Research Article Open Access
Generation of Reactive Oxygen Species (Ros) and Pro-Inflammatory Signaling in Human Brain Cells in Primary Culture
Walter J. Lukiw1, Surjyadipta Bjattacharjee1, Yuhai Zhao2, Aileen I. Pogue3 and Maire E. Percy4*
1LSU Neuroscience Center and Department of Ophthalmology, Louisiana State University Health Sciences Center, New Orleans LA 70112, USA
2University of Texas Health Science Center, Houston TX 77030, USA
3Alchem Biotek Corporation, Toronto ON M5T 1L8, Canada
4Surrey Place Centre, Toronto ON M5S 2C2 and University of Toronto Departments of Physiology and Obstetrics & Gynaecology, Toronto ON M5S 1A8, Canada
- Corresponding Author:
- Maire E. Percy
Neurological Disease Research Group
LSU Neuroscience Center and Department of Ophthalmology
Louisiana State University Health Sciences Center 2020 Gravier Street
New Orleans LA 70112 USA
Email: wlukiw@lsuhsc.edu
Received date: November 26, 2011; Accepted date: January 23, 2012; Published date: January 25, 2012
Citation: Lukiw WJ, Bjattacharjee S, Zhao Y, Pogue AI, Percy ME (2012) Generation of Reactive Oxygen Species (ROS) and Pro-Inflammatory Signaling in Human Brain Cells in Primary Culture. J Alzheimers Dis S2:001. doi:
Copyright: © 2012 Lukiw WJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Alzheimers Disease & Parkinsonism
Abstract
The cellular generation of reactive oxygen species (ROS) has been implicated in contributing to the pathology of human neurological disorders including Alzheimer’s disease (AD) and Parkinson’s disease (PD). To further understand the triggering and participation of ROS-generating species to pro-inflammatory and pathological signaling in human brain cells, in these experiments we studied the effects of 22 different substances (including various common drugs, interleukins, amyloid precursor protein, amyloid peptides and trace metals) at nanomolar concentrations, in a highly sensitive human neuronal-glial (HNG) cell primary co-culture assay. The evolution of ROS was assayed using the cell-permeate fluorescent indicator 2’,7’-dichlorofluorescein diacetate (H2DCFDA), that reacts with major ROS species, including singlet oxygen, hydroxyl radicals or superoxides (λEx 488 nm; λEm 530 nm). Western analysis was performed for cyclooxygenase-1 (COX-1), cyclooxygenase-2 (COX-2) and cytosolic phospholipase A (cPLA2) to study the effects of induced ROS on inflammatory gene expression within the same brain cell sample. The data indicate that apart from acetylsalicylic acid (aspirin) and simvastatin, several neurophysiologically-relevant concentrations of Aβpeptides and neurotoxic trace metals variably induced ROS induction, COX-2 and cPLA2 expression. These findings have mechanistic implications for ROS-triggered inflammatory gene expression programs that may contribute to AD and PD neuropathologic mechanisms.
Keywords
Alzheimer’s disease; Apoptosis; Fenton chemistry; Human neural cells; Inflammation; Metal sulfates; Synergistic effects
Introduction
Age-related neurological disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) have long been associated with free radical-induced oxidative stress, and these are driven by the nonhomeostatic production of reactive oxygen species (ROS) [1-8]. ROS are highly reactive and charged metabolic intermediates that attack DNA, RNA, protein and lipids to leave oxidized cellular components that are genotoxic or cytotoxic, and unable to perform their normal biological functions. While cellular systems have evolved elaborate anti-oxidant systems to neutralize the effects of ROS, degenerative disease processes such as those associated with AD and PD may overwhelm these neuroprotective anti-oxidant defenses [5-11]. The progressive generation of ROS during the course of human aging lies at the core of the free radical theory of aging originally proposed by Harman 55 years ago [10]; this theory implies that aging is associated with increased ambient levels of ROS, ROS-oxidized biomolecules and their deleterious biological effects [8-11].
Based on a previously verified and highly sensitive assay in vitro stress-test system for the effects of ROS on human brain cell pathogenic gene expression [9,12-18], in these experiments we tested the ROSinducing effects of the two most widely used non-prescription drugs – simvastatin and acetylsalicylic acid (aspirin); the pro-inflammatory cytokines – interleukin-6 (IL-6) and interleukin-1beta (IL-1β); tissue necrosis factor alpha (TNFα); amyloid precursor protein (βAPP) and the AD-associated neurotoxic peptides – Aβ40 and Aβ42; the neurotoxic metals – Hg, Cu, Zn, Mn, Fe and Al; and hydrogen peroxide at 50 nM or 100 nM ambient concentrations in the growth medium of human neuronal-glial (HNG) cells in primary culture. Several of these factors were tested in combination. Because brain cells sense applied physiological stress as a form of impending cellular injury, they respond through an up-regulation of immune and inflammatory gene expression which is, in part, neuroprotective [19,20]. However excessive stimulation of these immune and inflammatory pathways are highly detrimental to normal brain cell with the induction of stressresponsive, pro-inflammatory and pro-apoptotic gene expression programs that may initiate, enhance and/or accelerate brain cell decline [18-20].
Materials and Methods
Reagents and antibodies
Simvastatin (1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-hydroxy-6-oxotetrahydro- 2H-pyran-2-yl]ethyl} -3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen- 1-yl2,2-dimethylbutanoate; S6196) and acetylsalicylic acid (O-acetylsalicylic acid, ASA, aspirin; A5376) were dissolved in DMSO and ultrapure water, respectively, following instructions provided by the manufacturer (Sigma-Aldrich Chemical, St. Louis, MO). When appropriate, control HNG cells received DMSO at concentrations used in simvastatin and ASA assays. Solutions of βAPP, Aβ40 and Aβ42 were prepared as previously described [21-23]. All trace metals were used as ultrapure sulfates [14]. Briefly, Biochemika MicroSelect© ultrapure reagents for molecular biology, including MgSO4 (63133), Mn(II) sulfate (31425), Hg(II)SO4(83372), Cu(II)SO4 (35185), ZnSO4 (35392), FeSO4 (44970) and Al2(SO4)3 (11044; Sigma-Aldrich or Fluka Chemical, Milwaukee, WI), freshly prepared as 0.1 M stock solutions [9,16,17], were instilled into serum-containing HNG cell maintenance medium (HNGMM, pH 7.5; see section below for details) by gentle inversion, followed by filter sterilization using 0.2-μM spin filters (Millipore Corporation, Billerica, MA). Solutions were used at the concentrations shown in Table 1. HNG cells, HNGMM and bullet packs containing human epidermal and fibroblast growth factor (E/FGF), gentamicin/ amphotericin (G/A1000), neural survival factor-1 (NSF-1) and FBS were obtained from Clonetics-Lonza (Walkersville, MD). Western immunoblots were performed using human-specific primary antibodies against a beta-tubulin III (βTubIII T8660), glial fibrillary acidic protein (GFAP, G9269; Sigma-Aldrich Chemical). Antibodies to the control cytoskeletal filament β-actin (sc-81178), cyclooxygenase-1 (COX-1; sc-1752), cyclooxygenase-2 (COX-2; sc-1747) and cytosolic phospholipase A2 (cPLA2; sc-137089) were obtained from Santa Cruz Biotechnologies (Santa Cruz, CA). Hoechst 33258 bis-benzimide (H-1398), 2’,7’-dichlorofluorescein diacetate (H2DCFDA; D399) was obtained from Molecular Probes-Invitrogen (Eugene, OR) and used according to the manufacturer’s instructions. All other reagents were of the highest ultrapure grades commercially available and were used without further purification [18-20].
| Physiological stressor | Concentration | Degree of induction of ROS** | COX-1*** | COX-2*** | cPLA2**** | Referencest |
|---|---|---|---|---|---|---|
| simvastatin | 50 nM | 0 | - | - | - | 25,26 |
| aspirin | 50 nM | 0 | - | - | - | 27 |
| βAPP | 100 nM | 0 | - | - | - | 13,14,20 |
| Aβ40 | 10 nM | 1 | - | - | - | 13,14 |
| Aβ40 | 50 nM | 1 | - | - | - | 13,14 |
| Aβ40 | 100 nM | 2 | - | -/+ | -/+ | 13,14 |
| Aβ42 | 10 nM | 3 | - | + | - | 13,14 |
| Aβ42 | 50 nM | 4 | - | + | -/+ | 13,14 |
| Aβ42 | 100 nM | 5 | - | ++ | + | 13,14 |
| IL-6 | 50 nM | 5 | - | -/+ | -/+ | 32 |
| IL-1β | 50 nM | 6 | - | + | + | 28,33 |
| TNFα | 50 nM | 6 | - | + | + | 35 |
| Aβ42+IL-1β | 50 nM (each) | 7 | - | + | + | 33,36 |
| Aβ42+TNFα | 50 nM (each) | 7.5 | - | + | + | 31,35 |
| Hg | 50 nM | 1.5 | - | - | - | 16,37,39 |
| Cu | 50 nM | 3 | - | + | - | 16,37,40 |
| Zn | 50 nM | 4 | - | + | + | 16,37,41 |
| Mn | 50 nM | 4.5 | - | -/+ | + | 16,38 |
| Fe | 50 nM | 5 | - | -/+ | + | 16,31,37,42 |
| Al | 50 nM | 9 | - | ++ | ++ | 16-19,31,37,42 |
| Al+Fe | 50 nM (each) | 10 | + | +++ | +++ | 16-19,31,37 |
| H2O2 | 50 nM | 10 | + | +++ | +++ | 16,31,33 |
Table 1 Effects of different stressors, at nM concentrations, on reactive oxygen species (ROS) generation in human primary neuronal-glial (HNG) cell cultures, and induced inflammatory consequences as indicated by COX-2 and cPLA2 up-regulation*.
Table 1: *Depends on age of cell cultures and cell type; HNG cells cultured for 2 weeks under optimum growth conditions; each physiological stressor was assayed three times. ** scale of 1-10 based on these 20 evaluations ***detection of cycloxygenase-1 (COX-1), cycloxygenase-2 (COX-2) after 3 hrs treatment ****detection of cytoplasmic phospholipase A2 (cPLA2 ) after 3 hrs treatment tReferences quoted here include relevant research on metal-sulfate induced oxidative stress and inflammatory gene expression.
Human neuronal-glial (HNG) cells in primary culture
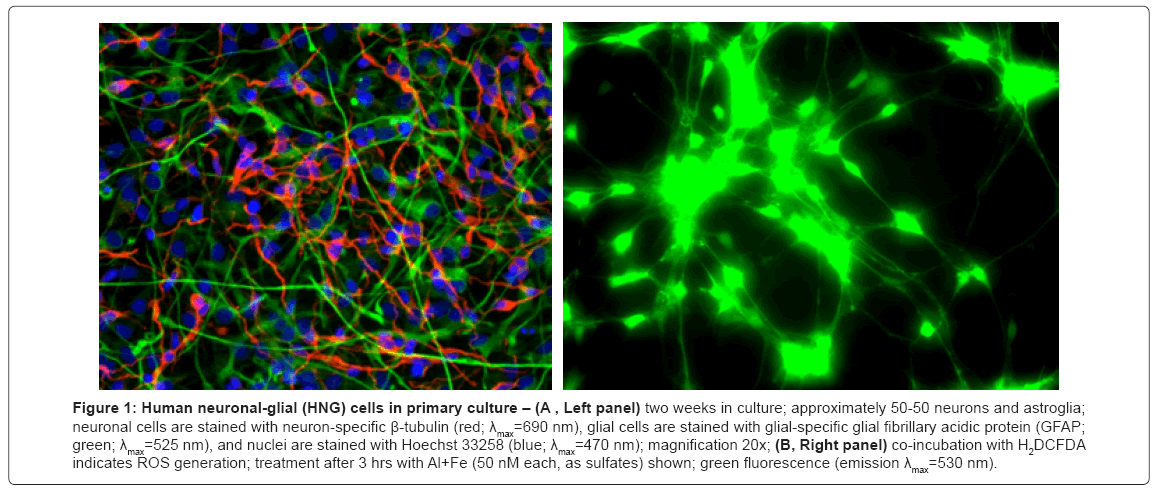
HNG cell lines, derived from normal human neural progenitor cells (PT-2599; Lonza Clonetics Cell Systems, Walkersville, MD) were cultured in 6-well (3.5 cm diameter) plates (Costar 3506, Corning Life Sciences, Acton, MA) at 5% CO2, 20% O2 and 37ºC in an HNG cell maintenance medium (HNGMM) supplemented with 2.5% serum containing hFGF (human fibroblast growth factor), NSF-1 (neuronal survival factor 1), hEGF (human epidermal growth factor) and GA- 1000 (gentamicin-amphotericin B G/A 1000) as previously described [9,12-19]. HNGMM was completely changed every 3.5 days. At 2 weeks of growth there were approximately 50% neurons and 50% astroglia (Figure 1). HNG cells tested positive for the neuronal- and glial-specific markers βTUBIII and GFAP, respectively, and tested negative for fibroblast contamination using antibodies against fibroblast-specific protein-1 (data not shown).
Figure 1: Human neuronal-glial (HNG) cells in primary culture – (A , Left panel) two weeks in culture; approximately 50-50 neurons and astroglia; neuronal cells are stained with neuron-specific β-tubulin (red; λmax=690 nm), glial cells are stained with glial-specific glial fibrillary acidic protein (GFAP; green; λmax=525 nm), and nuclei are stained with Hoechst 33258 (blue; λmax=470 nm); magnification 20x; (B, Right panel) co-incubation with H2DCFDA indicates ROS generation; treatment after 3 hrs with Al+Fe (50 nM each, as sulfates) shown; green fluorescence (emission λmax=530 nm).
Minimization of extraneous contamination
Throughout these experiments ultrapure water (18 megohm, Milli-Q, Millipore or Puriss 95305, Fluka) was employed in all cell culture, protein isolation and biochemical procedures to stringently exclude trace metal extraneous contamination; as analyzed by electro thermal atomic absorption spectroscopy, aluminum, copper, magnesium, mercury, iron and zinc content were <5 ppb. Coded isolation reagent and media samples were analyzed for potential trace metal contamination using a Perkin Elmer 5000PC Zeeman-type electro thermal atomic absorbance (EAA) spectrophotometer equipped with an automated sampler and IBM/AT-supported analysis package for trace metal analysis [9,18,19]. Wherever possible, ultrapure HNO3 washed polysulfone plasticware was used according to the URI-GSO protocols to stringently eliminate trace metal contamination [17-19].
Western analysis and immunostaining
To ascertain whether up-regulation in specific protein species was associated with ROS production, Western immunoblots were performed using human-specific primary monoclonal antibodies to COX-1, COX-2 and cPLA2 using the β-actin protein signal in the same sample as control [14-19]. Bound primary antibodies were detected with an anti-IgG fluor-linked secondary antibody (PA45007; Amersham Biosciences, Piscataway, NJ) and developed with an ECL+ Western blotting system (RPN2132; Amersham).
ROS Assay using 2’,7’-dichlorofluorescein diacetate (H2DCFDA)
Levels of reactive oxygen species (ROS) were assayed in metalion- treated and un-treated 2 wk old HNG cells (Figure 2) using 2’,7’-dichlorodihydro-fluorescein diacetate (H2DCFDA) at a 10μM ambient concentration in cell culture medium in the dark using protocols provided by the manufacturer (Molecular Probes). H2DCFDA, cell-permeate indicators that react with the highly reactive singlet oxygen, hydroxyl radicals or superoxide-generating fluorescent signals (collectively termed ROS), were quantified using electronic imaging photography under UV light (λEx 488 nm; λEm 530 nm) using a Zeiss Axioskop/Zeiss MC63 photo control unit coupled to a Nikon Optiphot 2 microscope equipped with an additional differential Interference Contrast/Nikon UFX DX photo control unit [9].
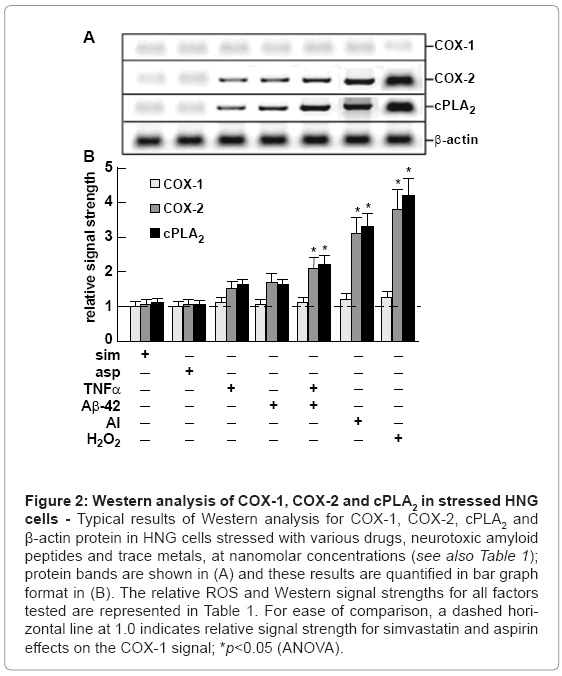
Figure 2: Western analysis of COX-1, COX-2 and cPLA2 in stressed HNG cells - Typical results of Western analysis for COX-1, COX-2, cPLA2 and β-actin protein in HNG cells stressed with various drugs, neurotoxic amyloid peptides and trace metals, at nanomolar concentrations (see also Table 1); protein bands are shown in (A) and these results are quantified in bar graph format in (B). The relative ROS and Western signal strengths for all factors tested are represented in Table 1. For ease of comparison, a dashed horizontal line at 1.0 indicates relative signal strength for simvastatin and aspirin effects on the COX-1 signal; *p<0.05 (ANOVA).
Western analysis data and statistical analysis
Western signal-intensity data were gathered by phosphor imaging onto molecular imaging screens using a Typhoon (Amersham- Pharmacia Biosciences) Molecular Imaging system. All statistical procedures were carried out using the programs and procedures in the SAS language (Statistical Analysis System, SAS Institute, Cary, NC). All p values were derived from protected t-tests or least square means from a two-way factorial analysis of variance (p, ANOVA); only p-values of less than 0.05 were considered to be statistically significant.
Results
A typical culture of HNG cells used in these experiments is shown in Figure1A, and a typical H2DCFDA-based ROS assay is shown in Figure 1B. The example shown in Figure 1B is from the intense degree of ROS generated in HNG cells stressed with Al+Fe (50 nM each, as sulfates). Fluorescent signals from stressed HNG cells were quantified using digital electronic imaging photography under ultraviolet (UV) light (λEx 488 nm; λEm 530 nm) employing an Axioskop/Zeiss MC63 photo control unit and a Nikon Optiphot 2 microscope equipped with an additional differential-Interference Contrast/Nikon UFX DX photo control unit.
The ROS signal intensity for 22 test compounds including Aβ40- and Aβ42peptides and trace metals are shown in Table 1; depending on ROS generated a scale from 1-10 was derived from the ROS signal obtained from control HNG cells as previously described [9,18]. HNG cells treated with MgSO4, simvastatin, aspirin or βAPP at any concentration tested for 3 hrs showed no generation of ROS above control values. Aβ40 peptide at 10, 50 and 100 nM ambient concentration showed relatively modest ROS generation; Aβ42 peptide at 10, 50 and 100 nM ambient concentration showed 3- to 5-fold the ROS induciblity as did Aβ40 peptide. Aβ40 and Aβ42 peptide together showed no significant synergistic effects (data not shown). Interleukin-6 (IL-6), interleukin 1-beta (IL-1β) and tissue necrosis factor alpha (TNFα) showed greater induction of ROS than Aβ40 or Aβ42 peptides alone. Interestingly the combination of Aβ42+IL-6 showed no synergistic effect of ROS generation while the combinations of Aβ42+IL-1β and Aβ42+TNFα showed a higher ROS generation than Aβ42, IL-1β or TNFα alone.
Concerning the ability of metal sulfates to generate ROS the order of effectiveness was Al>>Fe>Zn>Mn>Cu>Hg. The combination of Al+Fe (as sulfates) and H2O2 by itself showed the greatest ability to generate ROS at the concentrations tested.
Using Western assay the levels of COX-1, COX-2 and cPLA2 were also analyzed in these samples, and we observe a significant correlation between ROS generation and COX-2 and cPLA2 up-regulation; no significant induction was observed in the control COX-1. For example, the greatest inducers of ROS identified in this study, Al+Fe (as sulfates) and H2O2 by itself, associated with the highest induction of both COX- 2 and cPLA2, each to at least 3-fold or greater over controls.
Discussion
The original experimental design and aim of this study was to quantify the relative ROS-generating capability (and ensuing genetic toxicity) of several physiologically-relevant neurotoxic factors using human neuronal-glial (HNG) cells in primary co-culture. These primary cell cultures provide the basis for a highly sensitive and proven primary brain cell analytical assay that is representative of all human neocortical brain cell types. Although the present study was limited to study of ROS formation and activity of COX-1, COX-2 and cPLA2 levels, the ROS-induced expression of COX-2 and cPLA2 (but not COX-1) by different neurotoxins is by itself highly indicative of ROSmediated activation of the arachidonic acid cycle in primary HNG cells.
Excessive ROS generation in brain cells and CNS tissues promotes cellular oxidative stress that progressively renders normally functioning DNA, RNA, proteins and lipids incapable of performing their normal cellular functions: this is the basis of the free-radical theory of aging [1,4,5,10,11]. Aging is the greatest known risk factor for the onset of neurodegenerative diseases such as AD and PD, progressive, incurable neurological disorders with a prevalence of 67 AD and 10 PD cases per 1000 elderly persons [24]. In the aging brain, mitochondrial dysfunction increases with age, and increased production of ROS and oxidative stress is highly damaging to both neurons and glia in these common human neurodegenerative conditions [4,5,11,20]. The use of anti-oxidants and free radical trapping agents have shown significant benefit in reducing oxidative stress and ROS generation in these in vitro test systems and in human clinical trials [15,17,25,26].
Simvastatin, a generic statin that lowers cholesterol levels through inhibitory actions on HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) reductase, and aspirin, an non-steroidal anti-inflammatory drug that functions as a COX-1 and COX-2 inhibitor, are respectively, the two most widely prescribed and over-the-counter used drugs today in industrialized societies [27-29]. Both showed no significant promotion of ROS activity in the HNG cell assay system used here. Table 1 shows the relative degree of the induction of ROS for 20 other brain-relevant Aβ peptides, cytokines, toxic metals, and the synergistic effects of some of their combinations at 50 nM and 100 nM ambient concentrations in the HNG tissue culture medium. While the most potent single amyloid peptide inducer of ROS was Aβ42 peptide and the most potent cytokine inducer of ROS was TNFα, the combination of Aβ42+TNFα showed a synergistic enhancement in the production of ROS. The most potent single metal sulfate inducer of ROS was Al (as sulfate), and the most potent combinatorial metal sulfate inducer of ROS was Al+Fe that compared to an equivalent ROS-inducing capability by hydrogen peroxide (H2O2) by itself.
These findings underscore the idea that neurotoxic amyloid peptides and trace metals, at physiologically realistic nanomolar concentrations and either alone of in combination, are highly effective in inducing ROS and pathogenic and pro-inflammatory gene expression programs [9,15-19]. Interestingly, Mn induced ROS generation and neurological deficits have been recently implicated in the neuropathogenesis of idiopathic Parkinson’s disease (IPD), amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD) and prion disease [30]. Highly complex mixtures of Aβ40, Aβ42, cytokines and neurotoxic metals (as might be expected in vivo) are likely to induce synergistic effects in promoting stress and neurodegeneration [14-16]; unpublished observations. While the ‘housekeeping cyclooxygenase’ COX-1 was not found to be induced by any treatment in these studies, COX-2 and cPLA2 were found to be significantly up-regulated in ROS-stressed HNG cells. This may be particularly significant for pro-inflammatory signaling as COX-2 and cPLA2 represent the initial and rate-limiting enzymes in the arachidonic acid cycle, and pathogenic prostaglandin signaling pathways. In addition, ROS is a potent inducer of the proinflammatory transcription factor NF-кB that is up-regulated in both AD and PD, and both COX-2 and cPLA2 are at least under partial NF-кB-mediated regulation [31-33]. Highly specific chelators and innovative chelation strategies may be useful to neutralize the effects of neurotoxic metals in human brain cells [17,25,26]. Lastly, the HNG cell system described here may also serve as a suitable test platform to compare the effects of other physiologically relevant stressors and chelators, and other inhibitory molecules that may be useful in quenching ROS-induced pro-inflammatory signaling that contribute to neuropathogenic events.
Studies involving of human neuronal-glial (HNG) cells in primary co-culture carried out over the past 20 years have proved highly informative in neurodegenerative disease mechanistic studies [21,34- 36]. These and the present study should encourage other researchers to experiment with the organic and inorganic neurotoxic factors utilized and characterized in this study, and further explore their effects in HNG cells and other more specific human neural cell systems. A major challenge in neurodegenerative disorders is the connection between intracellular protein inclusions, which are different for different neurodegenerative diseases, neuroinflammation, and the functional loss of neurons and synapses [35-38]. Laboratories that have access to fresh human brain tissue, might consider co-culturing dopaminergic, midbrain, or hippocampal cells (mostly affected in IPD and AD, respectively) in parallel with astrocytic or glial cells in order to explore the relation between exposure to ROS-inducing factors and formation of intracellular inclusions. To also consider is the fact that it now is possible to induce functional dopaminergic neurons from human embryonic stem cells and pluripotent adult stem cells [38]. Although the use of human cells is likely to shed better light on the origin and pathogenesis of AD, IPD and other human neurodegenerative disorders than rodent cells, co-culture of human cells will benefit from recent advances in glial-neuronal sandwich co-culture systems [39-41].
Acknowledgements
The studies were presented in part at the Alzheimer’s Association International Conference on Alzheimer’s disease (AAICAD 2011) in Paris France 16-21 July 2011. Thanks are extended to Drs. Jian-Guo Cui, Yuan Li and to Darlene Guillot for expert technical assistance, and to the anonymous reviewers for the constructive comments provided. Research on the contribution of ROS to inflammatory gene expression in the Lukiw laboratory were supported through Translational Research Initiative (TRI) Grants from LSU Health Sciences Center New Orleans (WJL), a bioinformatics grant from the Louisiana Biotechnology Research Network (LBRN), an Alzheimer Association Investigator-Initiated Research Grant IIRG-09-131729 (WJL), and NIH NIA Grants AG18031 and AG038826 (WJL). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health. Dr. Percy acknowledges infrastructure support provided by Surrey Place Centre.
References
- Shibata N, Kobayashi M (2008) The role for oxidative stress in neurodegenerative diseases. Brain Nerve 60: 157-170.
- Allsop D, Mayes J, Moore S, Masad A, Tabner BJ (2008) Metal-dependent generation of reactive oxygen species from amyloid proteins implicated in neurodegenerative disease. Biochem Soc Trans 36: 1293-1298.
- Nunomura A, Hofer T, Moreira PI, Castellani RJ, Smith MA, et al. (2009) RNA oxidation in Alzheimer disease and related neurodegenerative disorders. Acta Neuropathol 118: 151-166.
- Patten DA, Germain M, Kelly MA, Slack RS (2010) Reactive oxygen species: Stuck in the middle of neurodegeneration. J Alzheimers Dis 20: 357-367.
- Facecchia K, Fochesato LA, Ray SD, Stohs SJ, Pandey S (2011) Oxidative toxicity in neurodegenerative diseases: role of mitochondrial dysfunction and therapeutic strategies. J Toxicol 2011: 683728.
- Jomova K, Valko M (2011) Importance of iron chelation in free radical-induced oxidative stress and human disease. Curr Pharm Des 3460-3473.
- Srivastava S, Haigis MC (2011) Role of sirtuins and calorie restriction in neuroprotection: implications in Alzheimer's and Parkinson's diseases. Curr Pharm Des 3418-3433.
- Tufekci KU, Genc S, Genc K (2011) The endotoxin-induced neuroinflammation model of Parkinson's disease. Parkinsons Dis 2011: 487450.
- Alexandrov PN, Zhao Y, Pogue AI, Tarr MA, Kruck TP, et al. (2005) Synergistic effects of iron and aluminum on stress-related gene expression in primary human neural cells. J Alzheimers Dis 8: 117-127.
- Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11: 298-300.
- Oliveira BF, Nogueira-Machado JA, Chaves MM (2010) The role of oxidative stress in the aging process. ScientificWorldJournal 10: 1121-1128.
- Cui JG, Kuroda N, Chandrasekharan NV, Pelaez RP, Simmons DL, et al. (2004) Cyclooxygenase-3 gene expression in Alzheimer hippocampus and in stressed human neural cells. Neurochem Res 29: 1731-1737.
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, et al. (2005) A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest 115: 2774-2783.
- Bazan NG, Lukiw WJ (2002) Cyclooxygenase-2 and presenilin-1 gene expression induced by interleukin-1beta and amyloid beta 42 peptide is potentiated by hypoxia in primary human neural cells. J Biol Chem 277: 30359-30367.
- Lukiw WJ, Percy ME, Kruck TP (2005) Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J Inorg Biochem 99: 1895-1898.
- Pogue AI, Li YY, Cui JG, Zhao Y, Kruck TP, et al. (2009) Characterization of an NF-?B-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J Inorg Biochem 103: 1591-1595.
- Kruck TP, Cui JG, Percy ME, Lukiw WJ (2004) Molecular shuttle chelation: the use of ascorbate, desferrioxamine and Feralex-G in combination to remove nuclear bound aluminum. Cell Mol Neurobiol 24: 443-459.
- Lukiw WJ, Percy ME, Kruck TP (2005) Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J Inorg Biochem 99: 1895-1898
- Lukiw WJ, Krishnan B, Wong L, Kruck TPA, Bergeron C, et al. (1992) Nuclear compartmentalization of aluminum in Alzheimer's disease (AD). Neurobiol Aging 13: 115-121.
- Lynch T, Cherny RA, Bush AI (2000) Oxidative processes in Alzheimer's disease: The role of Aß-peptide-metal interactions. Exp Gerontol 35: 445-451
- Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, et al. (2011) Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPAR?-mediated mechanisms in Alzheimer's disease models. PLoS One 6: e15816.
- Stine WB, Dahlgren KN, Krafft GA, LaDu MJ (2003) In vitro characterization of conditions for amyloid-beta peptide oligomerization and fibrillogenesis. J Biol Chem 278: 11612-11622.
- Stine WB, Jungbauer L, Yu C, LaDu MJ (2011) Preparing synthetic Aß in different aggregation states. Methods Mol Biol 670: 13-32.
- Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, et al. (2007) How common are the ‘‘common’’ neurologic disorders? Neurology 68: 326-337.
- Kruck TP, Percy ME, Lukiw WJ (2008) Metal sulfate-mediated induction of pathogenic genes and repression by phenyl butyl nitrone and Feralex-G. Neuroreport 19: 245-249.
- Percy ME, Kruck TPA, Pogue AI, Lukiw WJ (2011) Towards the prevention of potential aluminum toxic effects and an effective treatment for Alzheimer's disease. J Inorg Biochem 105: 1505-1512.
- Weber MS, Stuve O, Neuhaus O, Hartung HP, Zamvil SS (2007) Spotlight on statins. Int MS J 14: 93-97.
- Pawelczyk T, Kloszewska I, Sobów T (2005) Statins- the 21st century aspirin? Pol Merkur Lekarski 19: 111-114.
- Dineen PF, Curtin RJ, Harty JA (2010) A review of the use of common antiplatelet agents in orthopaedic practice. J Bone Joint Surg Br 92: 1186-1191.
- Bowman AB, Kwakye GF, Herrero Hernández E, Aschner M (2011) Role of manganese in neurodegenerative diseases. J Trace Elem Med Biol 191-203.
- Lukiw WJ, Bazan NG (2000) Neuroinflammatory signaling upregulation in Alzheimer's disease. Neurochem Res 25: 1173-1184.
- Lukiw WJ, Bazan NG (1998) Strong nuclear factor-?B-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer's disease superior temporal lobe neocortex. J Neurosci Res 53: 583-592.
- Flood PM, Qian L, Peterson LJ, Zhang F, Shi JS, et al. (2011) Transcriptional factor NF-?B as a target for therapy in Parkinson's disease. Parkinsons Dis 2011: 216-298.
- Lukiw WJ, Zhao Y, Cui JG (2008) An NF-?B-sensitive mi RNA-146a-mediated inflammatory circuit in Alzheimer disease and in stressed human brain cells. J Biol Chem 283: 31315-31322.
- Li YY, Cui JG, Dua P, Pogue AI, Bhattacharjee S, et al. (2011) Differential expression of miRNA-146a-regulated inflammatory genes in human primary neural, astroglial and microglial cells. Neurosci Lett 499: 109-113.
- Pogue AI, Percy ME, Cui JG, Li YY, Bhattacharjee S, et al. (2011) Up-regulation of NF-kB-sensitive miRNA-125b and miRNA-146a in metal sulfate-stressed human astroglial (HAG) primary cell cultures. J Inorg Biochem 105: 1434-1437.
- Goedert M, Clavaguera F, Tolnay M (2010) The propagation of prion-like protein inclusions in neurodegenerative diseases. Trends Neurosci 33: 317-325.
- Swistowski A, Peng J, Liu Q, Mali P, Rao MS, et al. (2010) Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 28: 1893-1904.
- Boraso M, Viviani B (2011) Glia-neuron sandwich cocultures: An in vitro approach to evaluate cell-to-cell communication in neuroinflammation and neurotoxicity. Methods Mol Biol 758: 135-152.
- Roqué PJ, Guizzetti M, Giordano G, Costa LG (2011) Quantification of synaptic structure formation in cocultures of astrocytes and hippocampal neurons. Methods Mol Biol 758: 361-390.
- Sundaram JR, Chan ES, Poore CP, Pareek TK, Cheong WF,et al. (2012) Cdk5/ p25-induced cytosolic PLA2-mediated lysophosphatidylcholine production regulates neuroinflammation and triggers neurodegeneration. J Neurosci 32: 1020-1034.
- Pogue AI, Cui JG, Li YY, Zhao Y, Culicchia F, et al. (2010) Micro RNA-125b (miRNA-125b) function in astrogliosis and glial cell proliferation. Neurosci Lett 476: 18-22.
- Cui JG, Li YY, Zhao Y, Bhattacharjee S, Lukiw WJ (2010) Differential regulation of interleukin-1 receptor-associated kinase-1 (IRAK-1) and IRAK-2 by microRNA-146a and NF-kappaB in stressed human astroglial cells and in Alzheimer disease. J Biol Chem 285: 38951-38960.
- Cui JG, Zhao Y, Lukiw WJ (2005) Isolation of high spectral quality RNA using run-on gene transcription; application to gene expression profiling of human brain. Cell Mol Neurobiol 25: 789-794.
- Monroe RK, Halvorsen SW (2005) Mercury abolishes neurotrophic factor-stimulated Jak-STAT signaling in nerve cells by oxidative stress. Toxicol Sci 94: 129-138.
- Bondy SC, Guo-Ross SX, Truong AT (1998) Promotion of transition metal-induced reactive oxygen species formation by beta-amyloid. Brain Res 799: 91-96.
- Chang CY, Chien HF, Jiangshieh YF, Wu CH (2003) Microglia in the olfactory bulb of rats during postnatal development and olfactory nerve injury with zinc sulfate: A lectin labeling and ultrastrucutural study. Neurosci Res 45: 325-333.
Relevant Topics
- Advanced Parkinson Treatment
- Advances in Alzheimers Therapy
- Alzheimers Medicine
- Alzheimers Products & Market Analysis
- Alzheimers Symptoms
- Degenerative Disorders
- Diagnostic Alzheimer
- Parkinson
- Parkinsonism Diagnosis
- Parkinsonism Gene Therapy
- Parkinsonism Stages and Treatment
- Stem cell Treatment Parkinson
Recommended Journals
Article Tools
Article Usage
- Total views: 15970
- [From(publication date):
September-2013 - Apr 02, 2025] - Breakdown by view type
- HTML page views : 11236
- PDF downloads : 4734