Research Article Open Access
Growth Kinetics of Acclimated Mixed Culture for Degradation of Isopropyl Alcohol (IPA)
Smita Raghuvanshi1*, Suresh Gupta1 and B. V. Babu21Assistant Professor, Department of Chemical Engineering, Birla Institute of Technology and Science (BITS), PILANI–333 031, Rajasthan, India
2Director, Institute of Engineering and Technology (IET), JK Lakshmipat University (JKLU) JAIPUR–302026, Rajasthan, India
- Corresponding Author:
- Smita Raghuvanshi
Department of Chemical Engineering
Birla Institute of Technology and Science (BITS)
PILANI–333 031 (Rajasthan) India
Tel: +91-1596-245073 ext. 215
Fax: +91-1596-244183
E-mail: smita@bits-pilani.ac.in; smitaraghuvanshi@gmail.com
Received date: November 06, 2012; Accepted date: November 29, 2012; Published date: December 03, 2012
Citation: Raghuvanshi S, Gupta S, Babu BV (2012) Growth Kinetics of Acclimated Mixed Culture for Degradation of Isopropyl Alcohol (IPA). J Biotechnol Biomaterial S13:002. doi:10.4172/2155-952X.S13-002
Copyright: © 2012 Raghuvanshi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Iso-propyl alcohol (IPA) is an organic chemical regarded as a potential pollutant. The permissible exposure limit (PEL) of IPA specified by Occupational Safety and Health Administration (OSHA) is 400 mg L-1. In this study the aerobic biodegradation of IPA is carried out by an acclimated mixed culture obtained by sewage treatment plant, for the range of 200-700 mg L-1 of initial IPA concentration. The batch degradation study demonstrated that the maximum growth rate obtainable is 0.337 h-1. To explain the inhibition effects, different kinetic growth models such as Haldane, Luong and Edward models are applied. The experimental data are found to fit well with inhibition models having the values of coefficient of determination (R2) of 0.989, 0.986 and 0.984 respectively for Haldane, Luong and Edward models. Based on the disappearance of IPA, degradation is modeled by the three-half-order kinetics and the resulting kinetic parameters are reported.
Keywords
Biodegradation; Isopropyl alcohol; Inhibition models; Growth kinetics; Mixed culture; Volatile Organic Compound (VOC)
Introduction
Volatile organic compounds (VOCs) are organic chemical compounds that have high enough vapor pressures under normal conditions to significantly vaporize and enter the atmosphere. Isopropyl Alcohol (IPA) is one such VOC having a boiling point of 83°C, which vaporizes at normal atmospheric conditions. It is flammable, clear, colorless liquid and is slightly odorous. IPA is also enlisted in the Organization for Economic Co-operation and Development (OECD) which maintains a list of 5,235 High Production Volume Chemicals (HPV) as compiled in 2000. The OECD list includes chemicals which have annual production volumes greater than one thousand metric tonnes (2.2 million pounds) in more than one economically developed country. IPA also comes under the category of HPV in US and other developed nations [1].
IPA has wide applications as a solvent in paint, pharmaceutical, lithography, and rubber manufacture industries. It is used as a cleaning agent in semiconductor and printing industries. It is also used for sterilizing and disinfecting surfaces in hospitals, dairy farms, food processing plants, household dwellings, veterinary institutions, farm structures, and poultry areas. It is one of the components of many antiseptic and cosmetic products [2]. The workspace exposure limit (WEL) and OSHA permissible exposure limit (PEL) is 400 mg L-1 for 8 h exposure [3].
IPA is released from above mentioned industries which lead to an adverse effect on the water and air quality and thus endanger public health. Inhalation of IPA vapors can cause gastrointestinal pain, cramps, nausea, vomiting, and diarrhea. IPA vapors can also cause eye irritation, possible corneal burns and eye damage. Chronic exposure of IPA may cause skin effects. Those who have pre-existing skin disorders or impaired liver, kidney, or pulmonary function may be more susceptible to the exposure of IPA [4]. The exposure of high concentrations of IPA can cause depression in central nervous system, unconsciousness and possibly death [4].
Due to its adverse effects on environment, more stringent requirements for removing such VOCs from aquatic environment need the development of innovative and cost effective techniques. The biological treatment of VOCs has been attracting more attention these days than physical and chemical methods as they are cost effective and create almost negligible secondary pollutant. Biological methods include variety of microorganisms’ community which is known to utilize VOCs as a carbon source for their growth [5].
Few studies have reported on the aerobic degradation of IPA with pure cultures such as Bacillus pallidus [6,7]. Bioconversion of high concentration of IPA was investigated by a solvent tolerant strain of bacteria identified as Sphingobacterium mizutae. This strain of bacteria exhibited the ability to utilize high concentration of IPA as the sole carbon source [8]. Some of the studies were reported for biodegradation of IPA using mixed culture as it can be predominantly used for large scale bio based treatment plants [9,10].
It was observed from earlier studies that mixed community of microbes attained from the sludge of sewage treatment plants gave better results for the degradation of significant pollutants such as methyl ethyl ketone (MEK), methyl isobutyl ketone (MIBK), 2, 4, 6 tricholorophenol, phenol, etc [11-16]. Quesnel and Nakhla (2006) reported the biodegradation kinetics of acetone and MIBK from acclimatized activated sludge [17]. There is a lack of biodegradation studies for IPA (inspite of being a biodegradable compound) using mixed culture and lack of kinetic data (biokinetics constants) as well.
Hence in the present study, IPA was chosen as the model contaminant and a detailed biodegradation study was carried out. The aim of this study was to understand the biodegradation of IPA with better quantitative experimental data. Thus the work involved the development of acclimated mixed culture for the biodegradation of IPA. The effect of time on initial concentration of IPA and biomass concentration ranging from 200-700 mg L-1 was also studied. The information collected from these experiments was used for the development of different growth kinetic models. This information is essential for determining and predicting the potential risk that a compound has on the degradation in the natural environment and the biodegradation time and size of equipment required for ex-situ treatment of pollutants.
Materials and Methods
Chemical reagents
All the materials used (IPA and various nutrient media) were of analytical grade and were brought from Merck (India) brand.
Preparation of media
Stock glucose solution was prepared by dissolving 10 g of glucose in 100 ml distilled water. The media, Minimal Salt Medium (MSM), was prepared having the following composition (in g/l): K2HPO4–0.8, KH2PO4–0.2, CaSO4.2H2O–0.05, MgSO4.7H2O–0.5, (NH4)2SO4–1.0, FeSO4–0.01 in distilled water and was autoclaved.
Microorganism culture conditions
An aerobic mixed microbial culture was obtained from the Municipal Sewage Treatment Plant of Birla Institute of Technology & Science (BITS) Pilani. The sludge was kept for settling for almost 3-4 hours in a room at around 25°C (away from sunlight). 10 gm of settled sludge was taken and thoroughly mixed with 100 mL of distilled water. The shaking was carried out gently and then sludge was allowed to settle. 50 mL of supernatant was taken and centrifugation was carried out for 2 minutes at 10,000 rpm in a centrifuge. The pellet obtained after the centrifugation was further used for the microbial growth and the supernatant was discarded.
Inoculation procedure
The Inoculation was carried out in laminar hood chamber. A loop full of sludge obtained after centrifugation was added to 100 mL of MSM. The solution was then kept in rotary shaker at 37°C for 48 hours. The sufficient microbial culture (measured by optical density value of more than 0.1 at 540 nm) was obtained. This obtained microbial culture was then used for obtaining the microbial culture acclimated with IPA. For this, 100mL of MSM was added with 1 mL of glucose and 20 μL of IPA to start with and was kept in rotary shaker for almost 48 hours. This procedure was followed for 3 weeks by decreasing the amount of glucose from 1 to 0 mL with a decrement of 200 μL every time and simultaneously increasing IPA amount from 20 to 60 μL with an increment of 10 μL. The conical flasks are sealed with the stoppers to avoid the loss of IPA during shaking. Hence the acclimated mixed culture was obtained after 3 weeks cycle. The final enrichment culture showed extensive growth in IPA which substantiates the fact that IPA is a biodegradable compound.
Biodegradation study
Batch biodegradation experiments were conducted in the concentration range of 200 700 mg L-1 with the values of 200, 300, 400, 500, 600 and 700 mg L-1 individually in 250 mL Erlenmeyer flasks sealed with cotton plugs. 100 mL of MSM was autoclaved and 2 mL of pre-cultured suspension (acclimated mixed culture) and known amount of IPA were added to it to maintain the required concentration. The amount of IPA added was 27, 40, 54, 68, 82 and 95 μL (based on density and boiling point of IPA) to maintain 200, 300, 400, 500, 600 and 700 mg L-1 of IPA concentration respectively. All experiments were carried out in an orbital shaking incubator set at 150 rpm and 37°C. The samples were collected at different intervals for different concentrations ranging from 200 to 700 mg L-1 based on visual observation (turbidity). The time interval was different for different samples. The time was not before 6 hours for any sample and final time was decided by observing the constant value of biomass concentration for 2 or 3 consecutive samples.
Analytical techniques
The optical density (OD) of the microbial culture was measured at 540 nm with respect to MSM using UV-VIS Spectrophotometer (Model 119- Systronics, India). Samples were then centrifuged at 10,000 rpm for two minutes to separate biomass and supernatant (aqueous IPA solution) [18]. Dry weight of biomass was obtained and concentrations of IPA in aqueous samples were measured using a gas chromatograph (Model 5700 series, Nucon Engineers, India). The temperatures of injection port, detector and oven were maintained at 150°C, 150°C and 200°C, (based on boiling points of the compound and specified ranges of gas chromatograph) respectively. Nitrogen was used as the carrier gas. All the experiments and measurements were carried out twice and the arithmetic averages were taken for calculations and data analysis.
Results and Discussion
In the present study, biodegradation of IPA was studied for the initial concentration range of 200-700 mg L-1 using acclimated mixed culture. The effect of influencing parameter such as time on initial IPA concentration and biomass concentration was studied. The different biodegradation growth models and kinetic rate models were applied to understand the mechanism of biodegradation of IPA. The results obtained are discussed in the following sections.
Effect of initial IPA concentration on time of biodegradation
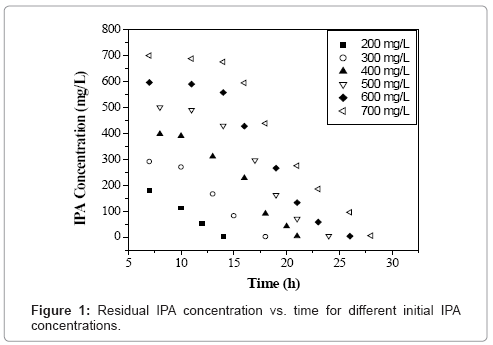
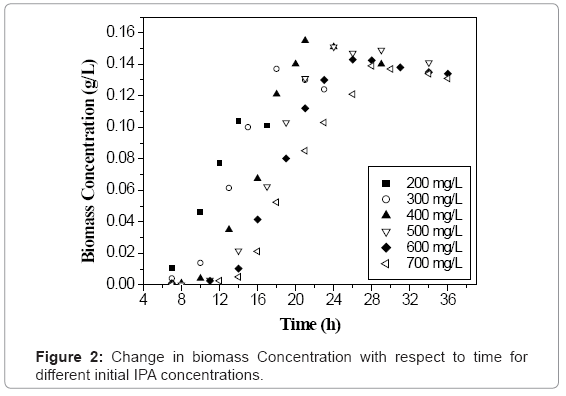
Initial concentration of any VOC has a significant influence on the growth of microbial culture [18]. Figure 1 shows the time profile of IPA biodegradation for concentration ranging from 200-700 mg L-1 using the acclimated mixed culture. It was found that the acclimated mixed culture degraded IPA in 14 h, 18 h, 21 h, 24 h, 26 h and 28 h for 200, 300, 400, 500, 600 and 700 mg L-1 of initial IPA concentration respectively. The IPA concentration was found decreasing with time showing the utilization of IPA by the microorganisms as it acts as a carbon source for their growth. It can be seen from figure 1, that at initial IPA concentration values of 200 to 400 mg L-1, a small lag phase was observed which indicates that there was no inhibition effect up to 400 mg L-1 of initial IPA concentration. Similar trends were observed with biomass concentration with time for initial IPA concentration values ranging from 200-700 mg L-1 as shown in figure 2. Biomass concentration increases with increase in time until all IPA was utilized by microorganisms. The maximum biomass concentrations were obtained as 0.104, 0.137, 0.155, 0.151, 0.143 and 0.139 g L-1 for initial IPA concentrations of 200, 300, 400, 500, 600 and 700 mg L-1, respectively. The biomass concentration increased with increase in initial IPA concentration from 200 to 400 mg L-1. This indicated that IPA has less inhibition effect on growth of microorganisms as shown by small lag phase. This indicated that above 400 mg L-1 of initial IPA concentration, substrate inhibition effect was predominant. The longer lag time corresponds to an increase in initial IPA concentration as reported for mixed culture and is attributed to substrate inhibition [18].
Initially, there was no increment in the biomass concentration with time giving the lag phase which is due to the reason that microbes take some time for acclimation to the new environment. In log phase, the biomass concentration increased exponentially where most of the substrate (IPA) was utilized by the microbes for their growth. After that, there was no increment in biomass concentration which is the stationary phase of growth curve. An exponential decrease in biomass concentration was observed during death phase which was around 5% of maximum biomass concentration [19]. Similar trends were obtained for initial IPA concentration of 200, 300, 400, 500, and 700 mg L-1 as can be seen from figure 2.
Biodegradation growth kinetics
Carbon substrates are most often utilized by microorganisms simultaneously under the carbon and energy controlled environmental conditions. Since growth is a result of catabolic and anabolic enzymatic activities, these processes, i.e., substrate utilization or growth-associated product formation, can also be quantitatively described on the basis of growth models. The relationship between the specific growth rate (μ) of a population of microorganisms and the substrate concentration (S) is a valuable tool in biodegradation processes. This relationship is expressed by a set of empirically derived rate laws which are considered as theoretical models. Various theoretical models such as Monod kinetic model [20], Powell kinetic model [21], Haldane model [22], Luong model [23] and Edwards model [24] are reported in the literature. These models are helpful in the understanding of the behavior of the biological processes and predicting the IPA concentrations in the treatment systems.
The contaminant degradation leads to the formation of biomass. As contaminant degradation is the result of the microbial activity, the kinetics of contaminant degradation is closely related to the kinetics of microbial growth. The obtained biomass concentrations at various initial values of IPA concentration are used to calculate the specific growth rate using eq. 1 [19].
 (1)
(1)
where, μ is the specific growth rate (h-1); x is the biomass concentration (g L-1) at time t (h); S is the substrate (IPA) concentration (mg L-1).
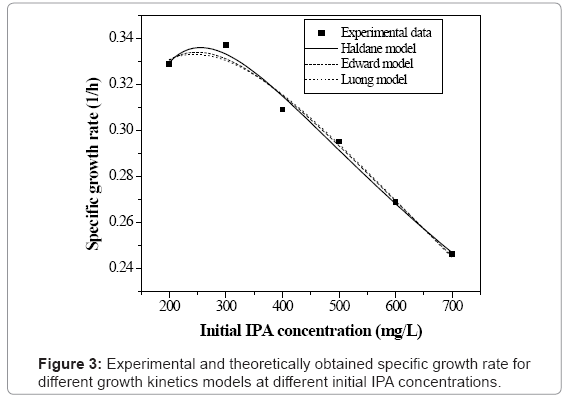
Figure 3 shows the specific growth rate values at different values of initial IPA concentration. The values of specific growth rate were obtained as 0.329, 0.337, 0.309, 0.295, 0.269 and 0.246 h-1 for the initial IPA concentration values of 200, 300, 400, 500, 600 and 700 mg L-1 respectively. The specific growth rate increases up to 300 mg L-1 of initial IPA concentration and then decreases with increase in concentration (see figure 3). In microbial processes, some higher concentrations of the substrate inhibit the growth of the microorganisms due to which the growth rate falls. In the present study, it is observed that the inhibition effect on microorganisms starts at low initial concentration values of IPA as compared to earlier studies [12,13]. Hence in this study, basic growth models such as Monod and Powell models were not considered. Monod and Powell models are simple models and have certain limitations. The limitation of classical Monod`s equation is that it does not account for the fact that cells may need substrate or may synthesize product even when they do not grow. Also, the Monod and Powell models do not consider the self inhibition effect which was exhibited during the (IPA) biodegradation process. So the present work includes the application of various substrate inhibition models such as Haldane, Luong and Edward models and discussed in the following sections.
Haldane’s model
IPA biodegradation was clearly subjected to the self-inhibition as shown by the decrease in specific growth rate at the start of the biodegradation of IPA with increasing initial IPA concentration as discussed in the earlier section (Biodegradation kinetics). In such cases, substrate inhibition is considered by incorporating the substrate inhibition constant (KI) in Monod’s Model. Among the various substrate inhibition models, Haldane’s model has been widely used [25–27,18]. Haldane model was originally proposed for substrate inhibition in 1968. According to Haldane model, the specific growth rate can be represented by eqn. (2).
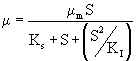 (2)
(2)
The model equation is non linear and it was solved using a professional graphics software package ORIGIN (version 6) to obtain the kinetic constants and were listed in table 1. Figure 3 shows the fit of Haldane model with the experimental results and predicted values of specific growth rate at different concentration are listed in table 2. The value of coefficient of determination (R2=0.989) showed that the present data confirmed well to the Haldane model. The obtained value of affinity constant, Ks, and substrate inhibition constant, KI, were 241.6 and 270.75 mg L-1 respectively. For self-inhibitory compounds, there is a critical substrate concentration, Ccrt, which is defined by eq. (3), above which the substrate removal rate falls due to self-inhibitory effect [28].
| S No | Model | µm (h-1) | Ks (mg L-1) | KI (mg L-1) | Sm (mg L-1) | K | n | R2 |
| 1 | Haldane | 0.972 | 241.60 | 270.752 | - | - | - | 0.989 |
| 2 | Luong | 1.279 | 5389.63 | - | 9.189 | - | 371.63 | 0.848 |
| 3 | Edward | 0.413 | 38.405 | 24832.43 | - | 32.64 | - | 0.984 |
Table 1: Growth kinetic parameters obtained from different growth models.
| S No | S0 (mg L-1) | Exp. µ (h-1) | Values of predicted specific growth rate, µ, (h-1) | ||
| Haldane | Luong | Edward | |||
| 1 | 200 | 0.27319 | 0.329 | 0.330 | 0.331 |
| 2 | 300 | 0.33827 | 0.333 | 0.331 | 0.330 |
| 3 | 400 | 0.36016 | 0.315 | 0.315 | 0.316 |
| 4 | 500 | 0.36158 | 0.291 | 0.293 | 0.293 |
| 5 | 600 | 0.32993 | 0.267 | 0.269 | 0.269 |
| 6 | 700 | 0.31507 | 0.247 | 0.245 | 0.245 |
Table 2: Experimental and predicted values of specific growth rate using different growth kinetic models.
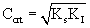 (3)
(3)
Critical IPA concentration was obtained as 255 mg L-1. The corresponding specific growth rate was 0.3361 h-1. The obtained value of Ks (241.6) was less than the critical IPA concentration which also confirms the self inhibition effect. Observed KI value was also low, which means that IPA is toxic to the cells even at low concentration ranges. In Haldane model, only the substrate utilization term was considered and the biomass production from the utilization of substrate was neglected [29].
Luong model
The inhibitory effect of substrate on biomass growth under batch conditions can also be represented by a mathematical expression which was proposed by Luong (1987). The inhibitory effect of IPA at higher concentration for mixed culture is given by eqn. 4.
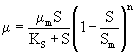 (4)
(4)
where Sm (mg L-1) is the critical inhibitor concentration above which biodegradation stops. The kinetic constants for Luong model were obtained using professional graphics software package ORIGIN (version 6). Experimentally found specific growth rate at different initial IPA concentration was fitted with Luong model and is shown in figure 3. The obtained value of coefficient of determination (R2=0.986) and comparison between the experimental and predicted value of specific growth rate (Table 2) indicated that the experimental results fit reasonably well to the Luong model. This model incorporated the IPA utilization by the mixed culture associated with some product formation during its biodegradation.
In this study, Haldane model (R2=0.989) was found slightly better than Luong model (R2=0.986) which indicates that product formation effect on biomass growth could not be completely neglected. The obtained value of maximum IPA concentration at which the culture ceased to grow was 88.91 mg L-1 according to Luong model. The higher value of critical inhibitor concentration indicated that the higher efficiency of the microbial culture to grow in the presence of IPA, and hence, a complete degradation of IPA was possible.
Edward model
High substrate concentrations inhibit microbial growth and may distort the metabolism of microorganisms [24]. Edward (1970) proposed a mathematical model which describes the mechanisms causing substrate inhibition for a wider concentration range and is given by eqn. 5
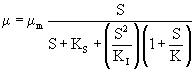 (5)
(5)
where K is a constant. This model was also solved by performing non-linear regression analysis using a professional graphics software package ORIGIN (version 6). The experimental data on specific growth rate, μ, obtained at initial IPA concentration ranging from 200 to 700 mg L-1 were used for estimating the kinetic parameters for the Edward model and listed in table 1. The obtained value of Ks was 38.4 mg L-1 which is less than the substrate concentration range (200-700 mg L-1). This confirms the substrate inhibition effect.
The values of kinetic constants (μm and Ks) predicted from Luong model differed from Edward model. This difference in the predicted values could be reasoned based on the fact that the two models were originally developed for systems containing different microorganisms and substrate [30].
Based on the above analysis, it may be concluded that all the three inhibition models (Haldane, Luong and Edward) explain the growth kinetics of IPA degradation reasonably well. However, Haldane model fits the experimental data the best.
Biodegradation rate kinetics
Several kinetic approaches for describing the transformation of organic compounds such as IPA into biomass by suspended microorganisms were being evaluated for biodegradation processes [31,32]. The rate of disappearance of substrate is dependent on the substrate concentration. The substrate concentration which changes with time can be described by zero-order, first-order and second-order rate kinetics. In biodegradation processes biomass is produced simultaneously as substrate concentration decreases [33]. The first-order and second-order kinetics were not widely preferred as they suffered from the limitation that they do not take into account of the biomass growth. Therefore in biodegradation processes, generally three-half-order kinetics is used [33]. The model is based on the assumption of first-order model with the introduction of an additional term for explaining the biomass formation. The rate of IPA degradation is given by eq. 6:
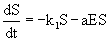 (6)
(6)
where k1 is the proportionality constant (time-1), E is the cell concentration (g L-1) and is the proportionality constant (1 (biomass concentration)-1(time)-1). After integration and simplification, Eq. 6 reduces to eq. 7 [33].
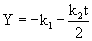 (7)
(7)
where
 (8)
(8)
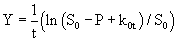 (9)
(9)
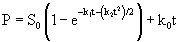 (10)
(10)
k1 and k2 were found by plotting Y against t which gave a straight line (eq. 8). k0 and S0 are zero-order rate constant and substrate concentration at zero time, respectively (eq. 9).
Eqn. 10 contains four unknown parameters and is highly nonlinear. It also requires the initial estimates of S0and k0 for the calculation of kinetic parameters (k1 and k2). In this equation, S0 and k0 can be obtained by the zero-order kinetics which is represented by differential and integral form as given by eq. 11 and 12 respectively:
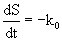 (11)
(11)
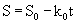 (12)
(12)
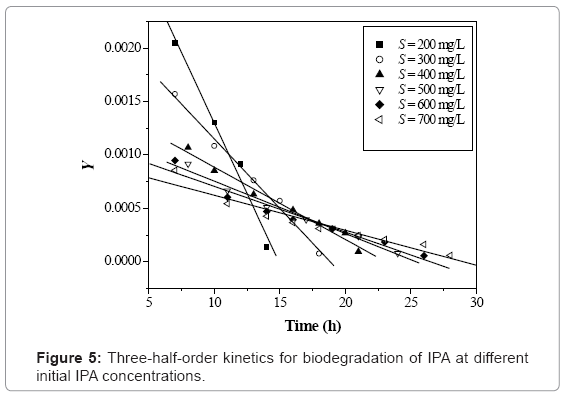
The zero-order and three-half-order kinetic constants were evaluated and listed in table 3 and the corresponding plots are shown in figure 4 and 5 respectively. The coefficient of determination obtained for zero-order kinetics was found in the range of 0.958-0.998 for various initial IPA concentration values.
| S No | Initial IPA Concentration (mg L-1) | Zero order kinetics | Three half order kinetics | ||||
|---|---|---|---|---|---|---|---|
| k0 | S0 | R2 | k1 | k2 ´ 103 | R2 | ||
| 1 | 200 | 25.63 | 362.29 | 0.998 | 2.64x 10-4 | 3.94 | 0.991 |
| 2 | 300 | 28.11 | 516.67 | 0.979 | 1.30 x 10-4 | 2.45 | 0.995 |
| 3 | 400 | 32.42 | 699.91 | 0.980 | 6.77 x 10-4 | 1.56 | 0.991 |
| 4 | 500 | 34.96 | 848.29 | 0.970 | 4.90 x 10-5 | 1.24 | 0.990 |
| 5 | 600 | 37.03 | 962.96 | 0.958 | 4.28 x 10-5 | 1.13 | 0.975 |
| 6 | 700 | 37.74 | 1094.11 | 0.962 | 3.27 x 10-5 | 0.95 | 0.959 |
Table 3: Parameters of zero-order and three-half-order kinetic models at different initial IPA concentrations.
The obtained values of three-half-order rate constants, (k1 and k2) were found decreasing with increase in the initial IPA concentration. The obtained value of coefficient of determination (R2=0.959-0.995) indicated that the three-half-order kinetic model is suitable to explain the IPA biodegradation rate kinetics using acclimated mixed culture over a wide range of operating conditions.
Conclusions
The present study was focused on the microbial utilization of one of the organic pollutants named IPA and the biodegradation kinetics of acclimated mixed culture for IPA was studied. It showed that the time required for utilizing IPA using acclimated mixed culture increased by increasing the initial concentration of IPA. The specific growth rate was found at various initial concentrations of IPA. The maximum specific growth rate was obtained at 300 mg L-1 of initial IPA concentration. Substrate inhibition occurred for more than 300 mg L-1 of IPA concentration. The different growth kinetic inhibition models such as Haldane, Luong and Edward model were also examined. The obtained values of coefficient of determination and closeness of experimental and predicted values of specific growth rate confirmed the suitability of all the three models for representing the biodegradation kinetics of IPA. However, Haldane model was found to fit the experimental data the best. The higher values obtained for critical inhibitor concentration indicated that the higher efficiency of the microbial culture to grow in the presence of IPA. Biodegradation rate kinetics using zero-order and three-half-order kinetic models were tested and three-half-order kinetic model was found suitable for the biodegradation of IPA.
Nomenclatures
a: proportionality constant, l g-1 h-1;
E: cell concentration, g L-1;
k1: three half order rate constant;
k2: three half order rate constant;
k0: zero order rate constant;
KI: substrate inhibition constant, g L-1;
Ks: half saturation constant, g L-1;
S: substrate (IPA) concentration, mg L-1;
S0: substrate concentration at zero time, mg L-1;
t: time, h;
x: biomass concentration, g L-1;
Ccrt: critical IPA concentration, mg L-1;
Sm: critical inhibitor concentration, mg L-1;
K: Edwards constant;
Greek;
μ: specific growth rate, h-1;
μm: maximum specific growth rate, h-1;
References
- http://high production volume (HPV) Chemicals.mht
- Mackay D, Wan, SY, Kuo, MC (1990) Illustrated Handbook of Physical-chemical Properties and Environmental Fate for Organic Chemicals. (Vol. III), Lewis Publishers, USA.
- http:// ISOPROPYL ALCOHOL.mht.
- http://www.shieldmedicare.com
- Quan X, Shi H, Wang J, Qian Y (2003) BBiodegradation of 2,4-dichlorophenol in sequencing batch reactors augmented with immobilized mixed culture. Chemosphere. 50: 1069-1074.
- Bustard MT, Meeyoo V, Wright PC (2001) Biodegradation of isopropanol in a three phase fixed bed bioreactor: start up and acclimation using a previously-enriched microbial culture. Environ. Technol 22: 1193-1201.
- Bustard MT, Whiting S, Cowan DA, Wright PC (2002) Biodegradation of high-concentration isopropanol by a solvent-tolerant thermophile, Bacillus pallidus. Extremophiles 6: 319-323.
- Mohammad BT, Wright PC, Bustard MT (2006) Bioconversion of isopropanol by a solvent tolerant Sphingobacterium mizutae strain. J Ind Microbiol Biotechnol 33: 975-983.
- Rene ER, Murthy DVS, Swaminathan T (2005) Performance evaluation of a compost biofilter treating toluene vapours. Process Biochemistry. 40: 2771–2779.
- Andreoni V, Baggi G, Colombo M, Cavalca L, Zangrossi M, Bernasconi S (1998) Degradation of 2,4,6-trichlorophenol by a specialized organism and by indigenous soil microflora: bioaugmentation and self-remediability for soil restoration.Lett. Appl. Microbiol.27: 86-92.
- Snyder CJ, Asghar M, Scharer JM, Legge RL (2006) Biodegradation kinetics of 2,4,6-trichlorophenol by an acclimated mixed microbial culture under aerobic conditions. Biodegradation 17: 535-544.
- Raghuvanshi S, Babu BV (2006) Removal of Methyl Ethyl Ketone (MEK) using Biofiltration. Proceedings of National Conference on Environmental Conservation (NCEC-2006).
- Raghuvanshi S, Babu BV (2006) Biofiltration for the Removal of Methyl Isobutyl Ketone (MIBK). Proceedings of International Symposium & 59th Annual Session of IIChE in association with International Partners (CHEMCON-2006).
- Deshusses MA, Hamer G, Dunn IJ (1996) T Transient-state behavior of a biofilter removing mixtures of vapors of MEK and MIBK from air. Biotechnol Bioeng. 49: 587-598.
- Geoghegan DP, Hamer G, Dehusses MA (1997) Effects of unsteady state conditions on the biooxidation of methyl ethyl and methyl isobutyl ketone in continuous flow liquid phase cultures. Bioprocess Biosyst Eng 16: 315-322.
- Buitrón G, González A (1996) Characterization of the microorganisms from an acclimated activated sludge degrading phenolic compounds. Water Sci Technol 34: 289-294.
- Quesnel D, Nakhla G (2006) Removal kinetics of acetone and MIBK from a complex industrial wastewater by an acclimatized activated sludge. J Hazard Mater 132: 253-260.
- Saravanan P, Pakshirajan K, Saha P (2008) Growth kinetics of an indigenous mixed microbial consortium during phenol degradation in a batch reactor. Bioresour Technol 99: 205-209.
- Bailey JE, Ollis DF (1986) Biochemical Engineering Fundamentals. (2nd edn) McGraw-Hill Inc, New York.
- Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3: 371-394.
- Evans CGT, Strange RE, Tempest DW (1967) Microbial physiology and continuous culture. HMSO, London, United Kingdom.
- Andrews JF (1968) A mathematical model for the continuous culture of micro- organisms utilizing inhibitory substance. Biotechnol Bioeng 10: 707-723.
- Luong JH (1987) Generalization of monod kinetics for analysis of growth data with substrate inhibition. Biotechnol Bioeng 29: 242-248.
- Edwards VH (1970) The influence of high substrate concentrations on microbial kinetics. Biotechnol Bioeng 12: 679-712.
- Sokol W (1987) Oxidation of an inhibitory substrate by washed cells (oxidation of phenol by Pseudomonas putida). Biotechnol. Bioeng 30: 921-927.
- Tang WT, Fan LS (1987) Steady state phenol degradation in a draft tube gas-liquid-solid fluidized bed bioreactor. AIChE J 33: 239-249.
- Peyton BM, Wilson T, Yonge DR (2002) Kinetics of phenol biodegradation in high salt solutions. Water Res 36: 4811-4820.
- Tomei MC, Annesini MC, Bussoletti S (2004) 4-Nitrophenol biodegradation in a sequencing batch reactor: kinetic study and effect of filling time. Water Res 38: 375-384.
- Monteiro1 AA, Boaventura RA, Rodrigues AE (2000) Phenol biodegradation by Pseudomonas putida DSM 548 in a batch reactor. Biochem Eng J 6: 45-49.
- Kumar A, Kumar S, Kumar S (2005) Biodegradation kinetics of phenol and catechol using Pseudomonas putidaMTCC 1194. Biochem Eng J 22: 151-159.
- Paris DF, Steen WC, Baughman GL, Barnett, JTJr (1981) Second-order model to predict microbial degradation of organic compounds in natural waters. Appl Environ Microbiol 41: 603-609.
- Simkins S, Alexander M (1984) Models for mineralization kinetics with the variables of substrate concentration and population density. Appl Environ Microbiol 47: 1299-1306.
- Brunner W, Focht DD (1984) Deterministic three-half-order kinetic model for microbial degradation of added carbon substrates in soil. Appl Environ Microbiol 47: 167-172.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15738
- [From(publication date):
specialissue-2013 - Mar 31, 2025] - Breakdown by view type
- HTML page views : 11109
- PDF downloads : 4629