Review Article Open Access
Gelatine Tannate for the Treatment of Acute Diarrhoea in Adults
Alessandro Allegrini1* and Massimo Costantini2
1Department of Oncology and Neuroscience, c/o Hospital Clinicizzato “SS Annunziata”, Via dei Vestini 31, 66100 Chieti, Italy
2Pharmacist Manager I level University of Oregon Hospital Pharmacy Hospital “G. Mazzini”, Padre Pio Square, 64100 Teramo, Italy
- *Corresponding Author:
- Dr. Alessandro Allegrini
Department of Oncology and Neuroscience
c/o Hospital Clinicizzato “SS Annunziata”
Via dei Vestini 31
66100 Chieti, Italy
E-mail: allegrinia@gmail.com
Received date: October 17, 2011; Accepted date: April 25, 2012; Published date: April 27, 2012
Citation:Allegrini A, Costantini M (2012) Gelatine Tannate for the Treatment of Acute Diarrhoea in Adults. J Gastrointest Dig Syst 2:110. doi:10.4172/2161-069X.1000110
Copyright: © 2012 Allegrini A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Gelatine tannate has been recently licensed as a medical device for the treatment of acute diarrhoea. Gelatine tannate is thought to act locally on the gut wall by forming a protective protein-based film and allowing the precipitation of pro-inflammatory mucoproteins from the intestinal mucus responsible for local inflammation, and subsequently eliminated through the faeces. In order to assess safety and efficacy of gelatine tannate, one randomised, parallel, double-blinded and placebo-controlled study was conducted by general practitioners in 40 adult patients diagnosed with acute diarrhoea (test formulation: capsules containing 500 mg of gelatine tannate). Safety was evaluated by monitoring adverse events (frequency, intensity and relation of adverse events to the administered treatments), laboratory parameters and vital signs between the pre- and post-study visits (primary study endpoints). Efficacy was evaluated in terms of mean decrease of both the daily frequency of watery stools (Stool Decrease Index, SDI) and the severity of the abdominal pain assessed using a 100-mm visual analogue scale (Pain Decrease Index, PDI). Statistical analyses were performed according to the principles of intention-to-treat and perprotocol. Gelatine tannate showed a good safety profile. No adverse events were reported in either active treatment or placebo arms. Gelatine tannate was significantly more effective than placebo (p < 0.01). In fact, adult patients treated with gelatine tannate had significantly less watery stools and less abdominal pain compared to patients treated with placebo. Gelatine tannate is an effective and safe treatment for acute diarrhoea in adults. The introduction of a viable treatment option based on a mechanical action could be an alternative to existing drugs for the treatment of acute diarrhoea. In fact, gelatine tannate is not absorbed systemically and acts locally to produce its effects on the gut wall, and thus may provide greater safety and tolerability compared to existing drug therapies.
Keywords
Acute diarrhoea; Capsules; Gelatine tannate; Safety; Efficacy; Randomised clinical trial
Abbreviations
AEs: Adverse Effects; CT: Cholera Toxin; ITT: Intent-to-Treat; LOCF: Last Observation Carried Forward; PDI: Pain Decrease Index; PP: Per Protocol; SD: Standard Deviation; SDI: Stool Decrease Index; VAS: Visual Analogue Scale
Introduction
Diarrhoea is the passing of increased amounts of loose or watery stools. Normal stools are usually solid because the small intestine and colon are highly efficient in absorbing nutrients, fluid and salts from the liquid, upper gut contents [1]. Diarrhoea occurs when these processes are impaired [2,3] typically due to increased intestinal secretion of fluid and electrolytes, predominantly in the small intestine; and decreased absorption of fluid, electrolytes, and more rarely of nutrients that can involve the small and large intestines. It can be acute (short-term) or chronic (long-term) if symptoms persist more than three weeks. Most people are affected by diarrhoea at some point in their lives. It is often accompanied by abdominal pain, nausea and vomiting.
Gelatine tannate (Tasectan™) has recently been approved in Europe as a medical device to control and reduce the symptoms associated with diarrhoea in infants, children and adults. Gelatine tannate is stable in the acid environment of the stomach, decomposing into tannic acid and gelatine once in the alkaline medium of the intestines. The binding of tannic acid with albuminoidal and mucilaginous substances from the intestinal content neutralises many inflammatory molecules being produced and released by the gastrointestinal walls, thereby protecting the digestive organs from injurious attack. All tannins act as astringents that shrink tissues and contract structural proteins in the skin and mucosa, actions which contribute to their antidiarrhoeal effect. Tannins are also attributed antibacterial and antioxidant properties. On the other hand, the gelatine in the compound is thought to provide mechanical protection to the inflamed gut walls by forming a biofilm that lines the gut walls, protecting it against the acids and alkaloids resulting from bacterial fermentation or putrefaction during gastrointestinal transit.
In vitro biological activities, such as inhibition of bacterial toxins, have been also assigned to tannins [3,4]. It has been shown that tannins are capable of inhibiting Vibrio cholerae toxins; they reduce intracellular cAMP formation capacity by inhibiting ADP ribosylation, which in turn decreases the intestinal secretion of chlorine and water [5]. The cholera toxin (CT) is an oligomeric protein formed by a single A unit and five B subunits. It becomes biologically active once the B unit binds to receptors (ganglioside GM) on the apical membrane of the enterocyte, inducing a cascade of changes in the CT molecule culminating with the subunit A being inserted in the cell. This is followed by activation of adenylyl cyclase and subsequent prostaglandin activation, resulting in the accumulation of sodium and water within the intestine [4]. Tannins are also capable of inhibiting the ADP-ribosyltransferase activity, [3] probably by forming macromolecular aggregates with the CT, preventing it from binding to the GM receptors of the apical portion of the enterocyte and thus inhibiting cAMP synthesis [4]. Tannins seem to also have antiviral effects, with at least two studies showing tannin to be inhibitory to viral reverse transcriptase [6,7].
Tannic acids have undesirable gastrointestinal effects; indeed, they are known to induce digestive symptoms such as nausea and vomiting, whilst inhibiting the absorption of iron and other metals. The use of tannic complexes such as albumin tannate and gelatine tannate, which are hydrolysed to albumin or gelatine, respectively, and tannic acid in the intestine, prevents the aforementioned adverse effects affecting the gastric mucosa or the metal chelating effects of tannic acid. There are very few studies on the effect of tannins on intestinal motility, but those that exist appear to confirm the absence of an inhibiting effect [8].
Natural polyphenols are abundant micronutrients in our diet and products rich in vegetable tannins, like herbal medications, have long been used to treat diarrhoea; [9,10] whilst there is plenty of evidence to support the antidiarrhoeal effect of medicinal plants found to be rich in tannins, there is little clinical evidence available so far to support its efficacy and safety in human patients [11-14].
The introduction of a viable treatment option based on a mechanical action could be an alternative to existing drugs for the treatment of acute diarrhoea. In fact, gelatine tannate is not absorbed systemically and acts locally to produce its effects on the gut wall, and thus may provide greater safety and tolerability compared to existing drug therapies. The aim of the present placebo-controlled study was to further assess both efficacy and safety of a new formulation (capsules containing 500 mg of gelatine tannate) in comparison with placebo in adults with acute diarrhoea.
Materials and Methods
Patients
Forty adults of both sexes were enrolled in the study. To be eligible, patients had to have been diagnosed with acute diarrhoea caused by intestinal infection (at least three watery stools in the 24 hours prior to inclusion into the study, n0 ≥ 3 and basal abdominal pain of at least 20 mm evaluated through a 100-mm visual analogue scale).
Patients were excluded if they had either known hypersensitivity or allergy to any of the active ingredients or excipients in the medical device, or concomitantly treated with other antidiarrhoeal at the time of inclusion in the study.
Study design
The study was a randomised, placebo-controlled, parallel-group clinical trial carried out in Italy by general practitioners experienced in clinical trials. The ethics committee for Biomedical Research of the University “G. D’Annunzio” in Chieti, Italy, approved the clinical study protocol and the related material. The study was conducted in accordance with the Declaration of Helsinki and the European guideline for Good Clinical Practice Guideline [15,16]. All patients gave written informed consent before their participation in the trial.
Screening procedures included: assessment of inclusion/exclusion criteria and collection of demographic characteristics, medical history, physical examination, past and current medication and assessment of vital signs such as blood pressure and pulse rate. In addition, urine screening for drugs of abuse and routine haematology, biochemistry, and urinalysis tests were conducted. Following the baseline evaluation (study day 0), participants were randomly assigned to receive either the active substance or the placebo. The randomisation scheme was created by using the randomisation functions in SPSS 13.0, and participants were allocated to either the active treatment or placebo following the order of admission into the trial. Forty adults were randomly assigned to one of two arms: patients in group A (n = 20) were given one capsule containing 500 mg of gelatine tannate administered six times daily for two consecutive days [batch number: NOV 07-08]; whilst patients in group B (n = 20) were given one capsule containing 500 mg of blank odourless and biologically inactive powder (rice starch, magnesium stearate) administered six times daily for two consecutive days [batch number: No. C-03].
The active treatments were pre-packed, labelled and supplied by the sponsor (Novintethical Pharma Sagl, Lugano, Switzerland) in accordance with applicable Good Manufacturing Practice. All patients were instructed to take the assigned treatment for two consecutive days after the first administration (study day 1) to the subsequent one (study day 2). Patients were requested to attend the practitioner ambulatory clinic for a final visit on study day 2.
Safety and efficacy assessment
The primary outcome was safety assessment and included the recording of adverse effects (AEs), spontaneously reported and monitored by a co-investigator(s) either during each visit or via a telephone questionnaire, and monitoring of laboratory parameters before and after the assigned treatment. AEs were analysed with regard to the number, severity, intensity, causal relation with treatment, and outcome (corrective treatment, premature study withdrawal). Serious AEs were defined as any untoward occurrence that resulted in death or was life-threatening, or required inpatient hospitalisation, or resulted in persistent or significant disability.
The secondary outcome was efficacy measured as the decrease (%) of both: the daily number of watery stools as defined by a Stool Decrease Index (SDI) calculated as follows: SDI1 = (n0 – n1)/n0*100, SDI2 = (n0 – n2)/n0*100 where n0, n1, and n2 indicate the number of watery stools on study day 0, study day 1 and study day 2, respectively; and the severity of the abdominal pain evaluated on a 100 mm VAS (defined by a Pain Decrease Index (PDI) calculated as follows: PDI1 = (VAS0 – VAS1)/VAS0 *100, PDI2 = (VAS0 – VAS2)/VAS0 *100 where VAS0, VAS1, and VAS2 indicate the severity of abdominal pain on study day 0, study day 1 and study day 2, respectively [12]. The VAS is a self-administered tool widely used to measure pain by asking the patient to indicate his/her perceived pain intensity (most commonly) along a 100 mm horizontal line; the rating is converted to a score by measuring the distance of the mark from the left edge [17-19].
Safety and efficacy variables
The criteria for safety comprised the difference between the frequency of AEs occurrence in the two study arms, measurement of vital signs (blood pressure and pulse rate) and routine clinical laboratory tests (biochemistry, haematology, urinalysis) between the pre- and post-study visits.
The criterion for efficacy evaluation was the responder rate to treatment. A responder is defined as a patient that exhibits an SDI2 and a PDI2 of at least of 30%. The primary efficacy analysis performed was the assessment of the difference in responder rate between two study arms.
Statistical analysis
A sample size of 20 patients for each of the study arms (active treatment and placebo groups) achieved 88% power at a 5% significance level using a two-sided equivalence test of proportions when both proportions in the active treatment and placebo group tested for equivalence is 1%; the maximum allowable difference between these proportions that still results in equivalence (the range of equivalence) is 20%. Therefore, the total sample size required was 40 adult patients.
Descriptive analyses including means, standard deviation (SD), maximum and minimum values were performed. The difference between frequencies of occurrence of AEs was assessed by constructing a 95% confidence interval around the difference between their mean frequencies in the two studied groups. Any difference in intensity and relation with treatments administered was assessed using a chi-square test. The presence (or lack) of any significant difference between the values of vital signs and routine clinical laboratory tests in pre and poststudy visits was assessed using the paired-samples Student’s t-test.
The efficacy of the active treatment was assessed through SDI and SPI. The difference in the responder rate between the two study groups was assessed using a chi-square test. Parametric and non-parametric tests were used to further describe the presence (or lack) of significant differences. The analysis was conducted on the intent-to-treat (ITT) population (i.e., including all randomised patients with at least one assessment of efficacy on study day 0 after randomisation). The last observation carried forward (LOCF) approach was used for patients who did not complete the study according to the protocol. A secondary analysis was planned on the per protocol (PP) population, which comprised patients who fulfilled the protocol requirements with no major deviation at inclusion or during follow-up [20-22]. The statistical analyses were performed using SPSS 13.0 for Windows.
Results
Study population
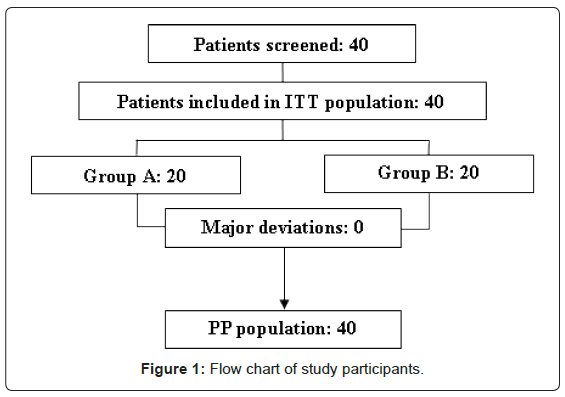
Forty (40) patients were included for participation in the study. The available ITT population comprised 40 patients with a mean age of 43 years (SD = 13). A total of 20 patients were randomised to receive the active treatment with capsules containing 500 mg of gelatine tannate (Group A), while the remaining 20 were administered placebo (Group B).
It was not necessary to exclude any patients because major deviations did not occur nor was there any dropout. Therefore, the ITT population and the PP population were the same (Figure 1).
Adherence to treatment was considered very good as 100% of the patients were compliant at the end of both scheduled treatments.
Evaluation of safety
Gelatine tannate proved to be a very safe treatment since no adverse events were reported in either active treatment or placebo arms. Measurement of vital signs (blood pressure and pulse rate) and results for routine clinical laboratory tests (biochemistry, haematology and urinalysis) between pre- and post-study visits did not differ clinically either.
Evaluation of efficacy
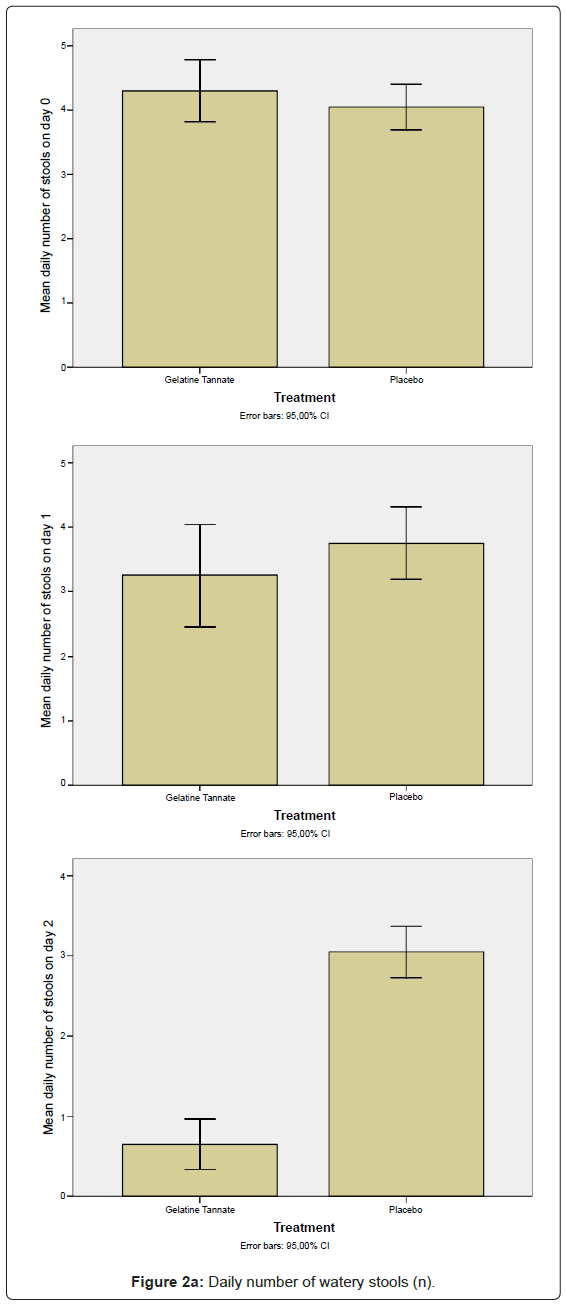
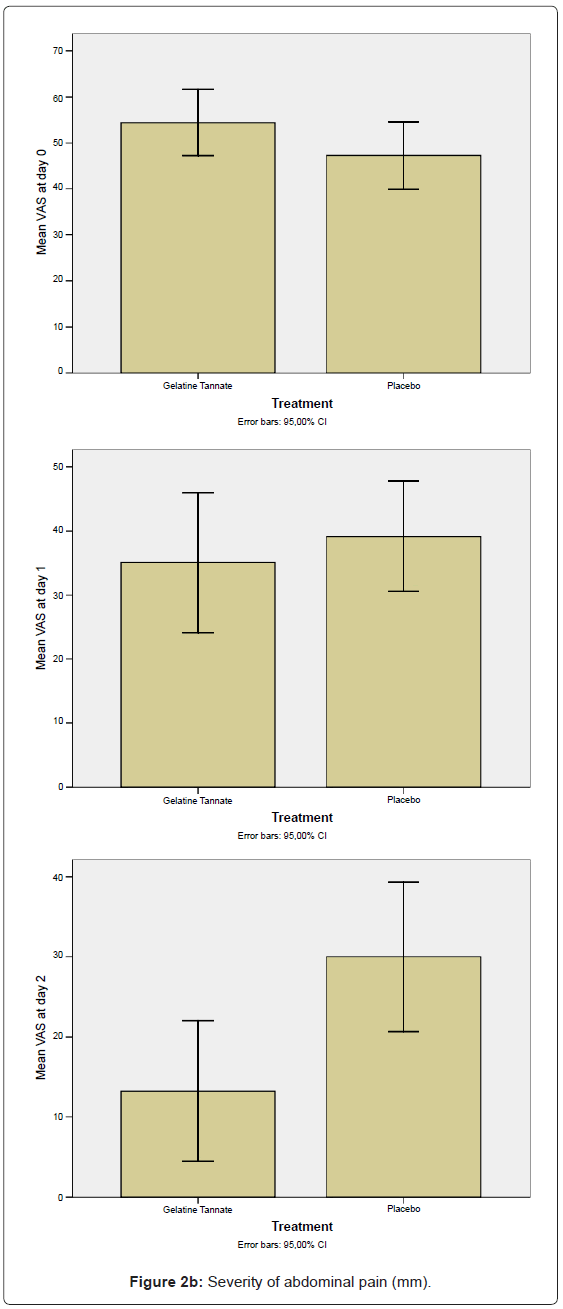
The secondary outcome measures to determine efficacy included both the decrease of the daily number (n) of watery stools (Figure 2a) and the decrease in abdominal pain as measured using the VAS-score (Figure 2b) between the baseline (n0,VAS0) and the final (n2,VAS2) visits, calculated in the ITT/PP population (Figure 2). The differences between study arms were statistically significant (p < 0.01) on study day 2: gelatine tannate showed a greater reduction than placebo in both the frequency of watery stools and the severity of abdominal pain (Figure 2).
Negative values for both SDI and PDI (relative to patients that exhibited an increase of n and VAS value between the baseline and the final visits) were not been considered in the analysis, the reason being negative percentages generate biases (for frequency of watery stools: on day 1, three patients exhibited an increase in each one of the two study arms; on day 2, only one patient exhibited an increase in the placebo group; for VAS scores: on day 1, one patient exhibited an increase in the gelatine tannate group while four patients exhibited an increase in the placebo group; two patients exhibited an increase on day 2 in the placebo group only). Even considering the negative values of SDI and PDI, the differences between the study arms remained statistically significant. It is noteworthy that, on day 2, only the placebo group exhibited an increase in frequency of watery stools and severity of abdominal pain compared to the baseline.
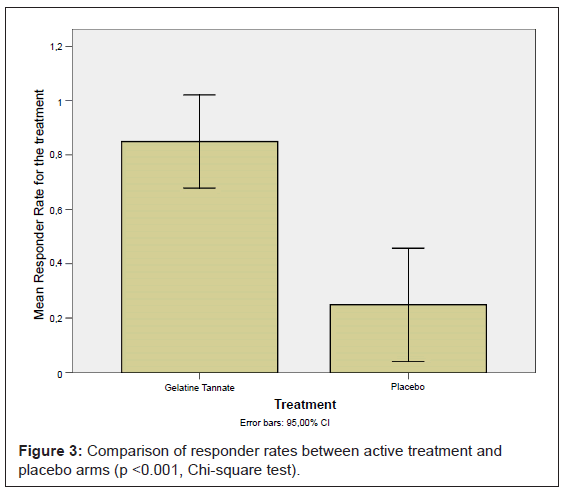
Gelatine tannate showed a responder rate on day 2 of 85% (17 out of 20 patients), while the placebo had a responder rate of 25% (5 out of 20 patients); the difference was statistically significant (p < 0.001) (Figure 3).
Discussion
The aim of the present placebo-controlled study was to assess both efficacy and safety of a new formulation (capsules containing 500 mg of gelatine tannate) in comparison with placebo in adults with acute diarrhoea. We consider the relevance that a compound containing polyphenols and tannins which has an effect on reducing the inflammatory status of the intestinal epithelium to have been extensively exhausted. Previous studies in vitro have shown that polyphenols and polyphenol-derived compounds or alkaloids may act on the biochemical mediators that activate vasodilation and production of exudate in the intercellular compartments of the intestines [4,5]. This could be due to a decrease of the transcriptional activation of target genes, adhesion molecules and chemokines that determine the activation of inflammatory pathways in the intestinal mucosa. However, well-conducted studies are needed to delineate such molecular events.
Gelatine tannate is a mixture of tannic acid and gelatine with antidiarrhoeal effect. The mechanism of action for gelatine tannate is not completely clear but is thought to act locally on the gut wall by forming a protective protein-based film and allowing the precipitation of proinflammatory mucoproteins from the intestinal mucus responsible for local inflammation, and subsequently eliminated through the faeces. Gelatine tannate is also attributed antibacterial and antioxidant properties, which could represent an advantage to the conventional use of antibiotics for the empirical treatment of diarrhoea as these have been reported as contributing to the development of resistance and dysbacteriosis [23]. Furthermore, unlike other antidiarrhoeal agents such as loperamide, gelatine tannate has no effects on the central nervous system, therefore can be considered as safe to use in children, particularly those under 2 years of age [24]; also, no undesirable effects such as reactive constipation have been reported. No gelatine tannaterelated adverse effects were recorded during the present study either, and the product was well tolerated.
The results presented here indicate that gelatine tannate is safe and effective for the treatment of acute diarrhoea in adults. Gelatine tannate proved more effective than placebo in reducing the daily number of watery stools and relieving abdominal pain caused by acute diarrhoea. The difference observed between the percentages of responders in the gelatine tannate vs. placebo groups was statistically significant (responder rate of 85% and 25%, respectively; p < 0.001). Both efficacy and safety/tolerability shown for gelatine tannate were consistent with previous observations [12,13]. Moreover, the data presented here validates the study design as it demonstrates that the selected clinical outcome measures were sufficiently sensitive to detect the observed difference between the active treatment and placebo arms as statistically significant.
The authors therefore concluded that the studied medical device (capsule containing 500 mg of gelatine tannate, batch number: NOV 07-08) provides an effective alternative to currently marketed antidiarrhoeals with the advantage of a favourable safety profile.
References
- Acute diarrhoea. British Society of Gastroenterology.
- Casburn-Jones AC, Farthing MJ (2004) Management of infectious diarrhoea. Gut 53: 296-305.
- Oi H, Matsuura D, Miyake M, Ueno M, Takai I, et al. (2002) Identification in traditional herbal medications and confirmation by synthesis of factors that inhibit cholera toxin-induced fluid accumulation. Proc Natl Acad Sci U S A 99: 3042-3046.
- Field M (2003) Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest 111: 931-943.
- Morinaga N, Iwamaru Y, Yahiro K, Tagashira M, Moss J, et al. (2005) Differential activities of plant polyphenols on the binding and internalization of cholera toxin in vero cells. J Biol Chem 280: 23303-23309.
- Kaul TN, Middleton E Jr, Ogra PL (1985) Antiviral effect of flavonoids on human viruses. J Med Virol 15: 71-79.
- Norton MR, Addy M (1989) Chewing sticks versus toothbrushes in West Africa. A pilot study. Clin Prev Dent 11: 11-13.
- Brun Y, Wang XP, Willemot J, Sevenet T, Demenge P (1998) Experimental study of antidiarrheal activity of Salicairine. Fundam Clin Pharmacol 12: 30-36.
- Haslam E (1996) Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J Nat Prod 59: 205-215.
- Palombo EA (2006) Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother Res 20: 717-724.
- Loeb H, Vandenplas E, Wursch P, Guesry P (1989) Tannin-rich carob pod for the treatment of acute-onset diarrhea. J Pediatr Gastroenterol Nutr 8: 480-485.
- Esteban Carretero J, Durbán Reguera F, López-Argüeta Alvarez S, López Montes J (2009) A comparative analysis of response to vs. ORS + gelatin tannate pediatric patients with acute diarrhea. Rev Esp Enferm Dig 101: 41-48.
- Allegrini A, Costantini M (2011) Tasectan (gelatine tannate) for the treatment of acute gastroenteritis in children. Proceedings of the 39th Annual Congress of the Belgian Society of Pediatrics.
- Aksit S, Caglayan S, Cukan R, Yaprak I (1998) Carob bean juice: a powerful adjunct to oral rehydration solution treatment in diarrhoea. Paediatr Perinat Epidemiol 12: 176-181.
- World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. 52nd WMA General Assembly, Edinburgh, Scotland, October 2000.
- European Medicines Agency. Note for Guidance on Good Clinical Practice. July 2002 CPMP/ICH/135/95.
- Revill SI, Robinson JO, Rosen M, Hogg MI (1976) The reliability of a linear analogue for evaluating pain. Anaesthesia 31: 1191-1198.
- Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, et al. (1978) Studies with pain rating scales. Ann Rheum Dis 37: 378-381.
- Huskisson EC (1982) Measurement of pain. J Rheumatol 9: 768-769.
- U.S. Food and Drug Administration (1998) FDA Guidance for Industry. Providing Clinical Evidence of Effectiveness for Human Drugs and Biological Products.
- CPMP/EWP/482/99 (2000) Points to consider on switching between superiority and non-inferiority, European Agency for the Evaluation of Medicinal Products, London.
- CPMP/ICH/363/96 (1998) Note for guidance on statistical principles for clinical trials. European Agency for the Evaluation of Medicinal Products, London.
- Mensa L, Marco F, Vila J, Gascón J, Ruiz J (2008) Quinolone resistance among Shigella spp. isolated from travellers returning from India. Clin Microbiol Infect 14: 279-281.
- Li ST, Grossman DC, Cummings P (2007) Loperamide therapy for acute diarrhea in children: systematic review and meta-analysis. PLoS Med 4: e98.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 29927
- [From(publication date):
July-2012 - Oct 23, 2025] - Breakdown by view type
- HTML page views : 24860
- PDF downloads : 5067