Research Article Open Access
Green Ionic Liquid Albumin Glassy Carbon Biosensor (ILAGC) for On-line Binding Studies of Human Serum Albumin with Retinol
Deia Abd El-Hady1,2* and Hassan M Albishri3
1Chemistry Department, Faculty of Science-North Jeddah, King Abdulaziz University, Saudi Arabia
2Chemistry Department, Faculty of Science, Assiut University, 71516-Assiut, Egypt
3Chemistry Department, Faculty of Science, King Abdulaziz University, Saudi Arabia
- *Corresponding Author:
- Deia Abd El-Hady
Department of Chemistry
Faculty of Science-North Jeddah
King Abdulaziz University, Saudi Arabia
Tel: +966544136236
Fax: +966544136236
E-mail: deiaabdelhady@yahoo.com
Received date: February 22, 2013; Accepted date: March 24, 2013; Published date: March 26, 2013
Citation: El-Hady DA, Albishri HM (2013) Green Ionic Liquid Albumin Glassy Carbon Biosensor (ILAGC) for On-line Binding Studies of Human Serum Albumin with Retinol. J Anal Bioanal Tech S7:004. doi: 10.4172/2155-9872.S7-004
Copyright: © 2013 El-Hady DA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A new biosensor composed of 1-butyl-3-methylimidazolium hexafluorophosphates (BMIMPF6)-human serum albumin (HSA) film coated on glassy carbon electrode (GCE) was produced. In the current work, the conjugation of ionic liquid (IL) with HSA improved the stability and binding affinity of protein onto GCE. A rapid and reliable voltammetric method for the on-line protein binding studies of retinol with HSA was developed by hyphenating ionic liquid albumin glassy carbon (ILAGC) biosensor with differential pulse anodic stripping voltammetry under physiological conditions. Detection limit and quantitation limit of 4.5 and 15.0 nmol L-1 were achieved, respectively. This gave us the opportunity to study the high binding constant between retinol and HSA by their detection in nanomolars. The electrochemical behavior of retinol onto ILAGC biosensor was monitored by cyclic voltammetry. The surface coverage of retinol on the proposed biosensor was calculated by double potential-step chronocoulometry. The binding constant of retinol with HSA was estimated to be 1.30×105 L mol-1 giving acceptable precision (SD ≤ 0.02) and good agreement with literature values. The proposed electrochemical biosensor opened a new venue for cheap, green and rapid on-line binding studies of small molecules with protein. As well, our proposed method could use to study either high or low binding affinities.
Keywords
Biosensor; Ionic liquid; Protein; Binding studies; Retinol; Green chemistry
Introduction
Retinol is considered one of the critical vitamin A components for many biological processes such as normal growth, vision, reproduction and epithelial differentiation [1]. Recently, retinol also exhibited an antioxidant power against damaging of free radicals [1]. Therefore, it is important to study its binding with plasma protein under physiological conditions. Human serum albumin (HSA) is the major part of plasma protein and the main target of several micronutrients. The determination of retinol binding constant with HSA was performed by FTIR, UV-Vis, CD and fluorescence spectroscopy [2]. As well, our previous work was used to calculate the binding constant of retinol with HSA by affinity capillary electrophoresis [3]. Retinol is also sensitive to any external stress by oxygen and could subject to oxidation [1,4]. Voltammetry is the best technique to study the redox behavior of retinol and has excellent features such as less cost, easy automated, rapid analysis and good precision. As well, it is consider a good alternative technique for the binding studies of protein with small molecules [5,6].
Room temperature ionic liquids (RTILs) have taken on a life of their own and now occupy the attention of scientists worldwide. RTILs are liquid ionic compounds at ambient temperature consisting of organic cations and various anions [7]. Recently, RTILs were used as electrolytes or electrode modifiers in the area of electroanalysis. Unfortunately for electrochemists, there are a limited number of RTILs that are really suitable for electrochemistry. When they have sufficient conductivity, in another side, they exhibit meager electrochemical windows and/or show limited chemical stability toward reactive materials. As well, it is necessary to select an appropriate type and size of working electrode to reduce the specific current and compensate of the ‘‘IR-drop’’ or ‘‘ohmic drop’’ [8].
A major advance in the last few years was the discovery of 1-butyl-3-methylimidazolium hexafluorophosphates (BMIMPF6) that has attracted widespread attention as nonaqueous polar, moderate hydrophobic, nonvolatile, high ionic mobility, chemical and thermal stable [9]. As well, it was found that BMIMPF6 on glassy carbon electrode (GCE) gave a wide electrochemical window (EW) ranged from -2.0 to 2.5 V and the IR-drop was almost negligible (<100 mV) [8]. It was already known that contamination of IL with water shrinks EW [10]. However, it was found that GCE was insensitive to water content in BMIMPF6 [8]. Another major impurity was oxygen which should be eliminated by purging with inert gas during the experiment.
Electrochemical methods were used with RTILs to develop new generation of ion selective sensors, voltammetric biosensors and gas biosensors [11]. BMIMPF6 was tested as binder in carbon composite electrode by Safavi et al. [12,13]. Moreover, a nano-ZnO and BMIMPF6 composite matrix was fabricated to study the electrochemistry of hemoglobin [14]. BMIMPF6 was used as both plasticizers and ion exchangers for electrodes with plasticized membranes [15]. Authors of [16] used BMIMPF6 as ionic additive in plasticized membranes of ionselective electrodes for decreasing of membrane’s dielectric constant.
The direct electron transfer between proteins and the electrode surface has received considerable attention recently, because it can use in fabricating new generation of biosensor devices. The covalent binding technique was often used for protein immobilization [17,18], but the process of covalent immobilization was somewhat complex and might induce protein denaturation. Recently, we have fabricated an ionic liquid albumin glassy carbon (ILAGC) biosensor and used it for the chiral discrimination of fenoprofen enantiomers [19]. In this work, ILAGC exhibits a stable biosensor for electrochemical measurements due to the moderate hydrophobicity of IL and its possibility to adsorb on the electrode substrate or attractive interactions with protein.
Therefore, in the present study, for the first time, a rapid, simple, precise and green differential pulse stripping voltammetric method coupled with ILAGC biosensor was used for the on-line binding studies of retinol with HSA.
Experimental
Apparatus
All voltammetric measurements were obtained using a voltammetric analyzer manufactured by Metrohm (Switzerland). A 10.0 mL glass voltammetric cell using commercial available electrode stand was used. The electrode was connected via IME-663 module (Netherlands). Potentials were controlled using a working electrode of a rotating ionic liquid albumin glassy carbon disc (3.0 mm diameter, Metrohm), a reference electrode of Ag/AgCl (3.0 M KCl) and a counter electrode of platinum wire. The pH’s were controlled using a pH meter model 810 (Fischer Scientific).
Chemicals and reagents
Retinol (97%) was obtained from Acros (Geel, Belgium). Human serum albumin (HSA) was purchased from sigma (St. Louis, MO, USA) and was used as received without further purification. Ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate was synthesized as described elsewhere [20]. Purity of the ionic liquid was checked with an elemental analysis and FTIR. Disodium hydrogen phosphate-2-hydrate and potassium dihydrogen phosphate were obtained from Riedel-deHaën. Ethanol was obtained from Fluka. Other chemical reagents were analytical grade, and double-distilled water was used throughout this study.
The 1.0 mg mL-1 stock solution of HSA was prepared by dissolving it directly in phosphate buffer, pH 7.4. A concentration of 67.0 mmol L-1 phosphate buffer was used to control the pH at 7.40. Stock solutions of retinol (100.0 mmol L-1) were prepared in 4 mL ethanol and dilute to 10.0 mL by phosphate buffer. The standards of retinol were stored at -20.0°C in dark bottles till starting the analyses.
Preparation of the electrochemical biosensor
The glassy carbon electrode (GCE) was prepared as described in our previous work [21] with little modifications. The electrode was polished at the beginning of the experiments with 0.05 μm aluminum oxide (particle size=0.1 μm, Metrohm, Switzerland). Subsequently, electrode was thoroughly rinsed with water to obtain a clean electrode surface. Then, the electrode was placed in the buffer solution; the potential was cycled 50x between -800.0 to +800.0 mV using cyclic voltammetry at a scanning rate of 100.0 mV s-1. The cleaned electrodes were dried in a room temperature. The ionic liquid human serum albumin modified GCE was simply prepared by following the procedure described in our previous work [19]. The HSA solution was mixed with BMIMPF6 and then the mixture was dripped on the glassy carbon electrode surface and dried by passing very slow rate of hot air for 10.0 min to form a stable gel-like film. The composite film modified electrode was stored in a refrigerator with fit cap when not in use. The refreshment of the bare glassy carbon surface was achieved by sonicating the modified electrode surface in 0.1 mol L-1 hydrochloric acid for 5.0 min and then acetonitrile for 5.0 min. The fabricated biosensor gave good reproducibility (n=7, RSD=4.6%) for the measurement of retinol binding with HSA.
Electrochemical measurements
Into a 10.0 mL volumetric cell, variant volumes of 100.0 mmol L-1 stock retinol solutions was diluted with 67.0 mmol L-1 phosphate buffer, pH 7.40 and mixed homogeneously by rotation for 5 s. Subsequently, the differential pulse or cyclic voltammetric curves were recorded to show the electrochemical changes of the reaction system in the potential range from 0.1 to 1.0 V and all the experiments were performed at room temperature 25 ± 2°C. Differential pulse experiments were performed at pulse height 25.0 mV, equilibrium time 5.0 s and scan rate 5.0 mV s-1. All the tested solutions were purged with nitrogen for 10.0 min before the experiments. During the analysis, a nitrogen environment was kept over the solution to prevent the possibility of oxidation of retinol by oxygen.
Results and Discussion
ILAGC biosensor and retinol electrochemical behavior
The electrochemical response before and after the ILAGC biosensor inserted into the retinol solution was investigated by cyclic voltammetry (CV). It was found that the redox current peak increased after the ILAGC was immersed in 700.0 nmol L-1 of retinol. This behavior could be attributed to the interaction between retinol and HSA making more molecules move to the biosensor surface.
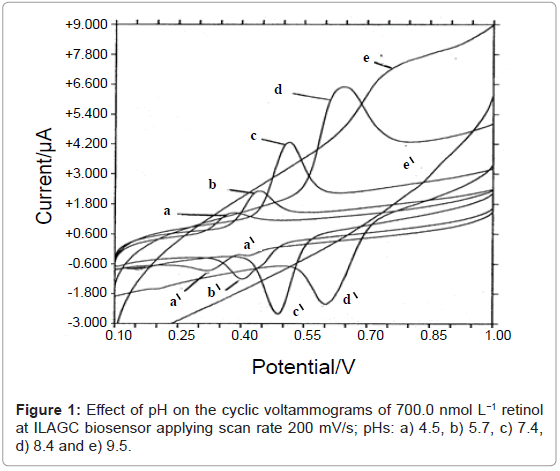
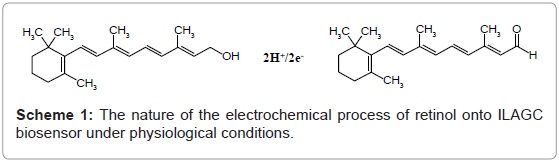
The electrochemical process of retinol onto ILAGC biosensor was investigated by cyclic voltammetry at different pH values. Figure 1 shows the effect of pH on cyclic voltammograms of retinol at ILAGC surface. One reduction peak at about 0.51 V and one oxidation peak at about 0.48 V are obtained at pH 7.4 (peak c) with scan rate of 200.0 mV s-1 validating the 2H+/2e- redox process (scheme 1). The peaks were completely disappeared in alkaline media (above pH 9.5) while another small oxidation peak at 0.33 V starting to disappear from neutral medium. The effect of scan rate was tested on 500.0 nmol L-1 of retinol, pH 7.4. A linear relationship is obtained between scan rate and peak height with a slope tends to unity (0.98 μA s V-1) and a correlation coefficient of 0.999. This behavior confirmed the possibility of retinol adsorption on ILAGC electrode. By applying a repetitive scan, the peaks were decreased rapidly indicating fast desorption of retinol molecules from ILAGC surface.
These electrochemical measurements could be based on the possibility of inclusion of small molecules inside macrocyclic protein/IL phase followed by electrochemical redox process on GC substrate and then analyte molecules excluded into the bulk. This suggested electrochemical mechanism opened a new venue for diverse applications of proposed biosensor for the determination of small molecules.
Therefore, the current results indicated that ionic liquid BMIMPF6 is considered a suitable mediator and promoter for the electrochemical activity of protein on GCE [19]. Therefore, it could be considered that BMIMPF6 enhanced for the stability and electrochemical activity of HSA on GCE. As well, the duration time of immersing biosensor in the cell solution affected the stability behavior of measurements. Biosensor gave good stability during the voltammetric time scale while its character went to be bad when it was continued contact with aqueous surrounding electrolyte for more than 8 hours. Therefore, it is recommended to remove the working electrode from cell solution during the breaks between measurements.
Method optimization and validation
The influence of amount of the mixture of BMIMPF6 and HSA on the peak current of retinol was investigated in the range of 2.0-12.0 μL of BMIMPF6 or HSA in the presence of fixed volume of another component. As shown in figure 2, with the amount of HSA increased in the presence of 6.0 μL of ionic liquid, the peak current was increased up to 8.0 μL and then became stable. This could be due to that the interaction of HSA and retinol had reached saturation, and the continuous increase of HSA has no effect. For further experiments, we chose the 8.0 μL HSA as the optimal amount. As well, the amount of ionic liquid BMIMPF6 was studied at a constant volume of HSA. The best results were also achieved in the presence of 6.0 μL of ionic liquid. Therefore, the mixture contained 8.0 μL of HSA and 6.0 μL of BMIMPF6 was used for the voltammetric discrimination of retinol.
At pH 7.4, a comparison between the voltammetric modes of differential pulse stripping voltammetry (DPSV), Osteryoung square-wave stripping voltammetry (OSWSV) and linear sweep stripping voltammetry (LSSV) was studied. It was obvious that DPSV is more sensitive with a good peak shape compared to others for the determination of retinol.
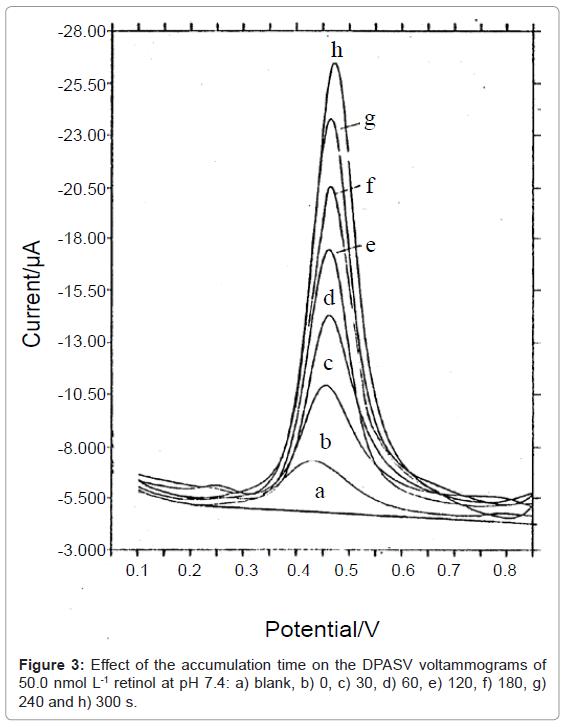
The effect of different experimental parameters e.g. pulse height, scan rate, etc has been tested. The optimum values were found to be 25.0 mV, 5.0 mV s-1 and 5.0 s for pulse height, scan rate and quiescent time, respectively. Under these parameters, a large response after 60.0 s accumulation time for a concentration of 200.0 nmol L-1 retinol is obtained under physiological conditions compared with 0 s. This indicates the rapid adsorption of retinol molecules and giving the possibility of ultra trace determination of retinol. The effect of accumulation times (tacc.) on the anodic current of retinol was investigated in figure 3. A linear relationship is obtained up to 300.0 s in the presence of 50.0 nmol L-1 retinol. In another hand, the linearity was decreased down to 120 s in the presence of 500.0 nmol L-1 retinol. This phenomenon could be attributed to the dependence of the equilibriums at the ILAGC surface on the amount of retinol species, which was subsequently dependent on the accumulation time. Therefore, 120.0 s was employed as the best time for retinol determination.
The ILAGC biosensor was also used to measure the current response of a series of retinol from 10-7 to 10-9 mol L-1 under optimal conditions by DPASV. It was found that larger the current gradually increased along with the increasing of retinol concentration in a good linear relationship. The linear equation was: Conc. (nmol/L)=0.061 Current (μA)+0.075 with correlation coefficient (r2)=0.998 and standard deviation (SD)=0.151. Reproducibility was checked by twenty one measurements within three consecutive days of 100.0 nmol L-1 retinol under optimal conditions; the relative standard deviation (RSD) of 1.52 was obtained. Detection limit and quantitation limit [22] of 4.5 and 15.0 nmol L-1 were achieved for retinol, respectively. The accuracy of the proposed method was determined by applying the optimized analytical approaches with three spiking replicates at three concentration levels of retinol covering the linearity range giving recoveries ranged from 99.8 to 100.5%. Therefore, the proposed ILAGC sensor could be used for the direct determination of retinol in different matrices with good precise data.
The charge of the double layer (Qdl) was calculated by applying a double potential-step chronocoulometric technique for the supporting electrolyte without retinol and with 300.0 nmol L-1 retinol. The adsorbed charge value (Qads) of retinol at the ILAGC biosensor was calculated and found to be 6.5 μC. The surface coverage was calculated by dividing the number of coulombs transferred on the conversion quantity (nFA) and found to be 6.98×10-11 mol cm-1. Thus, every mole retinol occupies an area of 2.764 nm2 at ILAGC biosensor.
Determination of the binding constant between retinol and HSA
In order to confirm that the interactive effect of HSA to retinol, it was important to calculate the binding constant between HSA and retinol. The value of binding constant for the interaction of retinol with HSA was determined by using the equation mentioned in our published work [19]; the binding number (m) and binding constant (K) of HSA–m retinol were calculated.
Keeping the HSA concentration constant inside the proposed biosensor but changing the concentration of retinol, the plot of log (ΔIi/(ΔIi max–ΔIi)) was obtained with the value of the peak current change. Where ΔIi means the change of the peak current of ILAGC biosensor after interacting with retinol and ΔIi max is the maximal peak current change. It was found that the plot of log (ΔIi/(ΔIi max–ΔIi)) against log [retinol] results in a straight line. From the intercept of the linear plot, binding constant (K) was calculated to be 1.31×105 L mol-1. However, from the slope of the linear plot, m≈1 for retinol was obtained. Therefore, the stoichiometry of HSA with retinol was 1:1.
The precision of calculated binding constants of retinol on ILAGC biosensor was studied. It was found that the calculated binding constant was 1.31 ± 0.02×105 L mol-1. The binding constant value of retinol-HSA was compared with literature value (1.28 ± 0.04×105 L mol-1) [3]. F-test between two values was performed at 95% confidence level (α=0.05) showing no large difference or good agreement.
Conclusions
The present work provides a new green hyphenation of ILAGC biosensor with differential pulse stripping anodic voltammetry for the on-line binding studies between retinol and HSA. The advantages of ease and reproducible preparation of biosensor, the small consumption of chemicals, eco-friendly and rapid estimation opened a new avenue for the high-throughput of small molecules-protein binding studies and a good alternative for other cost effective and non-green techniques. As well, our proposed method gave us a great chance to estimate the high binding constant between retinol and HSA by detecting the interaction in the nano level concentration. The studied binding of retinol with HSA gave an acceptable precision and a good agreement with literature value.
References
- Genestar C, Grases F (1995) Determination of vitamin A in pharmaceutical preparations by High-performance liquid chromatografy with diode-array detection. Chromatographia 40: 143-146.
- N'soukpoé-Kossi CN, Sedaghat-Herati R, Ragi C, Hotchandani S, Tajmir-Riahi HA (2007) Retinol and retinoic acid bind human serum albumin: stability and structural features. Int J Biol Macromol 40: 484-490.
- El-Hady DA, Albishri HM (2012) Hyphenated affinity capillary electrophoresis with a high-sensitivity cell for the simultaneous binding study of retinol and retinoic acid in nanomolars with serum albumins. J Chromatogr B Analyt Technol Biomed Life Sci 911: 180-185.
- Rizzolo A, Polesello S (1992) Chromatographic determination of vitamins in food. J Chromatogr 624: 103-152.
- Abd El-Hady D, Youssef AK (2013) Adsorptive and affinity linear sweep voltammetry for the determination of singulair in tablets and its interaction with human serum albumin. J Anal Chem.
- Abd El-Hady D, Youssef AK (2013) Using of in-situ mercury film sensor hyphenated with affinity voltammetry for high throughput drug-protein binding studies. Am J Anal Chem.
- Wasserscheid P, Welton T (2002) Ionic liquids in synthesis. Wiley-VCH Verlag, 103.
- Ohne H (2005) Electrochemical aspects of ionic liquid, Wiley-VCH Verlag, 36.
- Hagiwara R, Ito Y (2000) Room temperature ionic liquids of alkylimidazolium cations and fluoroanions. J Fluorine Chem105: 221-227.
- Fukaya Y, Ohno H (2013) Hydrophobic and polar ionic liquids. Phys Chem Chem Phys 15: 4066-4072.
- Hasanzadeh M, Shadjou N, Eskandani M, de la Guardia M (2012) Room-temperature ionic liquid-based electrochemical nanobiosensors. Trends Anal Chem 41: 58-74.
- Safavi A, Maleki N, Honarasa F, Tajabadi F, Sedaghatpour F (2007) Ionic liquids modify the performance of carbon based potentiometric sensors. Electroanalysis 19: 582-586.
- Maleki N, Safavi A, Tajabadi F (2006) High-performance carbon composite electrode based on an ionic liquid as a binder. Anal Chem 78: 3820-3826.
- Lesniewski A (2010) Electrodes modified with imidazolium functionalized materials. IChf, Warsow.
- Chernyshov DV, Shvedene NV, Antipova ER, Pletnev IV (2008) Ionic liquid-based miniature electrochemical sensors for the voltammetric determination of catecholamines. Anal Chim Acta 621: 178-184.
- Coll C, Labrador RH, Manez RM, Soto J, Sancenon F, et al. (2005) Ionic liquids promote selective responses towards the highly hydrophilic anion sulfate in PVC membrane ion-selective electrodes. Chem Commun (Camb) 24: 3033-3035.
- Zhang X, Deckert V, Steiger B, Hirayama MK, Suter UW, et al. (2004) Covalent binding of biorecognition groups to solids using poly(hydromethylsiloxane) as linkage. Talanta 63: 159-163.
- Kharitonov AB, Zayats M, Alfonta L, Katz E, Willner I (2001) A novel ISFET-based NAD+-dependent enzyme sensor for lactate. Sensors Actuats B 76: 203-210.
- Abd El-Hady D, Youssef AK (2013) Hyphenation of ionic liquid albumin glassy carbon biosensor or protein label-free sensor with differential pulse stripping voltammetry for interaction studies of human serum albumin with fenoprofen enantiomers. Anal Chim Acta 772: 68-74.
- Shvedene NV, Chernyshov DV, Khrenova MG, Formanovsky AA, Baulin VE, et al. (2006) Ionic liquids plasticize and bring ion-sensing ability to polymer membranes of selective electrodes. Electroanalysis 18: 1416-1421.
- Abd El-Hady D, Abdel-Hamid MI, Seliem MM, El-Maali NA (2005) Adriblastina-ssDNA interaction with statistical analysis. Talanta 66: 1207-1218.
- Wu H, Zhao XJ, Wang P, Dai Z, Zou XY (2011) Electrochemical site marker competitive method for probing the binding site and binding mode between bovine serum albumin and alizarin red S. Electrochim Acta 56: 4181-4187.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15351
- [From(publication date):
specialissue-2013 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 10735
- PDF downloads : 4616