Research Article Open Access
GC-MS Analysis of Phytochemicals and Simultaneous Determination of Flavonoids in Amaranthus caudatus (Sirukeerai) by RP-HPLC
Paranthaman R1*, Praveen kumar P2 and Kumaravel S3
1Technical Assistant, Indian Institute of Crop Processing Technology, Ministry of Food Processing Industries, Govt. of India, Thanjavur, India
2Senior Research Fellow, Indian Institute of Crop Processing Technology, Ministry of Food Processing Industries, Govt. of India, Thanjavur, India
3Scientist & Head i/c, Indian Institute of Crop Processing Technology, Ministry of Food Processing Industries, Govt. of India, Thanjavur, India
- *Corresponding Author:
- R. Paranthaman
Technical Assistant
Indian Institute of Crop Processing Technology
Ministry of Food Processing Industries, Govt. of India
Thanjavur-613 005 (TamilNadu), India
Tel: 04362226676
E-mail: paranthhu@gmail.com
Received date: July 10, 2012; Accepted date: September 21, 2012; Published date: September 27, 2012
Citation: Paranthaman R, Praveen kumar P, Kumaravel S (2012) GC-MS Analysis of Phytochemicals and Simultaneous Determination of Flavonoids in Amaranthus caudatus (Sirukeerai) by RP-HPLC. J Anal Bioanal Tech 3:147. doi: 10.4172/2155-9872.1000147
Copyright: © 2012 Paranthaman R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
A sensitive and selective high performance liquid chromatography method (HPLC) for simultaneous analysis of following five flavonoids like Gallic acid (GA), Caffeic acid (CA), Rutin (RU), Ferulic acid (FA) and Quercetin (QU) in Amaranthus caudatus (Sirukeerai) leaves. The results demonstrated that the Amaranthus caudatus leaves were separately extracted and analyzed using RP HPLC method. The contents of Gallic acid (0.083 μg/gm), Caffeic acid (0.004 μg/gm), Rutin (0.019 μg/gm), Quercetin (0.001 μg/gm) and Ferulic acid (0.001 μg/gm) in Amaranthus caudatus leaves. The GC-MS study also carried out and it showed the presence of phytochemicals. Hence these plants extracts with the property of bioavailability and retention of certain minerals by polyphenolic compounds can be recommended for their use as an alternative anti-infective agent in natural medicine for the treatment of infectious diseases.
Keywords
Amaranthus caudatus; RP-HPLC; Flavonoids; Pytochemicals; GC-MS
Introduction
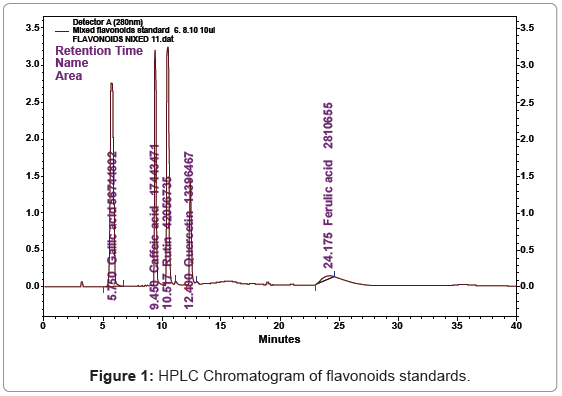
The genus Amaranthus belongs to the family Amaranthaceae and includes more than 60 species, of which three viz., Amaranthus hypochondriacus, A. cruentus and A. caudatus, are the essential grain species. Amaranth is a very versatile crop that is grown in a wide range of agro-climatic conditions; it resists drought, heat, and pests, and adapts readily to new environments, including some that are inhospitable to conventional cereal crops [1,2]. It is one of the few multi-purpose crops, which can supply grains and tasty leafy vegetables of high nutritional quality as a food and animal feed, and additionally an ornamental plant, because of an attractive inflorescence colouration [3,4]. The biomass of amaranth, both as green and dried, has high biological value, and is used as source of protein, amino acid and dietary fibre for fattened pigs, rabbits, and lambs [5,6]. Another article concerning the phenolic and flavonoid content in amaranth (Amaranthus hypochondriacus) has also recently been published [7]. Pasko et al. [8] analysed the total polyphenol content and anti-oxidant activity in two amaranth varieties (Amaranthus cruentus). Gallic acid (GA), Caffeic acid (CA), Rutin (RU), Ferulic acid (FA) and Quercetin (QU) are phenolic compounds. Structurally they have phenolic groups which serve as a source of readily available hydrogen atoms such that the subsequent radicals produced can be delocalized over the phenolic structure (Figure 1) [9,10]. Great focus has been put on health promoting secondary metabolites in food during the recent decade. A number of studies have been conducted on the health effects of polyphenols (flavonoids and phenolic acids). Flavonoids have been shown to inhibit certain types of cancer, dementia, cardiovascular diseases and diabetes [11- 14]. Ultraviolet detection (UV) [15] reversed phase high-performance liquid chromatography with ultraviolet detection (HPLC-UV) [16,17] have been used for analysis of flavonoids. The purpose of this study was to identify and quantify health promoting flavonoids in Amaranthus caudatus leaves. In Tamil Nadu state of India amaranth is consumed regularly as favourite dish where it is steamed and mashed with light seasoning salt, red chilies and cumin.
In this study, HPLC with UV detector was developed for simultaneous determination of Gallic acid (GA), Caffeic acid (CA), Rutin (RU), Ferulic acid (FA) and Quercetin (QU) in the leaf extracts of Amaranth caudatus.
Materials and Methods
RP-HPLC Analysis of flavonoids
Standard preparation: Standard stock solutions of five phenolic like Gallic acid (GA), Caffeic acid (CA), rutin (RU), Ferulic acid (FA) and Quercetin (QU) compounds were prepared in methanol, at concentrations of 2, 4, 6, 8 and 10 μg/ml, all standard solutions were filtered through HPLC filter 0.45 mm membrane filter (Millipore).
Sample preparation: The sample was prepared according to the procedure. The extraction was carried out using 2 ml of fermented broth with 50 mL of 95% ethanol under 80 KHz, 45°C in ultrasonic extraction device for 30 min, repeated twice. The extract was collected and filtered; the filtrate was dried at 50°C under reduced pressure in a rotary evaporator. The dried crude extract was dissolved in the 100 ml mobile phase. After filtering through a filter paper and a 0.45 mm membrane filter (Millipore), the extract was injected into HPLC.
HPLC conditions: Flavonoids were analysed using a RP-HPLC method [18], Shimadzu Corp., Kyoto, consisting of a LC-10ATVp pump, SCL 10A system controller and a variable Shimadzu SPD-10ATVp UV VIS detector and a loop injector with a loop size of 20 μl. The peak area was calculated with a CLASSVP software. Reverse-phase chromatographic analysis was carried out in isocratic conditions using a C-18 reverse phase column (250×4.6 mm i.d., particle size 5 μm, Luna 5μ C-18; phenomenex, Torrance, CA, USA) at 25°C. The gradient elution of solvent A [water-acetic acid (25:1 v/v)] and solvent B (methanol) had a significant effect on the resolution of compounds. As a result, solvent gradients were formed, using dual pumping system, by varying the proportion of solvent A [water-acetic acid (25:1, v/v)] to solvent B (methanol). Solvent B was increased to 50% in 4 min and subsequently increased to 80% in 10 min at a flow rate of 1.0 mL/min. Detection wavelength was 280 nm. Gallic acid (GA), caffeic acid (CA), rutin (RU), Ferulic acid (FA) and quercetin (QU) was used as internal and external standards. Phenolic acids present in each sample were identified by comparing chromatographic peaks with the retention time (Rt) of individual standards and further confirmed by co-injection with isolated standards. The amount of each phenolic acid is expressed as micrograms per gram of fresh weight unless otherwise stated.
Phytochemical analysis by GC-MS
Preparation of extract: Leaves of Amaranthus caudatus (Sirukeerai) were shade dried. 20 g of the powdered leaves were soaked in 95% ethanol for 12 h. The extracts were then filtered through Whatmann filter paper No. 41 along with 2 gm sodium sulfate to remove the sediments and traces of water in the filtrate. Before filtering, the filter paper along with sodium sulphate was wetted with 95% ethanol. The filtrate was then concentrated by bubbling nitrogen gas into the solution. The extract contained both polar and non-polar phytocomponents of the plant material used. 2 μl of these solutions was employed for GC/MS analysis [19].
GC analysis: GC-MS analysis was carried out on a GC CLARUS 500 PerkinElmer system comprising a gas chromatograph interfaced to a mass spectrometer (GC-MS) instrument employing the following conditions: column Elite-1 fused silica capillary column (30×0.25 mm ID×1EM df, composed of 100% Dimethyl poly siloxane), operating in electron impact mode at 70 eV; helium (99.999%) was used as carrier gas at a constant flow of 1ml/min and an injection volume of 0.5 EI was employed (split ratio of 10:1) injector temperature 250°C; ion-source temperature 280°C. The oven temperature was programmed from 110°C (isothermal for 2 min), with an increase of 10°C/min, to 200°C, then 5°C/min to 280°C, ending with a 9 min isothermal at 280°C. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 40 to 550 Da [20].
Results and Discussion
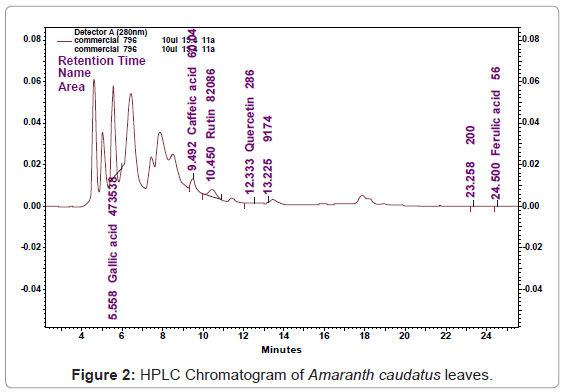
Phenolic compounds can be defined as a large series of chemical constituents possessing at least one aromatic ring, bearing hydroxyl and other sub-constituents. RP-HPLC analysis is the most used method for the identification of plant phenolic compounds. Because of the diversity and complexity of natural phenolics in medicinal plants, it is difficult to characterize every compound and elucidate its structure. However; it is not difficult to identify the major categories of phenolic compounds. The chromatographic separations of Retention time (Rt), Gallic acid (Rt- 5.750), Caffeic acid (Rt-9.450), Rutin (Rt-10.517), Quercetin (Rt-12.400) and Ferulic acid (Rt-24.175) standard shown in Figure 1. The content of each flavonoid was calculated from the corresponding calibration curve and presented as the mean of five determinations as shown in Table 1. The HPLC Result shows based on the Retention time (Rt), Gallic acid (Rt-5.558), Caffeic acid (Rt-9.492), Rutin (Rt-10.450), Quercetin (Rt- 12.333) and Ferulic acid (Rt-24.50) content in Amaranthus caudatus leaves was found to be 0.083 μg/gm, 0.004 μg/gm, 0.019 μg/gm, 0.001 μg/gm, and 0.001 μg/gm. The obtained value was compared with standard (Figure 2).
| SPD10Avp (280nm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pk # | Retention Time | Area | Height | Area Percent | Height Percent | concentration | Units | Name |
| 1. | 5.750 | 56744802 | 2757981 | 42.84 | 29.72 | 10.000 | μg/ml | Gallic acid |
| 2. | 9.450 | 17443471 | 1882880 | 13.17 | 20.29 | 10.000 | μg/ml | Caffeic acid |
| 3. | 10.517 | 42056735 | 3198304 | 31.75 | 34.47 | 10.000 | μg/ml | Rutin |
| 4. | 12.400 | 13396467 | 1402866 | 10.11 | 15.12 | 10.000 | μg/ml | Quercetin |
| 5. | 24.175 | 2810655 | 36358 | 2.12 | 0.39 | 10.000 | μg/ml | Ferulic acid |
Table 1: HPLC Validation data for flavonoids standards.
GC-MS Analysis
Free radicals play a crucial role in the development of tissue damage in pathological events. The extraction method presented is simple, rapid and inexpensive, with reduced solvent consumption. GC-MS method used for the analysis of the obtained extracts can be an interesting tool for testing the amount of some active principles in herbs used in cosmetic, drugs, pharmaceutical or food industry. The acidic fractions were silylated and subjected to GC-MS investigation. It is evident from the Table 2 that all fractions have a complex chemical composition. Some of the GC-MS peaks remained unidentified, because of lack of authentic samples and library data of corresponding compounds.
| SPD10Avp (280nm) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Pk # | Retention Time | Area | Height | Area Percent | Height Percent | concentration | Units | Name |
| 1. | 5.558 | 473538 | 44270 | 82.61 | 90.74 | 0.083 | μg/gm | Gallic acid |
| 2. | 9.492 | 6924 | 678 | 1.21 | 1.39 | 0.004 | μg/gm | Caffeic acid |
| 3. | 10.450 | 82086 | 3646 | 14.34 | 7.46 | 0.019 | μg/gm | Rutin |
| 4. | 12.333 | 286 | 70 | 0.05 | 0.14 | 0.001 | μg/gm | Quercetin |
| 5. | 24.500 | 56 | 2 | 0.01 | 0.00 | 0.001 | μg/gm | Ferulic acid |
Table 2: HPLC Validation data for Amaranth caudatus leaves.
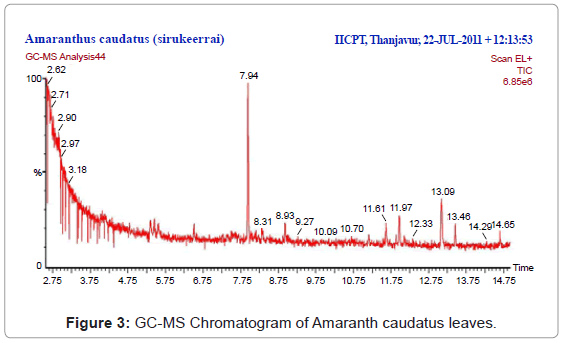
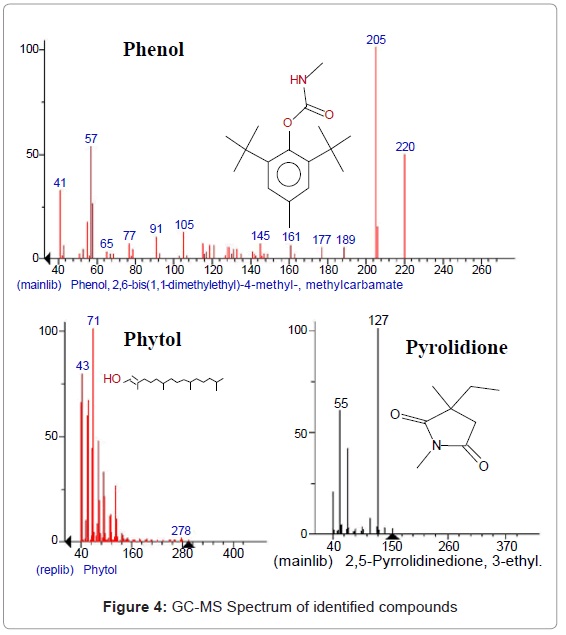
All the twelve compounds have been identified for the first time in Amaranth caudatus. The ethanol extract of the plant showed 12 constituents, the major constituents were Isoxazolidine, 5-hexyl-(+)-(peak area 0.47%), 1,2,4-Butanetriol (peak area 0.47%), trinitrate, N-Ethyl-N’-nitroguanidine (peak area 0.47%), Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl, methylcarbamate (peak area 2.37%), 2,5-Pyrrolidinedione, 3-ethyl-1, 3-dimethyl-(peak area 0.16%), 2-Piperidinone, N-[4-bromo- n-butyl]-(peak area 0.16%), 3-Hexadecyloxycarbonyl-5-(2-hydroxyethyl)-4-methylimidazolium ion (peak area 0.16%), 2-(3-Oxo-3-phenylpropyl)-3,5,6-trimethylpyrazine (peak area 0.47%), Pyridine-3-carboxamide, 4-dimethylamino-N-(2,4-difluorophenyl)-(peak area 1.11%), Phytol (peak area 84.97%), Pseudoephedrine, (+)-(peak area 2.06%), Aspidofractinine-3-methanol (peak area 7.12%) were reported in Table 3. The GC-MS chromatogram with peak area was given in Figure 3. The activity of important phyto-components identified in Amaranth caudatus leaf extract shown in Table 4 and Figure 4.
| No. | RT | Name of the compound | Molecular | MW | Peak Area % |
|---|---|---|---|---|---|
| 1. | 4.61 | Isoxazolidine, 5-hexyl-, (+)- | C9H19NO | 157 | 0.47 |
| 2. | 4.79 | 1,2,4-Butanetriol, trinitrate | C4H7N3O9 | 241 | 0.47 |
| 3. | 4.91 | N-Ethyl-N'-nitroguanidine | C3H8N4O2 | 132 | 0.47 |
| 4. | 7.94 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl-, methylcarbamate | C17H27NO2 | 277 | 2.37 |
| 5. | 8.31 | 2,5-Pyrrolidinedione, 3-ethyl-1,3-dimethyl- | C8H13NO2 | 155 | 0.16 |
| 6. | 8.93 | 2-Piperidinone, N-[4-bromo-n-butyl]- | C9H16BrNO | 233 | 0.16 |
| 7. | 11.16 | 3-Hexadecyloxycarbonyl-5-(2-hydroxyethyl)-4-methylimidazolium ion | C24H45N2O3 | 409 | 0.16 |
| 8. | 11.97 | 2-(3-Oxo-3-phenylpropyl)-3,5,6-trimethylpyrazine | C16H18N2O | 254 | 0.47 |
| 9. | 13.09 | Pyridine-3-carboxamide, 4-dimethylamino-N-(2,4-difluorophenyl)- | C14H13F2N3O | 277 | 1.11 |
| 10. | 14.96 | Phytol | C20H40O | 296 | 84.97 |
| 11. | 19.82 | Pseudoephedrine, (+)- | C10H15NO | 165 | 2.06 |
| 12. | 20.85 | Aspidofractinine-3-methanol, (2à,3á,5à)- | C20H26N2O | 310 | 7.12 |
RT=Retention time, MW=Molecular Weight,
Table 3: Phytocomponents identified in the methanolic extract of the leaves of Amaranth caudatus by GC-MS.
| No. | RT | Name of the compound | Uses |
|---|---|---|---|
| 4. | 7.94 | Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl-, methylcarbamate | Phenol is used as an oral anesthetic/analgesic, commonly used to temporarily treat pharyngitis. Phenol was widely used as an antiseptic, especially as Carbolic soap, from the early 1900s through the 1970s. |
| 9. | 13.09 | Pyridine-3-carboxamide, 4-dimethylamino-N-(2,4-difluorophenyl)- | It has demonstrated anti-inflammatory actions that may be of benefit to patients with inflammatory skin conditions. It has ability to block the inflammatory actions of iodides known to precipitate or exacerbate inflammatory acne |
| 10. | 14.96 | Phytol | It can be used as a precursor for the manufacture of synthetic forms of vitamin E and vitamin K1. In ruminants, the gut fermentation of ingested plant materials liberates phytol, a constituent of chlorophyll, which is then converted to phytanic acid and stored in fats |
| 11. | 19.82 | Pseudoephedrine, (+)- | Pseudoephedrine shrinks swollen nasal mucous membranes. It reduces tissue hyperemia, edema, and nasal congestion commonly associated with colds or allergies. Other beneficial effects may include increasing the drainage of sinus secretions, and opening of obstructed Eustachian tubes. The same vasoconstriction action can also result in hypertension, which is a noted side effect of pseudoephedrine |
Table 4: Activity of important phyto-components identified in Amaranth caudatus leaf extract by GC-MS.
Conclusion
This HPLC procedure provided excellent identification and quantification of these five flavonoids compounds presented in Amaranthus caudatus leaves. The HPLC results indicated that Amaranthus caudatus leaf extract contained an especially high concentration of gallic acid (0.083 μg/gm of dry weight). It was also shown that Caffeic acid, quercetin, rutin and ferulic acid were detected in Amaranthus caudatus leaves. Since these flavonoids have been of interest of health benefits, the present analytical study could be a potential application to identify and quantify the phenolic compounds in other medicinal plant extracts. In GC-MS results indicated twelve phytochemical constituents have been identified from ethanolic extract of the leaves of Amaranthus caudatus by Gas Chromatogram-Mass spectrometry (GC-MS) analysis. Phenolic compounds have also been known as antioxidant agents, which act as free radical terminators and have shown medicinal activity as well as exhibiting physiological functions. It was reported that compounds such as flavonoids, which contain hydroxyls, are responsible for the radical scavenging effects of most plants. The presence of these phytochemicals in Amaranthus caudatus root is a significant finding in this present study.
Funding
This work was supported by Indian Institute of crop processing technology, Pudukottai road, Thanjavur, Tamilnadu.
Acknowledgements
The reaches deeply thanks the Dr. K. Alagusundaram, Director, Indian Institute of Crop Processing Technology, Ministry of Food Processing Industries, Government of India, Thanjavur for Providing all the facilities and support used to carry out the work.
References
- Brenner DM, Baltensperger DD, Kulakow PA, Lehman JW, Myers RL, et al. (2000) Genetic resources and breeding of Amaranthus. In Janick Jules (Ed.), Plant breeding reviews 19: 227-285.
- Rana JC, Pradheep K, Yadav S K, Verma VD, Sharma PC (2007) Durga: A new variety of grain amaranth for cultivation in hill regions. Indian Farming 57: 27-28.
- Breene WM (1991) Food uses of grain amaranth. Cereal Foods World 36: 426-430.
- Mlakar SG, Turinek M, Jakop M, Bavec M, Bavec F (2009) Nutrition value and use of grain amaranth: Potential future application in bread making. Agricultura 6: 43-53.
- Andrasofszky E, Szöcs Z, Fekete S, Jelenits K (1998) Evaluation of the nutritional value of the amaranth plant. I. Raw and heat-treated grain tested in experiments on growing rats. Acta Vet Hung 46: 47-59.
- Zraly Z, Pisarikova B, Hudcova H, Trckova M, Herzig I (2004) Effect of feeding amaranth on growth efficiency and health of market pigs. Acta Veterinaria Brno 73: 437-444.
- Barba de la Rosa AP, Gueguen J, Paredes-López O, Viroben G (1992) Fractionation procedures, electrophoretic characterization, and amino acid composition of amaranth seed proteins. Journal of Agricultural and Food Chemistry 40: 931-936.
- Pasko P, Sajewicz M, Gorinstein S, Zachwieja Z (2008) Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromomatographica 20: 661-672.
- Robards K, Prenzler PD, Tucker G, Swatsitang P, Glover W (1999) Phenolic compounds and their role in oxidative processes in fruits. Food Chem 66: 401-436.
- Nikolic KM (2006) Theoretical study of phenolic antioxidants properties in reaction with oxygen-centered radicals. J Mol Struc: THEOCHEM774: 95-105.
- Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, et al. (2000) Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr 130: 2243-2250.
- Commenges D, Scotet V, Renaud S, Jacqmin-Gadda H, Barberger-Gateau P, et al. (2000) Intake of flavonoids and risk of dementia. Eur J Epidemiol 16: 357-363.
- Knekt P, Kumpulainen J, Jarvinen R, Rissanen H, Heliovaara M, et al. (2002) Flavonoid intake and risk of chronic diseases. Am J Clin Nutr 76: 560-568.
- Le Marchand L, Murphy SP, Hankin JH, Wilkens LR, Kolonel LN (2000) Intake of flavonoids and lung cancer. J Natl Cancer Inst 92: 154-160.
- Xi YL, Mou H, Shang YZ (2009) Determination of total flavonoids in Herba Scutellariae barbata by UV. Journal of ChengDe Medical College 26: 66-67.
- Chen XA, Yuan ZF, Tian YP, Li DQ, Zhang LT (2007) RP-HPLC determination of protocatechuic acid in Scutellaria barbara. Lishizhen Medicine and Materia Medica Research 18: 1405-1406.
- Qiao CF, Han QB, Song JZ, Mo SF, Tai C, et al. (2006) Determination of four main flavonoids in Herba Scutellariae barbata by HPLC. Chinese Pharmaceutical Journal 41: 1342-1344.
- Weerasak Samee, Suwanna Vorarat (2007) Simultaneous Determination of Gallic acid, Catechin, Rutin,Ellagic Acid and Quercetin in Flower Extracts of Michelia alba,Caesalpinia pulcherrima and Nelumbo nucifera by HPLC. Thai Pharm Health Sci J 2:131-137.
- Merlin NJ, Parthasarathy V, Manavalan R, Kumaravel S (2009) Chemical Investigation of Aerial Parts of Gmelina asiatica Linn by GC-MS. Pharmacognosy Res 1: 152-156.
- Praveen Kumar P, Kumaravel S, Lalitha C (2010) Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. African Journal of Biochemistry Research 4: 191-195.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 18431
- [From(publication date):
September-2012 - Apr 03, 2025] - Breakdown by view type
- HTML page views : 13268
- PDF downloads : 5163