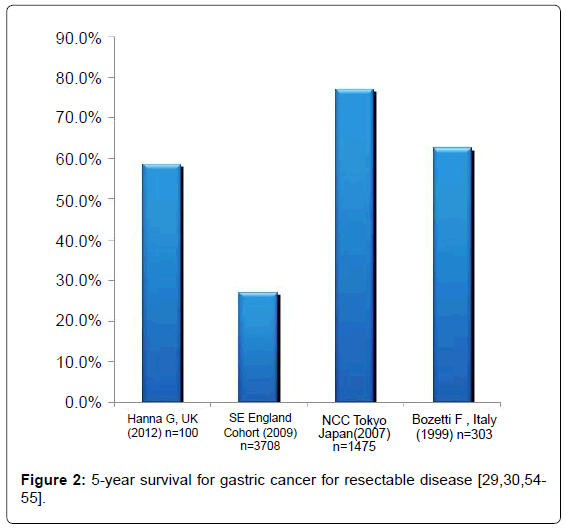Editorial Open Access
Gastro-Oesophageal Cancer
Yassar A Qureshi* and Ashish Rohatgi
Department Of Upper GI Surgery, The Royal London Hospital and Whipps Cross University Hospital, London, UK
- *Corresponding Author:
- Yassar A Qureshi
Department of Upper GI Surgery
The Royal London Hospital and Whipps Cross University Hospital
London, UK
E-mail: yassarqureshi@bartshealth.nhs.uk
Received date: August 20, 2013; Accepted date: August 21, 2013; Published date: August 24, 2013
Citation: Qureshi YA, Rohatgi A (2013) Gastro-Oesophageal Cancer. J Gastroint Dig Syst 3:e116. doi:10.4172/2161-069X.1000e116
Copyright: © 2013 Qureshi YA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Introduction
Gastro-oesophageal cancer remains a devastating diagnosis for the patient and a challenge for the clinician. Although rare, they cause disproportionate mortality in comparison to more commonly encountered malignancies. Survival figures remain relatively poor, partially due to the fact that significant proportion of patients present with advanced disease. The incidence of gastro-oesophageal cancer has striking geographical variation as does the gold standard treatment. Here we explore the epidemiology, aetiology and clinical aspects of this group of cancers. Further, we explore the treatment practices in different parts of the world and assess their impact on survival.
Oesophageal Cancer
Cancer of the oesophagus is the eight commonest worldwide, affecting more than 450 000 people. While incidence is increasing overall 5-year survival remains poor making this relatively rare cancer the 6th leading cause of cancer related death world-wide [1-3]. Incidence is highest in the Far East, and in China it is as high as 100 cases per 100 000 of the population [4,5]. In comparison, incidence is far less in the Western world, with 16 470 cases being diagnosed in the USA in 2009 and 14 530 mortalities in the same year [3]. Oesophageal cancer is group comprising several histological types, chiefly squamous cell (SCC), adenocarcinoma, leiomyosarcoma and other rarer types. SCC remains the commonest type worldwide, although adenocarcinoma is commoner in Western countries [6].
SCC is strongly associated with tobacco use and alcohol consumption. Other predisposing factors include a history of achalasia, caustic substance ingestion, radiation exposure and poor nutrition [7,8]. Additionally, recent studies have shown an association between oesophageal SCC and mutations in genes governing enzymes related to aldehyde metabolism [9]. In contrast to adenocarcinoma, SCC appears to be commoner in lower socioeconomic groups [10]. Oesophageal adenocarcinoma is also associated with tobacco use, with a higher incidence in men [7]. Interestingly, and perhaps accounting for its geographical distribution, it is associated with obesity, Barrett’s oesophagus, and chronic gastro-oesophageal reflux disease, largely diseases of the West [11,12]. The latter is a very strong risk factor, and likely related to the increasing prevalence of obesity. Barrett’s oesophagus has a prevalence of up to 6% in the general population. The protective changes in the lower oesophagus brought about by persistent acid exposure can result in dysplasia, with a 0.5% risk of development of adenocarcinoma per year [13]. Obesity, reflux and genetic abnormalities all themselves are risk factors for developing Barrett’s oesophagus [12].
Although symptoms are variable, most patients present with dysphagia. In the case of adenocarcinoma, this may be accompanied by symptoms relating to reflux disease. For SCC, weight loss is the most commonly associated symptom. Initial assessment by upper gastrointestinal endoscopy enables precise localization of the tumor and histopathological diagnosis by analysis of tissue samples. Adenocarcinomas have a predilection for the area from the distal third of the oesophagus to the gastro-oesophageal junction. SCCs are located more proximally. As part of pre-operative planning, the stomach is also assessed to ensure that a ‘neo-oesophagus’ can be fashioned from the stomach. Endoscopic ultrasonography is now routinely part of the staging protocol in most large centers. It allows a more detailed assessment of the dimensions of the tumor, and can assess for the presence of local pathological nodes. Using this technique, infiltration of the tumor into the wall of the oesophagus can be ascertained with an accuracy of up to 89% and nodal status up to 84% [14]. Computerised Tomography (CT) and Positron Emission Tomography (PET) is used to assess for metastatic disease. Staging laparoscopy can be performed pre-operatively enabling assessment for resectability, nodal status and possible intra-abdominal metastases in locally advanced disease particularly if lesions are under 1cm on scanning [15]. Staging laparoscopy is always performed if the tumor invades the stomach; in cases of tumor limited to the oesophagus, it is the surgeon’s choice whether or not to perform a laparoscopy. Staging facilitates a multidisciplinary decision about the best course of treatment. The TNM classification system is most widely used based on the consensus formulated by the American Joint Cancer Committee [16].
Treatment is multifaceted. For tumors staged up to T3 with or without nodal disease treatment intent is curative [17]. This invariably involves surgery. An open approach with an oesophagectomy with primary anastomosis can be performed by several techniques: thoracoabdominal approach, Ivor-Lewis (laparotomy or laparoscopy with right thoracotomy), the McKeown approach (laparotomy, right thoracotomy and neck dissection), or a transhiatal approach (laparotomy and neck anastomosis). Minimally invasive surgery is gaining favor in most units now with all or parts of the procedure performed laparosocpically [18]. The type of surgery performed will depend on the surgeon’s preference and the location of the tumor. There is no convincing evidence to favour one surgical technique above the others in terms of overall survival [19,20].
Minimally invasive surgery has been shown to reduce postoperative morbidity and time of hospital stay. Although debated, some studies have suggested reduced 30-day mortality and improved survival with laparoscopic techniques [18]. Further, laparoscopic surgery offers far less long-term morbidity when compared with laparotomy with a decreased incidence of incisional hernia and adhesions and so can therefore be argued to contribute towards improved quality of life long-term [21]. The extent of lymph node dissection also remains controversial. In the Far East, particularly in Japan, three-field dissection is performed (abdomen, chest and neck). In Western countries however only abdominal and chest nodes (two-field dissection) are cleared routinely. There is some debate as to additional complications associated with more extensive node dissection especially as there is no apparent survival advantage and no convincing evidence that threefield dissection provides more accurate sampling and therefore staging [22]. Oesophagectomy remains a difficult operation carrying significant morbidity and mortality, estimated to be between 1-23% [23].
The use of neoadjuvant chemotherapy has formed the basis of many trials. The aim of this treatment is to target systemic micrometastasis. However, results have been conflicting. A large US study by Kelsen et al. showed no difference in survival, whereas in the UK, both the MRC and MAGIC trials showed a survival advantage [24-26]. Thus, the use of neoadjuvant chemotherapy followed by surgery has become the standard in the UK and Europe, often with additional post-operative chemotherapy based on the final histology. In the USA, neoadjuvant chemoradiation is often administered, with the CROSS trial reporting a significant advantage [17]. Some studies have shown a benefit with surgery followed by adjuvant chemotherapy with or without radiation [27]. The Japanese Clinical Oncology Group determined that surgery and adjuvant chemoradiation yielded significantly improved survival, particularly for SCC [27]. In Asia, this approach has become the standard. Adjuvant radiotherapy alone has not shown any advantage, and is generally reserved for cases where residual tumor is left in situ, or for positive margins [28]. Chemoradiation as a definitive treatment has been assessed, particularly in the treatment of SCC, but surgery definitely improves local control. In cases of localized recurrence following chemoradiation a salvage oesphagectomy can be performed, although peri-operative morbidity and mortality remain high.
For unresectable disease or metastatic disease, external beam radiotherapy can be used to palliate symptoms. However, chemoradiotherapy in the palliative setting appears to control symptoms more effectively, in about 50% of patients [17]. Endoscopic stenting across tumours may be effectively utilised to treat obstruction or dysphagia [29-33].
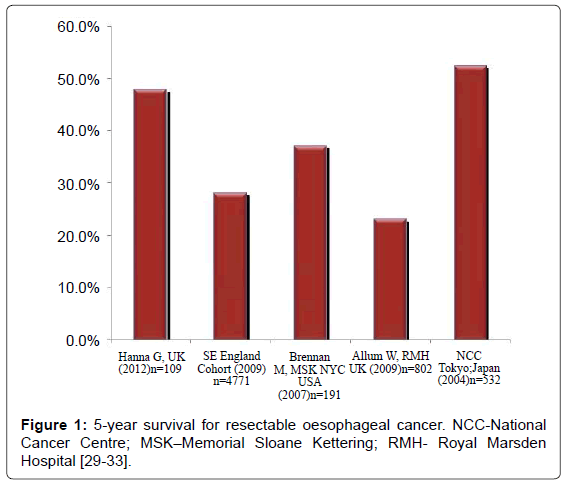
As figure 1 demonstrates, the 5-year survival remains poor despite optimal treatment in specialist centres. The studies in figure 1 demonstrate survival in patients undergoing curative treatment of resectable disease, but overall survival across all stages is estimated at a maximum of 25% [1].
Gastric Cancer
Gastric cancer has a worldwide incidence of 1 000 000 cases per year, and is the second leading cause of cancer related mortality [5,34]. It is most prevalent in the Far East, where the incidence is in the region of 70 cases per 100 000.36 It affects men twice as commonly as women [35]. By far most common subtype is adenocarcinoma; other subtypes include lymphoma and stromal tumors. In high incidence countries, screening programmes are well established. This has perhaps, in part, contributed to the variation in 5-year survival figures ranging from 52% in Japan to 25% in Europe and USA [36].
Helicobacter pylori are a gram-negative microaerophilic bacterium whose colonisation of the stomach may have devastating consequences. By varying mechanisms, H pylori stimulates gastrin related acid production, and when this becomes overwhelming, chronic inflammation ensues. Such changes may cause dysplasia and adenocarcinoma formation [37]. Studies have suggested a very strong link between H pylori and the development of gastric cancer: up to a 6 fold increase in risk in patients who are colonized with gastric H pylori [38]. Hereditary gastric cancer accounts for up to 3% of cases and is thought to relate to a mutation in the CDH1 gene, which regulates the activation of adhesion molecules [39]. The lifetime risk for carriers of this mutation for developing gastric cancer is 83% for women and 67% for men [34,40]. Other genetic conditions such as Lynch syndrome and Peutz-Jeghers disease may also infer an increased risk of developing cancer. Smoking, high salt intake, pernicious anaemia and chronic inflammation are all associated risk factors. It is interesting to note that although the incidence of distal gastric cancer has reduced, the incidence of proximal cancers has risen, and this may be associated with obesity [35].
There are no typical symptoms which will confer a diagnosis of gastric cancer, but patients may present with epigastric pain, weight loss, symptoms suggestive of anaemia, or with symptoms of advanced disease. Rarely, patients may present with significant complications, including perforation, obstruction or bleeding. Diagnosis is again confirmed by upper gastrointestinal endoscopy with tissue samples taken for histological diagnosis. Staging investigations are identical to those for oesophageal cancer. Again, laparoscopic staging with or without peritoneal fluid sampling is a useful adjunct and forms part of the staging protocol in most units. In Gastric cancer, PET is not routinely used as a staging investigation.
Surgery involves a total gastrectomy for resectable disease, or for distal cancers a subtotal gastrectomy is sufficient as long as clear margins are achieved. Randomized control trials have confirmed that there is no difference in survival in total or distal gastrectomies for distal cancers [41,42]. The extent of lymph node dissection remains both variable and debatable. Indeed, there are 16 lymph node compartments in close proximity to the stomach which may be resected [43]. Two general types are used practically: D1 resection, where the N1 group of nodes and the omentum are resected; and D2, where additionally, the N2 groups of nodes are dissected. Various trials and studies have been performed to ascertain whether there is any advantage with D2 resection, which may be associated with increased morbidity. As yet, there is no clear trial that has shown improved survival with D2 resection without increased mortality and morbidity [44-47]. Whereas in the USA and many European countries a resection and D1/D2node dissection is preferred, in Japan and the Far East, a D2 or even D3 (N3 group of nodes along the splenic artery, hepatoduodenal ligament and mesenteric root) resection is mostly performed [45,46]. Further, a locally advanced tumor may infiltrate into the spleen, colon or pancreas, and these organs often need to resected too, resulting in increased morbidity [48].
The MAGIC trial in the UK showed an overall survival advantage in administering pre- and post-operative chemotherapy for resectable disease of 13%.26Similarly, a French trial detected similar findings [49]. Trials assessing the use of chemoradiotherapy in the preoperative setting have been promising, but this has not yet translated into clinical practice. Trials have assessed the use of a short course of pre-operative radiotherapy, and these have had favorable results [50]. However, given that surgery alone has a prime outcome, the use of pre-operative treatment is still being determined and debated. The role of adjuvant treatment is better established. Radiotherapy has been used, with some studies showing better loco regional outcomes [51,52]. This is an especially effective modality where positive margins are found. For unresectable disease, palliative radiotherapy can provide good symptom relief, and it can also be used to treat bleeding from the tumor. Chemotherapy in the palliative setting also has been shown to improve symptoms, but survival is generally unaffected. Stents or palliative bypasses can be utilized in gastric outlet obstruction [53,54].
Long term survival is certainly improving for gastric cancer, but still remains worrying. Figure 2 shows the 5-year survival values for resectable disease with optimal treatment. Whereas these results are relatively good, overall survival across all stages remains worrying at between 25-52% [36]. It is this variability which needs to be addressed, as there is still no uniform strategy on the extent of surgery or on the administration of neo-adjuvant or adjuvant treatment.
Conclusions
It is imperative, bearing in mind that incidence is increasing, that we maintain research into both the mechanisms of gastro-oesophageal oncogenesis and optimizing the best standard of treatment. A number of trials are in progress either refining current standards or assessing new techniques, but these must be translated into clinical practice. Survival for this group of cancers remains poor, and we must redress this urgently. Newer studies assessing biological therapies, screening programmes or cancer prevention strategies must be supported, and we must learn from our past experiences. There is a geographical variation in both incidence and survival, and these patterns need to be studied further to elucidate the causes, with optimal treatment strategies being adopted. It is clear that, as ever, there is scope for improvement, and this must be enacted and focused, so that raw data can be transformed into good clinical practice with improved outcomes for patients.
References
- Enzinger PC, Mayer RJ (2003) Esophageal cancer. N Engl J Med 349: 2241-2252.
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917.
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225-249.
- [No authors listed] (2008) Esophageal cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract Gastroenterol Hepatol 5: 517-526.
- Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24: 2137-50.
- Pohl H, Welch HG (2005) The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst 97: 142-146.
- Vaughan TL, Davis S, Kristal A, Thomas DB (1995) Obesity, alcohol, and tobacco as risk factors for cancers of the esophagus and gastric cardia: adenocarcinoma versus squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 4: 85-92.
- Lee CH, Wu DC, Lee JM, Wu IC, Goan YG, et al. (2007) Carcinogenetic impact of alcohol intake on squamous cell carcinoma risk of the oesophagus in relation to tobacco smoking. Eur J Cancer 43: 1188-1199.
- Yang SJ, Wang HY, Li XQ, Du HZ, Zheng CJ, et al. (2007) Genetic polymorphisms of ADH2 and ALDH2 association with esophageal cancer risk in southwest China. World J Gastroenterol 13: 5760-5764.
- Brown LM, Hoover R, Silverman D, Baris D, Hayes R, et al. (2001) Excess incidence of squamous cell esophageal cancer among US Black men: role of social class and other risk factors. Am J Epidemiol 153: 114-22.
- Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, et al. (2003) Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst 95: 1404-1413.
- Anderson LA, Watson RG, Murphy SJ, Johnston BT, Comber H, et al. (2007) Risk factors for Barrett's oesophagus and oesophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol 13: 1585-1594.
- Pennathur A, Landreneau RJ, Luketich JD (2005) Surgical aspects of the patient with high-grade dysplasia. Semin Thorac Cardiovasc Surg 17: 326-332.
- Rösch T (1995) Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin N Am 5: 537-547.
- Luketich JD, Schauer P, Landreneau R, Nguyen N, Urso K, et al. (1997) Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg 114: 817-821.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL et al. (2010) AJCC cancer staging manual (7thedn) Springer, New York, NY.
- Pennathur A, Gibson MK, Jobe BA, Luketich JD (2013) Oesophageal carcinoma. Lancet 381: 400-412.
- Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, et al. (2012) Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 256: 95-103.
- Goldminc M, Maddern G, Le Prise E, Meunier B, Campion JP, et al. (1993) Oesophagectomy by a transhiatal approach or thoracotomy: a prospective randomized trial. Br J Surg 80: 367-370.
- Chu KM, Law SY, Fok M, Wong J (1997) A prospective randomized comparison of transhiatal and transthoracic resection for lower-third esophageal carcinoma. Am J Surg 174: 320-324.
- Pennathur A, Zhang J, Chen H, Luketich JD (2010) The "best operation" for esophageal cancer? Ann Thorac Surg 89: S2163-2167.
- Nishihira T, Hirayama K, Mori S (1998) A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 175: 47-51.
- Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, et al. (2002) Hospital volume and surgical mortality in the United States. N Engl J Med 346: 1128-1137.
- Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, et al. (1998) Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 339: 1979-1984.
- Medical Research Council Oesophageal Cancer Working Group (2002) Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 359: 1727-1733.
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, et al. (2006) Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355: 11-20.
- Ando N, Iizuka T, Ide H, Ishida K, Shinoda M, et al. (2003) Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study--JCOG9204. J Clin Oncol 21: 4592-4596.
- Kleinberg L, Gibson MK, Forastiere AA (2007) Chemoradiotherapy for localized esophageal cancer: regimen selection and molecular mechanisms of radiosensitization. Nat Clin Pract Oncol 4: 282-294.
- Hanna GB, Boshier PR, Knaggs A, Goldin R, Sasako M (2012) Improving outcomes after gastroesophageal cancer resection: can Japanese results be reproduced in Western centers? Arch Surg 147: 738-745.
- Anderson O, Ni Z, Møller H, Coupland VH, Davies EA, et al. (2011) Hospital volume and survival in oesophagectomy and gastrectomy for cancer. Eur J Cancer 47: 2408-2414.
- Barbour AP, Rizk NP, Gonen M, Tang L, Bains MS, et al. (2007) Adenocarcinoma of the gastroesophageal junction: influence of esophageal resection margin and operative approach on outcome. Ann Surg 246: 1-8.
- Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE (2009) Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 27: 5062-5067.
- Igaki H, Tachimori Y, Kato H (2004) Improved survival for patients with upper and/or middle mediastinal lymph node metastasis of squamous cell carcinoma of the lower thoracic esophagus treated with 3-field dissection. Ann Surg 239: 483-490.
- Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ (2009) Gastric cancer. Lancet 374: 477-490.
- Yamaoka Y, Kato M, Asaka M (2008) Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med 47: 1077-1083.
- Parkin DM (2001) Global cancer statistics in the year 2000. Lancet Oncol 2: 533-543.
- Correa P (1992) Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 52: 6735-6740.
- Helicobacter and Cancer Collaborative Group (2001) Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut 49: 347-353.
- Guilford PJ, Hopkins JB, Grady WM, Markowitz SD, Willis J, et al. (1999) E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat 14: 249-255.
- Brooks-Wilson AR, Kaurah P, Suriano G, Leach S, Senz J, et al. (2004) Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet 41: 508-517.
- Bozzetti F, Marubini E, Bonfanti G, Miceli R, Piano C, et al. (1999) Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 230: 170-178.
- Gouzi JL, Huguier M, Fagniez PL, Launois B, Flamant Y, et al. (1989) Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg 209: 162-166.
- Japanese Gastric Cancer Association (1998) Japanese Classification of Gastric Carcinoma - 2nd English Edition - Gastric Cancer 1: 10-24.
- Cuschieri A, Weeden S, Fielding J, Bancewicz J, Craven J, et al. (1999) Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 79: 1522-1530.
- Dent DM, Madden MV, Price SK (1988) Randomized comparison of R1 and R2 gastrectomy for gastric carcinoma. Br J Surg 75: 110-112.
- Wu CW, Hsiung CA, Lo SS, Hsieh MC, Chen JH, et al. (2006) Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 7: 309-315.
- McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J (2003) Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev 4: CD001964.
- Kitamura K, Nishida S, Ichikawa D, Taniguchi H, Hagiwara A, et al. (1999) No survival benefit from combined pancreaticosplenectomy and total gastrectomy for gastric cancer. Br J Surg 86: 119-122.
- Boige V, Pignon J, Saint-Aubert B, Lasser P, Conroy T, et al. (2007) Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin (P) to surgery alone in adenocarcinoma of stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin 25: 4510.
- Fiorica F, Cartei F, Enea M, Licata A, Cabibbo G, et al. (2007) The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev 33: 729-740.
- Kozak KR, Moody JS (2008) The survival impact of the intergroup 0116 trial on patients with gastric cancer. Int J Radiat Oncol Biol Phys 72: 517-521.
- Coburn NG, Guller U, Baxter NN, Kiss A, Ringash J, et al. (2008) Adjuvant therapy for resected gastric cancer--rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys 70: 1073-1080.
- Markar SR, Karthikesalingam A, Jackson D, Hanna GB (2013) Long-term survival after gastrectomy for cancer in randomized, controlled oncological trials: comparison between West and East. Ann Surg Oncol 20: 2328-2338.
- Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, et al. (2004) Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol 22: 2767-2773.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 14544
- [From(publication date):
October-2013 - Nov 19, 2025] - Breakdown by view type
- HTML page views : 9836
- PDF downloads : 4708