Review Article Open Access
Henipavirus Vaccine Development
Jackie Pallister1*, Deborah Middleton1, Christopher C. Broder2 and Lin-Fa Wang1
1CSIRO Livestock Industries, Australian Animal Health Laboratory, 5 Portarlington Road, Geelong, VIC, 3220, Australia
2Department of Microbiology and Immunology, Uniformed Services University, Bethesda, MD 20814, USA
- *Corresponding Author:
- Jackie Pallister
CSIRO Livestock Industries
Australian Animal Health Laboratory
5 Portarlington Road,Geelong
VIC, 3220, Australia
Tel: 61 3 5227 5277
Fax: 61 3 52275555
E-mail: jackie.pallister@csiro.au
Received Date: July 16, 2010; Accepted Date: September 07, 2011; Published Date: September 25, 2011
Citation: Pallister J, Middleton D, Broder CC, Wang LF (2011) Henipavirus Vaccine Development. J Bioterr Biodef S1:005. doi: 10.4172/2157-2526.S1-005
Copyright: © 2011 Pallister J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioterrorism & Biodefense
Abstract
The henipaviruses, Hendra virus and Nipah virus, belong to the family Paramyxoviridae which has long been a source of highly contagious pathogens for both humans and animals. Some notable paramyxoviruses such as measles virus have spilled over from animals into humans to cause significant morbidity and mortality. Since 1994 the henipaviruses have periodically emerged from their animal reservoir in flying foxes to cause disease in human and animal populations. The recent emergence of these viruses coupled with the high mortality rate associated with henipavirus infections and the lack of any licensed prophylactic or therapeutic treatments, makes them agents of particular concern in the area of both human and agricultural biodefense. Advances in our understanding of henipavirus infection and pathogenesis has led to the development of several promising vaccine candidates making it likely that vaccines for henipavirus infections may be available in the near future.
Keywords
Henipavirus; Vaccine; Hendra virus; Nipah virus
Introduction
The history of the interaction of man and animals is one involving a constant exchange of microorganisms; according to one survey an estimated 61% of human infections are caused by zoonotic organisms that have transferred from animals to humans [1]. The vast majority of new pathogens recognised in humans since 2001 are zoonotic [2] including those causing very high impact infections such as acquired immunodeficiency syndrome (AIDS), a result of infection with human immunodeficiency virus type-1 (HIV-1) [3], and severe acute respiratory syndrome (SARS) [4-6]. Plague is perhaps one of the best known and most terrifying of the zoonoses. The causative agent, the bacterium Yersinia pestis, is carried by rodents. The first recorded outbreaks were in the 6th and 7th centuries, and later the most notable one in the 14th century when, by some estimates, half the population of Europe died. The devastation caused by the natural spread of zoonotic agents such as these into human populations provides an insight into the destructive potential of deliberately introduced pathogens, particularly those to which humans have had little or no previous exposure, and for which there are no therapeutic treatments.
The use of biological agents as weapons is a time honoured tradition in the field of human conflict. Perhaps the earliest recorded instance is the catapulting of plague-ridden bodies into cities in the 14th century [7]. In World War I horses and mules were deliberately infected with glanders and anthrax [8], both agents capable of infecting humans as well as horses. More recently, in 2001, anthrax spores were posted in the United States mail and infected 22 people, of whom 11 contracted pulmonary anthrax and 5 died [9].
The virus family Paramyxoviridae, consisting of viruses possessing non-segmented, single stranded negative sense RNA genomes, is also the source of several highly contagious pathogens such as measles virus and mumps virus in humans and canine distemper virus in dogs [10]. Measles virus is most closely related to the etiologic agent of "cattle plague", rinderpest virus, and is thought to have been acquired from this species at the time of domestication of cattle, possibly around the 11th to 12th century [11]. On contact with naïve populations in the Americas in the 16th century the measles virus is reported to have killed 50% of certain human populations as well as two thirds of the population of Cuba in 1529. Some hundreds of years after measles virus is thought to have crossed into man, the paramyxoviruses as a group have continued to be a source of emerging zoonotic infections: two new additions to the paramyxovirus family, Nipah virus (NiV) and Hendra virus (HeV) emerged to cause infections among humans at the end of the last century. HeV and NiV were assigned to a new genus, the henipaviruses [12-14], based in part on both their broad host range and ability to cause mortalities in both humans and animals and their unique and distinctly large genomes size - 18,234 nucleotides for HeV [12], and 18,246 or 18,252 nucleotides for NiV Malaysia and NiV Bangladesh respectively [15,16] - which are approximately 15% larger than other paramyxovirus genomes.
Epidemiology of henipavirus infections
The source of these new henipavirus infections was not immediately apparent. In the first outbreak in 1994, HeV infected and caused mortalities in horses and humans [17]. In an effort to determine the reservoir species for HeV, a serological survey was carried out in eastern Queensland with sera collected from 46 species including 34 species of wildlife. No antibody was detected in this initial survey, but in a second survey targeting flying foxes and birds, antibodies capable of neutralizing HeV were detected in the 4 mainland species of pteropid bats found in eastern Australia [18] and virus was subsequently isolated from the reproductive tract and urine of wild-caught bats [19]. NiV appeared some 4 years later in an outbreak that primarily affected pigs and humans in peninsular Malaysia and Singapore [20], and was later shown to be closely related to HeV by immunological and molecular analyses [21,22]. Pteropid bats were the suspected reservoir host based on the similarities between HeV and NiV. Again surveillance of animal species identified neutralizing antibody to NiV mainly in flying foxes (Pteropus sp). Virus was isolated from urine of these animals and from partially eaten fruit [23] under trees in which bats foraged.
For both HeV and NiV a major mechanism of spillover infection is thought to be contamination of food sources by bats; such as pasture underneath fruit trees for horses in Queensland or contaminated date palm sap or fruits consumed by humans in Asia. Since 1994 there have been 31 outbreaks of HeV, unusually 17 of these have occurred in 2011 (Table 1). On each occasion, horses have been infected and, in 5 of these, transmission to humans has occurred. Although the number of known human infections is small, the mortality rate is high with 4 deaths recorded among 7 cases. In all instances the infection of humans has been through horses infected with HeV and no known cases of direct transmission from bats to humans have been reported [24]. In nature, HeV has so far only been isolated from bats, humans and horses, although other mammals replicate virus following exposure under laboratory conditions.
| Date | Location | Horses no. cases | Humans deaths/no. cases | Reference |
|---|---|---|---|---|
| 1994 Aug | Mackay , QLD | 2 | 1/1 (100%) | [75] |
| 1994 Sept | Hendra, QLD | 20 | 1/2 (50%) | [17] |
| 1999 Jan | Trinity Beach, QLD | 1 | 0/0 | [99] |
| 2004 Oct | Gordonvale, QLD | 1 | 0/1 | [100] |
| 2004 Dec | Townsville, QLD | 1 | 0/0 | [101] |
| 2006 June | Peachester, QLD | 1 | 0/0 | [100] |
| 2006 Oct | Murwillumbah, NSW | 1 | 0/0 | [102] |
| 2007 June | Peachester, QLD | 1 | 0/0 | [103] |
| 2007 July | Clifton Beach, QLD | 1 | 0/0 | [104] |
| 2008 July | Redlands, QLD | 5 | 1/2 (50%) | [105] |
| 2008 July | Proserpine, QLD | 3 | 0/0 | [106] |
| 2008 July | Rockhampton, QLD | 3 | 1/1 (100%) | [107] |
| 2009 Sept | Bowen, QLD | 3 | 0/0 | [108] |
| 2010, May | Tewantin, QLD | 1 | 0/0 | [109] |
| 2011, June | Logan Reserve, QLD | 1 | 0/0 | [110] |
| 2011, June | Kerry, QLD | 1 | 0/0 | [111] |
| 2011, June | McLeans Ridge, NSW | 2 | 0/0 | [112] |
| 2011, July | Mt Alford, QLD | 3+ 1 dog | 0/0 | [113,114] |
| 2011, July | Utungan, NSW | 1 | 0/0 | [115] |
| 2011, July | Park Ridge, QLD | 1 | 0/0 | [116] |
| 2011, July | Kuranda, QLD | 1 | 0/0 | [117] |
| 2011, July | Hervey Bay, QLD | 1 | 0/0 | [118] |
| 2011, July | Corndale, NSW | 1 | 0/0 | [119] |
| 2011, July | Boondall, QLD | 1 | 0/0 | [118] |
| 2011, July | Chinchilla, QLD | 1 | 0/0 | [120] |
| 2011, July | Mullumbimby, NSW | 1 | 0/0 | [121] |
| 2011, August | Newrybar, NSW | 1 | 0/0 | [122] |
| 2011, August | Pimlico, NSW | 2 | 0/0 | [122] |
| 2011, August | Mullumbimby, NSW | 1 | 0/0 | [122] |
| 2011, August | Currumbin Valley, QLD | 1 | 0/0 | [123] |
| Total | 64 | 4/7 (57%) |
Based on Smith et al. [124].
Table 1: Hendra virus outbreaks in Australia, August 1994-August 2011.
Since 1998 NiV has re-emerged more than a dozen times in Bangladesh and neighbouring parts of India (Table 2) and these outbreaks differed from the initial emergence of NiV in Malaysia. In the Malaysian outbreak the disease appeared to be largely encephalitic with respiratory signs recorded in only a small percentage of patients [25]. While there was no clinical evidence of human-to-human transmission, abnormal cerebral magnetic resonance imaging was seen in a nurse with asymptomatic NiV infection, indicating that human-tohuman spread may occur [26]. The mortality rate was approximately 40% [27]. By comparison the later episodes of human NiV infection in Bangladesh and India were characterized by a higher mortality rate and clear evidence of human-to-human spread [28-33]. Respiratory symptoms were more severe and the fatality rate approached 70% [34]. In the Faridpur outbreak in Bangladesh in 2004, 75% of patients developed respiratory difficulty and the associated fatality rate was 73% [35]. In addition, patients with respiratory symptoms were more likely to transmit the virus [36], and the case for the role of respiratory secretions in the human-to-human spread was further strengthened by the identification of NiV RNA in the respiratory secretions of infected patients [16,37]. In the most recent emergence of NiV in Bangladesh in early 2011, the mortality rate has exceeded 75% [38]. NiV infects humans, bats, pigs and dogs [39] in nature, and like HeV, other mammals replicate virus following exposure under laboratory conditions.
Viral infection
One of the distinguishing characteristics of the henipaviruses in comparison to all other paramyxoviruses is the ability to induce severe disease across a broad range of vertebrate hosts. Some of the reasons for this became clear with the identification of ephrin-B2 and ephrin-B3 as the host cell receptors for these viruses [40-43]. Ephrins act as a ligand for their receptors, Eph molecules, and both are members a large family of tyrosine kinase receptors. Both the ephrin ligands and their Eph receptor partners are highly conserved and evolutionarily ancient bi-directional signalling cell surface molecules [44,45]. Sequencing of the ephrin-B2 and ephrin-B3 genes of the human, pig, horse, cat, dog and bats showed over 95% identity at the amino acid level [46]. Binding of ephrins to the Eph receptor facilitates communication between cells by triggering cellular signalling pathways that regulate cell movement, positioning and adhesion (reviewed in [47]). They are expressed on most human tissues [48], but are most highly expressed on neurons, arterial endothelial cells and smooth muscle reflecting their role in development of the nervous and cardiovascular systems and in erythropoiesis [49-52]. Ephrins also play a role in many adult organ systems through regulation of cell migration and tissue assembly [53]. Ephrins have been found in all mammalian species examined and in a number of lower order species such as C. elegans [54]. However, ephrin expression alone is not sufficient to confer susceptibility to henipavirus infection. Mice have so far proved to be refractory to systemic infection despite expressing ephrin B2 [55], suggesting that other factors such as the ability of the host cell to replicate the virus or co-receptors may be important [14].
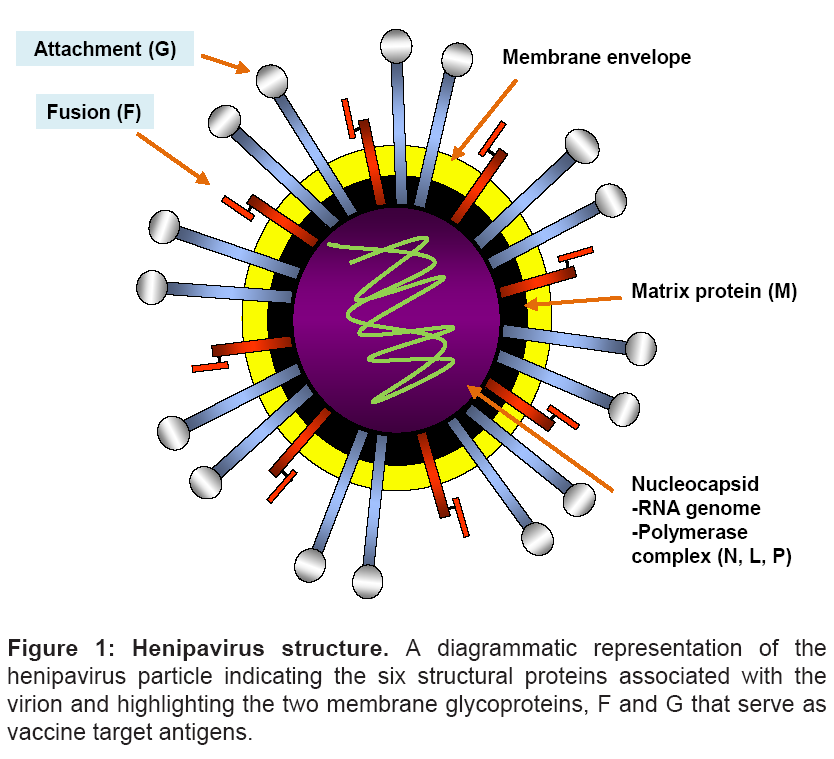
Similar to other paramyxoviruses, henipavirus infection of the host cell is mediated by two membrane anchored surface glycoproteins- and HeV and NiV possess an attachment (G) and fusion (F) glycoprotein (Figure 1) [10,56]. The G glycoprotein is present as tetramer anchored in the lipid membrane of the virus [57] which appears to be associated with the trimeric F glycoprotein prior to receptor binding [43]. The F glycoprotein is a typical class I viral fusion glycoprotein and its activity is dependent on the cleavage of the inactive F0 glycoprotein into two subunits, F1 and F2 by the proteolytic enzyme Cathepsin L [58]. Following binding of G to its ephrin receptors, conformational changes are speculated to occur within the G glycoprotein oligomer (reviewed in [59]). The receptor engagement of the G glycoprotein in turn triggers a conformational change in the F glycoprotein, the exact details of which remain ill-defined, leading to the exposure of the fusion peptide which inserts into the juxtaposed-host cell membrane to form a physical link between the viral and cellular membranes. This is followed by a dramatic refolding of the F glycoprotein structure and association of its two α-helical heptad repeat domains referred to as the 6-helix bundle formation which is believed to facilitate the merger of the viral and cellular membranes [59-61] (reviewed in Dutch 2010 [60]). The end result of the fusion process is entry of the nucleocapsid into the cytoplasm of the cell and the onset of viral replication.
Viral pathogenesis
The utilisation of ephrin-B2 and ephrin-B3 as the receptor on the host cell leads to fundamental similarities in the disease processes caused by HeV and NiV regardless of the species infected. Principally, tropism for the vascular endothelium is responsible for the widespread vasculitis seen in humans, monkeys, horses, hamsters, cats and ferrets infected with HeV [62-65]; and in humans, pigs, guinea pigs, cats, hamsters, ferrets and monkeys infected with NiV [66-70]. Viral tropism for neurons is reflected in the common finding of central nervous system (CNS) neuronal infection which may or may not result in encephalitis. Autopsy of fatally infected NiV patients, and isolation of virus from nasopharyngeal secretions of human patients infected with NiV [37] suggested that respiratory and lymphoid tissues could be the primary site of virus replication, followed by a viraemic phase [71]. Outcomes of virus infection studies in ferrets are consistent with this. Recently, ferrets were treated with a cross reactive and henipavirus neutralizing human monoclonal antibody (mAb) specific for the HeV G glycoprotein [72,73] to evaluate the therapeutic benefit of passively administered antiviral mAb on an otherwise lethal HeV infection scenario. Passive immunotherapy with mAb m102.4 reduced viral replication sufficiently to prevent lethal disease. However, viral RNA was detected in the nasal washes and oral swabs and at post mortem viral genome was detected in the retropharyngeal lymph nodes that drain the nasal cavity even where genome was not detected in any other tissues. These results suggested that the primary site of HeV replication could well be in respiratory and lymphoid tissue, in accordance with observations made for NiV [71] and the well characterized paramyxovirus, measles [74].
In the viraemic phase, viral antigen was found in the endothelial cells of small blood vessels and in arteriolar smooth muscle, with viral infection leading to systemic vasculitis including in the CNS (reviewed in [65,70]). Multinucleated syncytial endothelial cells were also seen both in HeV infections [17,75] and in the initial NiV outbreak in Malaysia, and are considered by some authors to be diagnostic of henipavirus infection [71]. The route of viral infection to the brain is thought to be via infection of endothelium, with local extension to neurons following infarction and resulting injury to nervous tissue [14]. In pigs exposed to NiV, anterograde infection of the brain has also been proposed [76] and data derived from experimentally infected ferrets also supports the possibility of this scenario under certain conditions (J. Pallister and D. Middleton, unpublished results).
Persistent infection with virus is thought to be responsible for henipavirus disease recurring sometime after an apparent recovery from a previous infection. In the initial NiV outbreak in Malaysia, 7.5% of those who survived acute encephalitis suffered recurrent neurological disease known as relapsed encephalitis and 3.4% suffered late-onset encephalitis where neurological manifestations were first seen some time after recovery from an acute non encephalitic or asymptomatic infection [25,77]. Both outcomes were reportedly due to recrudescence and rapid replication of virus that persisted following the initial infection [14,77] although NiV was not isolated from the neurological tissues of these patients [77]. A similar disease pattern occurred in a Mackay farmer who initially contracted HeV from infected horses. He recovered from the initial infection but developed encephalitis and died 13 months later. Again no virus was isolated from the brain although PCR, immunohistochemistry and electron microscopy indicated the presence of HeV [75].
In a small number of cases, an acute measles virus infection can also lead to a persistent infection which in turn leads to the development of subacute sclerosing pan-encephalitis (SSPE). SSPE is a progressive neurological disorder of children and young adults [78,79]) characterized by severe demyelination and infection of neurons leading to death. The disease appears on average 7-10 years post an acute infection with measles [80], but is now rare in western countries where it has largely been eliminated by vaccination [81]. The hallmark of viruses that persist to cause SSPE is the accumulation of mutations, particularly in the M protein and the cytoplasmic tail of the F protein; both proteins that play an integral role in viral budding [10,82]. In all SSPE measles strains the F glycoprotein loses the carboxy-terminal pentadecapeptide that is highly conserved in morbilliviruses and thought to be involved in budding [82]. In addition, the M protein is severely reduced or lacking in these viruses [83,84] and rapid posttranslational degradation of the M protein was shown to lead to defects in virus budding [85]. Together these observations have led to the suggestion that defective viral budding is a mechanism of persistence.
Threat to biosecurity
Henipaviruses have been classified as category C priority pathogens and Biosafety Level-4 (BSL-4) agents by the Centers for Disease Control and Prevention (CDC), and the National Institute of Allergy and Infectious Diseases (NIAID). The NIAID Strategic Plan for Biodefense Research (NIAID Biodefense Research Agenda) encompasses emerging animal pathogens considered as potential biothreats - like NiV which is designated as the example pathogen defining category C agents [86]. Some of the reasons for inclusion of henipaviruses are (i) the high mortality rate associated with henipavirus infection (greater than 50%) (ii) the absence of vaccines and post-exposure treatments - one of the reasons that these agents have been designated as Biosafety Level 4 (BSL-4) organisms (iii) there has been no co-evolution of humans and henipaviruses that might reduce the virulence of the infection in humans (iv) carriage of these viruses by wildlife and their relative ease of propagation means that the agent is theoretically accessible from nature (v) their very broad host range amongst mammalian species and (vi) possible confusion with other more common ailments leading to delayed diagnosis.
Vaccine development
The recent emergence of these viruses and the sporadic nature of disease outbreaks have made the development and testing of vaccines and therapeutics for henipavirus infections a low commercial priority. However, the development of such countermeasures is a crucial component of any preparedness plan against an outbreak or emergence whether deliberate or natural. Vaccines have been used very successfully to control other well known and debilitating paramyxovirus infections including measles and mumps infection of humans and rinderpest virus infection of cattle. Vaccination with an attenuated live measles virus vaccine began in 1963 and was highly successful in reducing the infection rate with measles virus. In the United States alone, the first 20 years of vaccination is estimated to have prevented 52 million cases of the disease, 17,400 cases of mental retardation and 5200 deaths [87]. As a result of vaccination the United States has been declared free of endemic measles [88]. Importantly, an historic announcement in May 2011 declared rinderpest as the first animal disease ever to be eradicated by humankind [89]. Vaccination was a central plank of the campaign to eradicate the virus.
Successful resistance to paramyxovirus infection that is conferred by vaccination is commonly mediated by an adaptive immune response to viral surface proteins/glycoproteins [90] particularly for infections associated with a viraemic phase such as those caused by the measles virus and the mumps virus [91,92]. Consequently, vaccine development for the henipaviruses has focused on the viral F and G envelope glycoproteins either expressed in a recombinant virus or as a recombinant subunit immunogen.
Hamsters vaccinated with recombinant vaccinia viruses encoding NiV G or F were protected against a lethal challenge with NiV. However a strong anamnestic response to the challenge virus suggested that vaccination did not prevent virus replication [93]. Similarly, pigs vaccinated with canarypox viruses encoding either NIV G or F were protected against a lethal NiV infection and although virus was not reisolated from any tissues low levels of viral RNA were detected in several samples [94].
Several studies have also been carried out with a HeV recombinant soluble G glycoprotein (sG)-based subunit immunogen (HeVsG). In one experimental study, cats survived a lethal NiV challenge with no clinical signs [95] and the data supported the development of sterilizing immunity in this animal model. In a second study carried out in cats, virus was reisolated from one vaccinated animal and viral RNA was detected in the brains of several animals receiving the two highest doses of vaccine [96]. The authors speculated that the detection of genome in the brain in the face of significant levels of neutralizing antibody prior to challenge indicated that 'a persistent infection might occur despite pre-existing immunity'. In a vaccine antigen dose sparing study, ferrets immunized with HeVsG survived an otherwise lethal HeV challenge. Here, all vaccine antigen doses prevented clinical disease and there was no anamnestic antibody response detected following challenge, nor could any challenge virus be reisolated from any animal [97]. While all three of these studies utilized HeVsG as the vaccine immunogen, variations in adjuvant used, immunogen dose and challenge virus dose make it difficult to directly compare the experimental outcomes. However, the results of two of three studies indicate that it is possible to prevent establishment of a HeV infection by vaccination, and indeed all three studies indicated that vaccination could prevent clinical illness.
Development of an effective vaccine ideally requires an understanding of how the agent in question interacts with the host to cause disease. Anterograde infection of the brain has been proposed in henipavirus infection, as well as infection via the systemic route. In addition to preventing systemic disease, an ideal vaccine would prevent infection of the CNS by either route and thus eliminate the possibility of recrudescent CNS disease - vaccination against measles virus did reduce the incidence of persistent infection manifested as SSPE. Clinical trials of a potential vaccine against a BSL-4 agent could not be carried out in humans; instead there is a requirement by the U.S. FDA that candidate vaccines be tested in at least two different animal models [98]. Relevant animal models that reproduce the nervous and systemic aspects of henipavirus infection and a thorough understanding of henipavirus pathogenesis in these animal models will be essential to this activity. To this end, the development of a model for henipavirus infection in a non-human primate (African green monkey) was an important step, and indeed disease progression mediated by either HeV or NiV in these animals essentially mirrors that seen in humans [64,69]. Other species that may be suitable include golden hamsters, ferrets and cats [70].
The strategy for the deployment of successful therapeutics is relatively straightforward but a successful vaccine may be deployed differently in different circumstances. While the outbreaks caused by henipaviruses remain sporadic in nature and involve relatively small numbers of people and animals (except in the NiV outbreak in Malaysia where over one million pigs were culled), mass vaccination is unlikely to be a viable approach. Vaccination of select human populations at risk may be warranted in some circumstances; one such population might be, for example, horse veterinarians and horse owners in north eastern Australia. However, the primary strategy for containing HeV outbreaks in Australia is to vaccinate horses in at-risk areas. Human infection with HeV is so far only known to have occurred via close contact with infected horses and so vaccination of horses would hopefully prevent the chain of transmission to humans. The same principle may apply if for instance, pigs (or any other animal) became a significant source of human infection, as seen in the initial NiV outbreak in Malaysia and Singapore. Should the nature of henipavirus outbreaks change or bioterrorism involving these agents become a reality then mass vaccination may become a viable option.
Conclusions
Experience with paramyxoviruses indicates that the opportunity for significant reduction in transmission risk by vaccination is great. There is potential for recrudescence of the virus but the potential for sterilizing immunity seen with some HeVsG candidate vaccines may circumvent this risk - as measles vaccine reduced the incidence of the persistent infection manifested as SSPE. The challenges in developing a vaccine against a BSL-4 agent are significant but not insurmountable. The requirement to carry out challenge experiments at BSL-4 imposes constraints on the speed with which the preliminary vaccine work can be conducted, and the process is further complicated by the rigorous testing required prior to release of a vaccine for human use. However, vaccine development is progressing and, in conclusion, it would seem that vaccines for henipavirus infections are likely to be available in the foreseeable future.
References
- Taylor LH, Latham SM, Woolhouse ME (2001) Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 356: 983-989.
- Christou L The global burden of bacterial and viral zoonotic infections. Clin Microbiol Infect 17: 326-330.
- Hahn BH, Shaw GM, De Cock KM, Sharp PM (2000) AIDS as a zoonosis: scientific and public health implications. Science 287: 607-614.
- Peiris JS, Guan Y, Yuen KY (2004) Severe acute respiratory syndrome. Nat Med 10: S88-97.
- Li W, Shi Z, Yu M, Ren W, Smith C, et al. (2005) Bats are natural reservoirs of SARS-like coronaviruses. Science 310: 676-679.
- Lau SK, Woo PC, Li KS, Huang Y, Tsoi HW, et al. (2005) Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc Natl Acad Sci U S A 102: 14040-14045.
- Derbes VJ (1966) De Mussis and the great plague of 1348. A forgotten episode of bacteriological warfare. JAMA 196: 59-62.
- Foxell JW (2001) Current Trends in Agroterrorism (Antilivestock, Anticrop, and Antisoil Bioagricultural Terrorism) and Their Potential Impact on Food Security. Studies in Conflict and Terrorism 24: 107-129.
- Day TG (2003) The autumn 2001 anthrax attack on the United States postal service: the consequences and response. Journal of contingencies and crisis management 11: 110-117.
- Griffin DE, Lamb RA, Straus SE, Howley PM,Lamb RA, et al. (2007) Paramyxoviridae: The viruses and their replication. In: Knipe DM, Griffin DE, Lamb RA, Straus SE, Howley PM et al., editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins. pp. 1449-1496.
- Furuse Y, Suzuki A, Oshitani H et al. Origin of measles virus: divergence from rinderpest virus between the 11th and 12th centuries. Virol J 7: 52.
- Wang LF, Yu M, Hansson E, Pritchard LI, Shiell B, et al. (2000) The exceptionally large genome of Hendra virus: support for creation of a new genus within the family Paramyxoviridae. J Virol 74: 9972-9979.
- Mayo MA (2002) A summary of taxonomic changes recently approved by ICTV. Arch Virol 147: 1655-1663.
- Eaton BT, Mackenzie JS, Wang L-F (2007) In: Knipe DM, Griffin DE, Lamb RA, Straus SE, Howley PM et al., editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins. pp. 1587-1600.
- Harcourt BH, Tamin A, Halpin K, Ksiazek TG, Rollin PE, et al. (2001) Molecular characterization of the polymerase gene and genomic termini of nipah virus. Virology 287: 192-201.
- Harcourt BH, Lowe L, Tamin A, Liu X, Bankamp B, et al. (2005) Genetic characterization of Nipah virus, Bangladesh, 2004. Emerg Infect Dis 11: 1594- 1597.
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, et al. (1995) A morbillivirus that caused fatal disease in horses and humans. Science 268: 94-97.
- Young PL, Halpin K, Selleck PW, Field H, Gravel JL, et al. (1996) Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg Infect Dis 2: 239-240.
- Halpin K, Young PL, Field HE, Mackenzie JS (2000) Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol 81: 1927- 1932.
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, et al. (2000) Nipah virus: a recently emergent deadly paramyxovirus. Science 288: 1432-1435.
- Wang L, Harcourt BH, Yu M, Tamin A, Rota PA, et al. (2001) Molecular biology of Hendra and Nipah viruses. Microbes Infect 3: 279-287.
- Daniels P, Ksiazek T, Eaton BT (2001) Laboratory diagnosis of Nipahand Hendra virus infections. Microbes Infect 3: 289-295.
- Chua KB, Lek Koh C, Hooi PS, Wee KF, Khong JH, et al. (2002) Isolation of Nipah virus from Malaysian Island flying-foxes. Microbes Infect 4: 145-151.
- Selvey LA, Taylor R, Arklay A, Gerrard J (1996) Screening of bat carers for antibodies to equine morbillivirus. Communicable Disease Intelligence 20: 477- 478.
- Goh KJ, Tan CT, Chew NK, Tan PS, Kamarulzaman A, et al. (2000) Clinical features of Nipah virus encephalitis among pig farmers in Malaysia. N Engl J Med 342: 1229-1235.
- Tan KS, Sarji SA, Tan CT, Abdullah BJJ, Chong HT, et al. (2000) Patients with asymptomatic Nipah virus infection may have abnormal cerebral MR imaging. Neurol J Southeast Asia 5: 69-73.
- Parashar UD, Sunn LM, Ong F, Mounts AW, Arif MT, et al. (2000) Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998-1999 outbreak of severe encephalitis in Malaysia. J Infect Dis 181: 1755-1759.
- ICDDRB (2004) Person-to-person transmission of Nipah virus during outbreak in Faridpur District. Health Science Bulletin 2: 5-9.
- Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, et al. (2006) Nipah virusassociated encephalitis outbreak, Siliguri, India. Emerg Infect Dis 12: 235-240.
- Gurley ES, Montgomery JM, Hossain MJ, Bell M, Azad AK, et al. (2007) Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis 13: 1031-1037.
- Hsu VP, Hossain MJ, Parashar UD, Ali MM, Ksiazek TG, et al. (2004) Nipah Virus Encephalitis Reemergence, Bangladesh. Emerg Infect Dis 10: 2082- 2087.
- Blum LS, Khan R, Nahar N, Breiman RF (2009) In-depth assessment of an outbreak of Nipah encephalitis with person-to-person transmission in Bangladesh: implications for prevention and control strategies. Am J Trop Med Hyg 80: 96-102.
- Homaira N, Rahman M, Hossain MJ, Epstein JH, Sultana R, et al. (2010) Nipah virus outbreak with person-to-person transmission in a district of Bangladesh, 2007. Epidemiol Infect 138: 1630-1636.
- Lo MK, Rota PA (2008) The emergence of Nipah virus, a highly pathogenic paramyxovirus. J Clin Virol 43: 396-400.
- Hossain MJ, Gurley ES, Montgomery JM, Bell M, Carroll DS, et al. (2008) Clinical presentation of nipah virus infection in Bangladesh. Clin Infect Dis 46: 977-984.
- Luby SP, Gurley ES, Hossain MJ (2009) Transmission of human infection with Nipah virus. Clin Infect Dis 49: 1743-1748.
- Chua KB, Lam SK, Goh KJ, Hooi PS, Ksiazek TG, et al. (2001) The presence of Nipah virus in respiratory secretions and urine of patients during an outbreak of Nipah virus encephalitis in Malaysia. J Infect 42: 40-43.
- Anonymous (2011) Nipah encephalitis, human - Bangladesh: Rangpur (05) 20110308.0756. International Society for Infectious Diseases.
- Mills JN, Alim AN, Bunning ML, Lee OB, Wagoner KD, et al. (2009) Nipah virus infection in dogs, Malaysia, 1999. Emerg Infect Dis 15: 950-952.
- Negrete OA, Levroney EL, Aguilar HC, Bertolotti-Ciarlet A, Nazarian R, et al. (2005) EphrinB2 is the entry receptor for Nipah virus, an emergent deadly paramyxovirus. Nature 436: 401-405.
- Negrete OA, Wolf MC, Aguilar HC, Enterlein S, Wang W, et al. (2006) Two Key Residues in EphrinB3 Are Critical for Its Use as an Alternative Receptor for Nipah Virus. PLoS Pathog 2: e7.
- Bonaparte MI, Dimitrov AS, Bossart KN, Crameri G, Mungall BA, et al. (2005) Ephrin-B2 ligand is a functional receptor for Hendra virus and Nipah virus. Proc Natl Acad Sci U S A 102: 10652-10657.
- Bishop KA, Stantchev TS, Hickey AC, Khetawat D, Bossart KN, et al. (2007) Identification of Hendra virus G glycoprotein residues that are critical for receptor binding. J Virol 81: 5893-5901.
- Drescher U (2002) Eph family functions from an evolutionary perspective. Curr Opin Genet Dev 12: 397-402.
- Suga H, Koyanagi M, Hoshiyama D, Ono K, Iwabe N, et al. (1999) Extensive gene duplication in the early evolution of animals before the parazoaneumetazoan split demonstrated by G proteins and protein tyrosine kinases from sponge and hydra. J Mol Evol 48: 646-653.
- Bossart KN, Tachedjian M, McEachern JA, Crameri G, Zhu Z, et al. (2008) Functional studies of host-specific ephrin-B ligands as Henipavirus receptors. Virology 372: 357-371.
- Poliakov A, Cotrina M, Wilkinson DG (2004) Diverse roles of Eph receptors and ephrins in the regulation of cell migration and tissue assembly. Dev Cell 7: 465-480.
- Hafner C, Schmitz G, Meyer S, Bataille F, Hau P, et al. (2004) Differential gene expression of Eph receptors and ephrins in benign human tissues and cancers. Clin Chem 50: 490-499.
- Wang B, Zhang N, Qian KX, Geng JG (2005) Conserved molecular players for axon guidance and angiogenesis. Curr Protein Pept Sci 6: 473-478.
- Zhang J, Hughes S (2006) Role of the ephrin and Eph receptor tyrosine kinase families in angiogenesis and development of the cardiovascular system. J Pathol 208: 453-461.
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, et al. (1999) Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13: 295-306.
- Suenobu S, Takakura N, Inada T, Yamada Y, Yuasa H, et al. (2002) A role of EphB4 receptor and its ligand, ephrin-B2, in erythropoiesis. Biochem Biophys Res Commun 293: 1124-1131.
- Pasquale EB (2008) Eph-ephrin bidirectional signaling in physiology and disease. Cell 133: 38-52.
- Wang X, Roy PJ, Holland SJ, Zhang LW, Culotti JG, et al. (1999) Multiple ephrins control cell organization in C. elegans using kinase-dependent and -independent functions of the VAB-1 Eph receptor. Mol Cell 4: 903-913.
- Westbury HA, Hooper PT, Selleck PW, Murray PK (1995) Equine morbillivirus pneumonia: susceptibility of laboratory animals to the virus. Aust Vet J 72: 278- 279.
- Lamb RA (1993) Paramyxovirus fusion: A hypothesis for changes. Virology 197: 1-11.
- Bossart KN, Crameri G, Dimitrov AS, Mungall BA, Feng YR, et al. (2005) Receptor binding, fusion inhibition and induction of cross-reactive neutralizing antibodies by a soluble G glycoprotein of Hendra virus. J Virol 79: 6690-6702.
- Pager CT, Dutch RE (2005) Cathepsin L is involved in proteolytic processing of the Hendra virus fusion protein. J Virol 79: 12714-12720.
- Hickey AC, Broder CC (2009) The mechanism of henipavirus fusion: examining the relationships beween the attachment and fusion glycoproteins. Virologica Sinica 24: 110-120.
- Dutch RE (2010) Entry and fusion of emerging paramyxoviruses. PLoS Pathog 6: e1000881.
- Bossart KN, Broder CC (2009) Paramyxovirus entry. In: Pohlmann S, Simmons G, editors. Viral Entry into Host Cells. Austin: Landes Bioscience / Eurekah
- Pallister J, Middleton D, Crameri G, Yamada M, Klein R, et al. (2009) Chloroquine administration does not prevent Nipah virus infection and disease in ferrets. J Virol 83: 11979-11982.
- Hooper P, Zaki S, Daniels P, Middleton D (2001) Comparative pathology of the diseases caused by Hendra and Nipah viruses. Microbes Infect 3: 315-322.
- Rockx B, Bossart KN, Feldmann F, Geisbert JB, Hickey AC, et al. (2010) A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment. J Virol 84: 9831-9839.
- Wong KT (2009) Pathology of henipavirus infection in humans and hamster model. Neurology Asia 14: 59-61.
- Middleton DJ, Westbury HA, Morrissy CJ, van der Heide BM, Russell GM, et al. (2002) Experimental Nipah virus infection in pigs and cats. J Comp Pathol 126: 124-136.
- Wong KT, Grosjean I, Brisson C, Blanquier B, Fevre-Montange M, et al. (2003) A golden hamster model for human acute Nipah virus infection. Am J Pathol 163: 2127-2137.
- Bossart KN, Zhu Z, Middleton D, Klippel J, Crameri G, et al. (2009) A neutralizing human monoclonal antibody protects against lethal disease in a new ferret model of acute nipah virus infection. PLoS Pathog 5: e1000642.
- Geisbert TW, Daddario-DiCaprio KM, Hickey AC, Smith MA, Chan YP, et al. (2010) Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS ONE 5: e10690.
- Williamson MM, Torres-Velez FJ Henipavirus: a review of laboratory animal pathology. Vet Pathol 47: 871-880.
- Wong KT, Shieh WJ, Zaki SR, Tan CT (2002) Nipah virus infection, an emerging paramyxoviral zoonosis. Springer Semin Immunopathol 24: 215-228.
- Zhu Z, Dimitrov AS, Bossart KN, Crameri G, Bishop KA, et al. (2006) Potent neutralization of Hendra and Nipah viruses by human monoclonal antibodies. J Virol 80: 891-899.
- Zhu Z, Bossart KN, Bishop KA, Crameri G, Dimitrov AS, et al. (2008) Exceptionally potent cross-reactive neutralization of Nipah and Hendra viruses by a human monoclonal antibody. J Infect Dis 197: 846-853.
- Griffin DE, Bellini WJ,Knipe DM, Griffin DE, Lamb RA, et al. (2007) Measles virus. Fields Virology. Philadelphia: Lippincott Williams & Wilkins. pp. 1551- 1585.
- O'Sullivan JD, Allworth AM, Paterson DL, Snow TM, Boots R, et al. (1997) Fatal encephalitis due to novel paramyxovirus transmitted from horses. Lancet 349: 93-95.
- Weingartl H, Czub S, Copps J, Berhane Y, Middleton D, et al. (2005) Invasion of the central nervous system in a porcine host by nipah virus. J Virol 79: 7528- 7534.
- Tan CT, Goh KJ, Wong KT, Sarji SA, Chua KB, et al. (2002) Relapsed and lateonset Nipah encephalitis. Ann Neurol 51: 703-708.
- Payne FE, Baublis JV, Itabashi HH (1969) Isolation of measles virus from cell cultures of brain from a patient with subacute sclerosing panencephalitis. N Engl J Med 281: 585-589.
- Horta-Barbosa L, Fuccillo DA, Sever JL, Zeman W (1969) Subacute sclerosing panencephalitis: isolation of measles virus from a brain biopsy. Nature 221: 974.
- Modlin JF, Jabbour JT, Witte JJ, Halsey NA (1977) Epidemiologic studies of measles, measles vaccine, and subacute schlerosing encephalitis. Paediatrics 59: 505-512.
- Bellini WJ, Rota JS, Lowe LE, Katz RS, Dyken PR, et al. (2005) Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis 192: 1686- 1693.
- Rima BK, Duprex WP (2005) Molecular mechanisms of measles virus persistence. Virus Res 111: 132-147.
- Lin FH, Thormar H (1980) Absence of M protein in a cell-associated subacute sclerosing panencephalitis virus. Nature 285: 490-492.
- Hall WW, Choppin PW (1979) Evidence for lack of synthesis of the M polypeptide of measles virus in brain cells in subacute sclerosing panencephalitis. Virology 99: 443-447.
- Sheppard RD, Raine CS, Bornstein MB, Udem SA (1985) Measles virus matrix protein synthesized in a subacute sclerosing panencephalitis cell line. Science 228: 1219-1221.
- NIAID Biodefense Research Agenda for Category B and C Priority Pathogens. https://biodefenseniaidnihgov.
- Bloch AB, Orenstein WA, Stetler HC, Wassilak SG, Amler RW, et al. (1985) Health impact of measles vaccination in the United States. Pediatrics 76: 524- 532.
- Orenstein WA, Papania MJ, Wharton ME (2004) Measles elimination in the United States. J Infect Dis 189 Suppl 1: S1-3.
- Promed 20110526.1603 (2011) Rinderpest - worldwide: global eradication.
- Graham BS, Crowe JE (2007) Immunization against viral diseases. In: Knipe DM, Griffin DE, Lamb RA, Straus SE, Howley PM et al., editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins. pp. 487-538.
- Plotkin SA (1999) Vaccination against the major infectious diseases. C R Acad Sci III 322: 943-951.
- Wolinsky JS, Waxham MN, Server AC (1985) Protective effects of glycoproteinspecific monoclonal antibodies on the course of experimental mumps virus meningoencephalitis. J Virol 53: 727-734.
- Guillaume V, Contamin H, Loth P, Georges-Courbot MC, Lefeuvre A, et al. (2004) Nipah virus: vaccination and passive protection studies in a hamster model. J Virol 78: 834-840.
- Weingartl HM, Berhane Y, Caswell JL, Loosmore S, Audonnet JC, et al. (2006) Recombinant nipah virus vaccines protect pigs against challenge. J Virol 80: 7929-7938.
- Mungall BA, Middleton D, Crameri G, Bingham J, Halpin K, et al. (2006) Feline model of acute Nipah virus infection and protection with a soluble glycoproteinbased subunit vaccine. J Virol 80: 12293-12302.
- McEachern JA, Bingham J, Crameri G, Green DJ, Hancock TJ, et al. (2008) A recombinant subunit vaccine formulation protects against lethal Nipah virus challenge in cats. Vaccine 26: 3842-3852.
- Pallister J, Middleton D, Wang L-F, Klein R, Haining J, et al. (2011) A Recombinant Hendra virus G Glycoprotein-Based Subunit Vaccine Protects Ferrets from Lethal Hendra virus Challenge. Vaccine: (in press).
- Snoy PJ Establishing efficacy of human products using animals: the US food and drug administration's "animal rule". Vet Pathol 47: 774-778.
- Promed 19990219.0218 (1999) Hendra virus, horse - Australia (Queensland).
- Hanna JN, McBride WJ, Brookes DL, Shield J, Taylor CT, et al. (2006) Hendra virus infection in a veterinarian. Med J Aust 185: 562-564.
- Promed 20041214.3307 (2004) Hendra virus - Australia (QLD).
- Promed 20061109.3222 (2006) Hendra virus, equine - Australia (New South Wales): suspected.
- Marsh GA, Todd S, Foord A, Hansson E, Davies K, et al. (2010) Genome sequence conservation of Hendra virus isolates during spillover to horses, Australia. Emerg Infect Dis 16: 1767-1769.
- Promed 20070903.2896 (2007) Hendra virus, human, equine - Australia (Queensland) (03): correction.
- Promed 20080821.2606 (2008) Hendra virus, human , equine - Australia (07): (Queensland).
- Promed 20080725.2260 (2008) Hendra virus, human , equine: Australia (04): (Queensland).
- Promed 20090903.3098 (2009) Hendra virus, human, equine - Australia (04): (Queensland) Fatal.
- Promed 20090910.3189 (2009) Hendra virus, human, equine - Australia (05): (Queensland).
- Promed 20100524.1724 (2010) Hendra virus, equine - Australia (04): (Queensland) human exposure.
- Promed 20110723.2220 (2011) Hendra virus, equine - Australia (16): (Queensland, New South Wales)
- Promed 20110630.1989 (2011) Hendra virus, equine -Australia (03): (Queensland) Human Exposure.
- Promed 20110702.2012 (2011) Hendra virus, equine - Australia (05) (New South Wales) Human Exposure.
- Promed 20110705.2036 (2011) Hendra virus, equine - Australia (06): (Queensland, New South Wales) Human Exposure.
- Promed 20110727.2257 (2011) Hendra virus, equine - Australia (18): (Queensland) Canine.
- Promed 20110710.2084 (2011) Hendra virus, equine - Australia (08): (Queensland, New South Wales) Human Exposure.
- Promed 20110706.2045 (2011) Hendra virus, equine - Australia (07): (Queensland, New South Wales) Human Exposure.
- Promed 20110713.2110 (2011) Hendra virus, equine - Australia (10): (Queensland, New South Wales) Human Exposure.
- Promed 20110716.2158 (2011) Hendra virus, equine - Australia (14): (Queensland, New South Wales).
- Promed 20110717.2167 (2011) Hendra virus, equine - Australia (15): (QueenslandNew South Wales)
- Promed 20110725.2243 (2011) Hendra virus, equine - Australia (17): (Queensland, New South Wales)
- Promed 20110729.2274 (2011) Hendra virus, equine - Australia (20): (Queensland, New South Wales) Canine.
- Promed 20110818.2512 (2011) Hendra virus, equine - Australia (23): ( New South Wales).
- Promed 20110823.2570 (2011) Hendra virus, equine - Australia (24): (Queensland)
- Smith I, Broos A, De Jong C, Zeddeman A, Smith C, et al. (2011) Identifying Hendra virus diversity in Pteropid bats. Emerg Infect Dis (in press).
- Promed 20040212.0472 (2004) Henipavirus - Bangladesh (Manikganj, Rajbari).
- ICDDRB (2005) Nipah virus outbreak from date palm juice. Health Science Bulletin 3: 1-5.
- Luby SP, Rahman M, Hossain MJ, Blum LS, Husain MM, et al. (2006) Foodborne transmission of Nipah virus, Bangladesh. Emerg Infect Dis 12: 1888-1894.
- Promed 20070508.1484 (2007) Nipah virus, fatal - India (West Bengal).
- ICDDRB (2007) Person-to-person transmission of Nipah infection in Bangladesh. Health Science Bulletin 5: 1-6.
- ICDDRB (2008) Outbreaks of Nipah virus in Rajbari and Manikgonj. Health Science Bulletin 6: 12-13.
- Promed 20100122.0250 (2010) Nipah virus, fatal - Bangladesh: (Faridpur).
- Promed 20110204.0402 (2011) Nipah virus, fatal - Bangladesh (Faridpur, Rajbari).
- Promed 20110204.0408 (2011) Undiagnosed encephalitis - Bangladesh (02): (Rangpur) Nipah virus confirmed.
Relevant Topics
- Anthrax Bioterrorism
- Bio surveilliance
- Biodefense
- Biohazards
- Biological Preparedness
- Biological Warfare
- Biological weapons
- Biorisk
- Bioterrorism
- Bioterrorism Agents
- Biothreat Agents
- Disease surveillance
- Emerging infectious disease
- Epidemiology of Breast Cancer
- Information Security
- Mass Prophylaxis
- Nuclear Terrorism
- Probabilistic risk assessment
- United States biological defense program
- Vaccines
Recommended Journals
Article Tools
Article Usage
- Total views: 17446
- [From(publication date):
specialissue-2011 - Apr 04, 2025] - Breakdown by view type
- HTML page views : 12719
- PDF downloads : 4727