposis, Serrated Polyposis, Hereditary Mixed Polyposis, Polymerase Proofreading Polyposis)
MUTYH (MYH)-Associated Polyposis (MAP)
Clinical features
MUTYH-associated polyposis (MAP) was originally identified in Welsh kindred in 2002 and is an autosomal recessive (unlike most other polyposis syndromes) adenomatous polyposis syndrome most commonly with an attenuated FAP-like phenotype [4,7,8,15,16]. The syndrome usually has from 10-100 adenomatous polyps and there are cases with less than 10 or no polyps [5,8,15]. It is now recognized that serrated polyps including hyperplastic polyps and sessile serrated adenomas also occur in approximately one-half of MAP patients [4,5,8]. Since this is an autosomal recessive syndrome it usually presents in a sporadic manner in the mid-50s which is later than patients with FAP or AFAP [4,7,8,15]. The risk of colorectal cancer is increased in MAP and the tumors occur at a younger age, tend to be right-sided and synchronous, and occur in those with a personal history of adenomas [5,17]. Duodenal and gastric polyps and duodenal adenocarcinoma can occur in MAP; however, the rate of extracolonic cancers appears to be lower than in FAP or AFAP but twice that of the general population [5,8,15].
Genetics
The MutY human homologue (MYH) gene is located on the short arm of chromosome 1, codes for a DNA glycosylase, and is part of the base excision repair (BER) system [4,5,8,15-17]. The BER system serves a vital role in the cellular defense against oxidative damage to DNA specifically the oxidation of guanine to 8-oxo-guanine (8-oxoG) [4,8,15-17]. After oxidative damage mis-pairing can occur between 8-oxoG and adenine, causing a G:C>T:A transversion [4,8,15,16]. The enzymatic activity of this gene is specifically designed to correct these transversions induced by the variant base 8-oxo-guanine (8- oxoG) [4,8,15,16]. Patients with MUTYH gene mutations have reduced or absent enzymatic activity, therefore, they are unable to repair this damage and accumulate other adverse secondary somatic mutations [8,15].
Two mutations account for approximately 80% of MYH mutations: Y165C and G382D missense mutations [4,7,8,15-17]. There are other variant mutations that occur in those of Italian, Indian, Asian, or Pakistani descent [4,8,15].
Clinical management
A surveillance protocol similar to that utilized for AFAP is utilized in MAP patients [15, please see above]. Notably, colonoscopy is utilized instead of flexible sigmoidoscopy in MAP due to the possibility of lower numbers of polyps [15]. Since the polyp burden is usually less colonoscopy with polypectomy is the preferred management for MAP with colectomy reserved for those with a high polyp burden, large adenomas, or advanced adenomas [15].
Serrated Polyposis Syndrome (SPS)
Clinical features
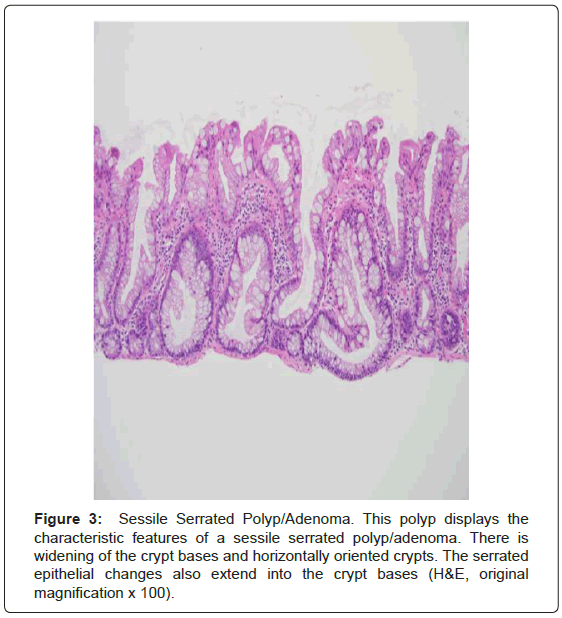
Serrated polyposis syndrome (SPS; formerly known as hyperplastic polyposis syndrome) is a rare, likely hereditary, polyposis syndrome most common in those of northern European ancestry. It is also more common among current smokers. The prevalence is estimated to range from 1:3,000 to 1:100,000, thought the syndrome may be underrecognized [2,4,5,8,18-20]. The median age at diagnosis is 51-yearsold [2,20]. The current WHO criteria for the diagnosis of SPS are as follows: (1) Five or more serrated polyps proximal to the sigmoid colon with at least two greater than 1 cm in size; (2) at least one proximal serrated polyp in an individual with a first-degree relative with SPS; or (3) Twenty or more serrated polyps, of any size, distributed throughout the colon (Figure 3) [5,8,18-20]. Conventional adenomas also occur in the syndrome in up to 85% of patients [4,8,18]. There is a 25-40% risk of colorectal carcinoma in patients with SPS and is significantly higher than that of the general population [2,4,5,8,18-20]. However, unlike the other polyposes there appears to be little if no risk of extracolonic malignancy and no extracolonic manifestations [8].
Genetics
The genetic basis of SPS has yet to be established and both autosomal dominant and recessive modes of inheritance have been proposed [5,8]. The mutations associated with the other polyposis syndromes (BMPR1A, SMAD4, PTEN, MUTYH, and GREM1) are not commonly mutated in SPS [21]. This syndrome is very heterogeneous and may have three phenotypes including (1) large sessile serrated polyps/ adenomas in the proximal colon with a high risk of colorectal cancer; (2) numerous small hyperplastic polyps in the colorectum with a lower risk of colorectal cancer; and (3) numerous small serrated polyps on the left side [4].
Clinical management
There are no uniform screening or surveillance guidelines established for SPS; however, some advocate colonoscopy with the use of narrow band imaging or chemoendoscopy every 1-2 years to improve polyp detection rates [5].
Hereditary Mixed Polyposis Syndrome (HMPS)
Clinical features
Hereditary mixed polyposis syndrome (HMPS) is characterized by the presence of polyps of several different types and/or individual polyps that are composed of more than one histologic type [4,8]. The syndrome appears to be inherited as an autosomal dominant trait [4,8]. The polyps are confined to the colon, are between 1-15 in number (and rarely exceed 50), and may resemble adenomas macroscopically [4,8]. The polyps vary both within a family and within an individual and may have an expanded lamina propria and resemble juvenile polyps [4,8]. These same polyps may have a serrated and villiform architecture resembling an “atypical” juvenile polyp [4,8]. The serrated polyps may or may not harbor dysplasia; conventional adenomatous polyps also occur [4,8]. There is an increased risk of colorectal carcinoma usually diagnosed at the mean age of 48 years that is most likely attributable to the sessile serrated adenomas, conventional adenomas, and “atypical” juvenile polyps [4,8]. No extracolonic features have been identified [4]. This syndrome is easily confused and clinically overlaps with HMPS and JPS [4,8].
Genetics
This syndrome is restricted to one large and extended Ashkenazi Jewish family and smaller Ashkenazi Jewish families [4,8]. Recently, the genetic defect that causes HMPS was identified as 40 kb duplication on the long arm of chromosome 15 upstream from the GREM1 gene [22]. GREM1 is involved in the BMP pathway and is a BMP antagonist [22]. This mutation leads to increased and ectopic expression of GREM1 mRNA and protein [22]. This is thought to possibly promote multiple stages of colorectal tumorigenesis [22].
Clinical management
The management of these patients is not clearly defined. Colonoscopy and polypectomy is recommended every 1-2 years and due to the proximal location of many polyps, sigmoidoscopy has no role in surveillance [9]. If surgery is contemplated, it has been proposed that total colectomy be performed when there is a family history of increased malignancy or adenomas on colonoscopy [9].
Polymerase Proofreading Associated Polyposis [PPAP]
Clinical features
A recently described, primarily adenomatous polyposis syndrome, polymerase proofreading associated polyposis (PPAP) is another autosomal dominantly inherited polyposis syndrome with a variable phenotype that may include 10-100 adenomas, often presenting before 60-years, with or without colorectal carcinoma, and/or isolated early onset colorectal carcinoma [8,23]. These patients may also have hyperplastic polyps although adenomas are the most common type of polyp [8]. The risks of colorectal and other forms of cancer have yet to be determined as there are too few reported families at this time [8].
Genetics
Monoallelic germline mutation of the DNA polymerase subunit genes POLD1 and POLE were identified by whole genome sequencing in a subset of patients with multiple adenomas and/or colorectal carcinoma [23]. This evidence suggests that the germline POLE mutation (L424V variant) causes tumors by decreased fidelity of replication-associated polymerase proofreading, leading to increased mutation rates through base substitution [23]. The POLD1 mutation (S478N) variant predisposes to colorectal cancer, endometrial cancer, and possibly, brain tumors [23]. The POLD1 mutation also is believed to have impaired proofreading as a mechanism for tumorigenesis [24]. More data are needed to clearly delineate the carcinogenic mechanisms of these mutations.
Clinical management
Although further data are required, it is probably best to manage mutation carriers with regular and frequent colonoscopic polypectomy and consideration of prophylactic surgery in a manner between the recommendations for Lynch syndrome and MAP [23].
Summary
We have provided a concise review of the well-known and the recently described hereditary polyposis syndromes (summarized in Table 1). This is truly an evolving field with expansion in the molecular and phenotypic characterization of these syndromes. In the near future, the genetic mechanism(s) of sessile serrated polyposis may be unraveled and new syndromes or genetic mechanisms for familial colorectal carcinoma will be discovered.
| Syndrome | Inheritance | Gene | Polyp and Cancer Location (s) | Extraintestinal Manifestations |
|---|---|---|---|---|
| FAP and variants | Autosomal Dominant | APC on 5q | Colorectum >> stomach and small bowel | Fibromatosis (desmoid), congenital hypertrophy of the retinal pigmented epithelium, skull and mandible osteomas, epidermal cysts, dental alterations |
| Peutz-Jeghers | Autosomal Dominant | STK11 on 19p | Polyps: Small bowel > stomach, colon Malignant Potential: 10-20%, entire GI tract High prevalence of pancreatic, gastric, endocervical, and bilateral breast cancers |
Mucocutaneous pigmentation, gonadal tumors |
| Juvenile Polyposis | Autosomal Dominant | SMAD4 on 18q or BMPRIA on 10q | Polyps: Colorectum > stomach > small bowel; may be diffuse Malignant Potential: Colon cancer 20-70%, occurs within polyps with dysplasia/cancer |
Sporadic forms only: hydrocephalus, hypertelorism, GU and cardiac defects, malrotation of the gut |
| PTEN Hamartoma | Autosomal Dominant | PTEN on 10q | Cowden Syndrome Polyps: throughout the GI tract Cowden Syndrome Malignant Potential: none for GI lesions, increased breast, thyroid, and endometrial cancers Bannayan-Riley-Ruvalcaba-Syndrome Polyps: Colon and tongue Bannayan-Riley-Ruvalcaba Syndrome Malignant Potential: as above for Cowden Syndrome |
Cowden Syndrome: Facial trichilemmomas, acral keratoses, oral fibromas, lipomas, hemangiomas, macrocephaly, Lhermitte-Duclos disease Bannayan-Riley-Ruvalcaba Syndrome: macrocephaly, lipomas, pigmented penile macules |
| MUTYH (MYH)-Associated Polyposis | Autosomal Recessive | MUTYH (MYH) on 1p | Colorectum > stomach and small bowel | |
| Serrated Polyposis | Currently undefined, likely hereditary | Currently undefined, likely hereditary | Colorectum with increased risk of colorectal cancer | None defined |
| Hereditary Mixed Polyposis | Appears to be autosomal dominant | GREM1 on 15q | Confined to colon with increased risk of colorectal cancer | None defined |
| Polymerase Proofreading Associated Polyposis | Autosomal Dominant | POLD1 and POLE | Colorectum with increased risk of colorectal cancer | None currently defined |
Table 1: Summary of the Hereditary Polyposis Syndromes.
References
- Haggitt RC, Reid BJ (1986) Hereditary gastrointestinal polyposis syndromes. Am J Surg Pathol 10: 871-887.
- Crowder CD, Sweet K, Lehman A, Frankel WL (2012) Serrated polyposis is an underdiagnosed and unclear syndrome: the surgical pathologist has a role in improving detection. Am J Surg Pathol 36: 1178-1185.
- Bronner MP (2003) Gastrointestinal inherited polyposis syndromes. Mod Pathol 16: 359-365.
- Jass JR (2007) Gastrointestinal polyposes: clinical, pathological and molecular features. Gastroenterol Clin North Am 36: 927-946.
- Patel SG, Ahnen DJ (2012) Familial colon cancer syndromes: an update of a rapidly evolving field. Curr Gastroenterol Rep 14: 428-438.
- Brosens LA, van Hattem WA, Jansen M, de Leng WW, Giardiello FM, et al. (2007) Gastrointestinal polyposis syndromes. Curr Mol Med 7: 29-46.
- Burt R, Neklason DW (2005) Genetic testing for inherited colon cancer. Gastroenterology 128: 1696-1716.
- Lucci-Cordisco E, Risio M, Venesio T, Genuardi M (2013) The growing complexity of the intestinal polyposis syndromes. Am J Med Genet A 161: 2777-2787.
- Calva D, Howe JR (2008) Hamartomatous polyposis syndromes. Surg Clin North Am 88: 779-817, vii.
- Gammon A, Jasperson K, Kohlmann W, Burt RW (2009) Hamartomatous polyposis syndromes. Best Pract Res Clin Gastroenterol 23: 219-231.
- van Lier MG, Westerman AM, Wagner A, Looman CW, Wilson JH, et al. (2011) High cancer risk and increased mortality in patients with Peutz-Jeghers syndrome. Gut 60: 141-147.
- Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, et al. (1998) Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet 18: 38-43.
- Latchford AR, Neale K, Phillips RK, Clark SK (2012) Juvenile polyposis syndrome: a study of genotype, phenotype, and long-term outcome. Dis Colon Rectum 55: 1038-1043.
- Howe JR, Roth S, Ringold JC, Summers RW, Järvinen HJ, et al. (1998) Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science 280: 1086-1088.
- Poulsen ML, Bisgaard ML (2008) MUTYH Associated Polyposis (MAP). Curr Genomics 9: 420-435.
- Nielsen M, Joerink-van de Beld MC, Jones N, Vogt S, Tops CM, et al. (2009) Analysis of MUTYH genotypes and colorectal phenotypes in patients With MUTYH-associated polyposis. Gastroenterology 136: 471-476.
- Cleary SP, Cotterchio M, Jenkins MA, Kim H, Bristow R, et al. (2009) Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 136: 1251-1260.
- Rosty C, Buchanan DD, Walsh MD, Pearson SA, Pavluk E, et al. (2012) Phenotype and polyp landscape in serrated polyposis syndrome: a series of 100 patients from genetics clinics. Am J Surg Pathol 36: 876-882.
- Caetano AC, Ferreira H, Soares J, Ferreira A, Gonçalves R, et al. (2013) Phenotypic characterization and familial risk in hyperplastic polyposis syndrome. Scand J Gastroenterol 48: 1166-1172.
- Jasperson KW, Kanth P, Kirchhoff AC, Huismann D, Gammon A, et al. (2013) Serrated polyposis: colonic phenotype, extracolonic features, and familial risk in a large cohort. Dis Colon Rectum 56: 1211-1216.
- Clendenning M, Young JP, Walsh MD, Woodall S, Arnold J, et al. (2013) Germline Mutations in the Polyposis-Associated Genes BMPR1A, SMAD4, PTEN, MUTYH and GREM1 Are Not Common in Individuals with Serrated Polyposis Syndrome. PLoS One 8: e66705.
- Jaeger E, Leedham S, Lewis A, Segditsas S, Becker M, et al. (2012) Hereditary mixed polyposis syndrome is caused by a 40-kb upstream duplication that leads to increased and ectopic expression of the BMP antagonist GREM1. Nat Genet 44: 699-703.
- Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, et al. (2013) Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 45: 136-144.
- Briggs S, Tomlinson I (2013) Germline and somatic polymerase ε and δ mutations define a new class of hypermutated colorectal and endometrial cancers. J Pathol 230: 148-153.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 19524
- [From(publication date):
December-2013 - Apr 05, 2025] - Breakdown by view type
- HTML page views : 14499
- PDF downloads : 5025