Commentary Open Access
Gastrointestinal Cancer
Kuo-Ching Yuan*
Trauma and Critical Care Center, Division of General Surgery, Department of Surgery, Chang-Gung Memorial Hospital, Chang-Gung University, Linkou, Taiwan
- *Corresponding Author:
- Kuo-Ching Yuan
Trauma and Critical Care Center
Division of General Surgery, Department of Surgery
Chang-Gung Memorial Hospital
Chang-Gung University, Linkou, Taiwan
Tel: 886-3-3281200, ext: 3651
Fax: 886-3-3289582
E-mail: traumayuan@gmail.com
Received date: May 20, 2013; Accepted date: July 23, 2013; Published date: July 25, 2013
Citation: Yuan KC (2013) Gastrointestinal Cancer. J Gastroint Dig Syst 3:125. doi:10.4172/2161-069X.1000125
Copyright: © 2013 Yuan KC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Introduction
Gastrointestinal (GI) cancer refers to malignant tumors arising within the gastrointestinal tract. A wide definition of gastrointestinal tract includes hollow organ, solid organ and biliary system. Our discussions here are limited to hollow organs, including the esophagus, stomach, small bowels, colon and rectum. The symptoms usually associated with the location, and include obstruction (difficulty swallowing or defecating), abnormal bleeding, or others. The diagnosis often requires endoscope with biopsy. The treatment is determined by the tumor site, the cell type and whether it has invaded other tissues or spread elsewhere in the body (staging). Staging also determines the prognosis.
Esophageal Cancer
Most of the esophageal cancer originates from the mucosa layer. It often grows out and through other layers of the esophagus tissue. There are two common esophageal cancer cell types:
1. Squamous cell carcinoma: The morphology of this cancer cell is flat. Most of the cancers occur on the upper and middle segment of the esophagus belong to this type.
2. Adenocarcinoma: The adenocarcinoma originates from the glandular cell. The cancer cell appeared in glandular arrangement. The adenocarcinoma is more commonly seen on the lower segment of esophagus.
Some risk factors associated with esophageal cancer have been proposed:
1. Smoking and alcohol drinking: Squamous cell carcinoma of esophagus is highly associated with smoking and alcohol drinking.
2. Diet habit: Taking more vegetables and fruits can reduce incidence of squamous cell carcinoma.
3. None-steroid anti inflammation drug: Patients who regularly take NSAID have lower incidence of esophageal cancer.
4. Gastroesophageal regurgitation with stimulate lower esophageal mucosa. Long term regurgitation can resulted in Barrett’s esophagus. Barrett’s esophagus means the lower esophageal mucosa are replaced by abnormal cell, which can lead to esophageal cancer.
Other risk factors include old age, and male gender.
Dysphagia (difficulty swallowing) and odynophagia (painful swallowing) are the most often symptoms of esophageal cancer. Dysphagia is seen in most patients. Fluids and soft foods are usually tolerated, while formed substances (such as bread or meat) cause much more difficulty. Significant body weight loss is a result of poor oral intake and the active cancer. Pain behind the sternum or in the epigastrium, often a heartburn sensation, may be obvious. Another signs include unusually husky, raspy, or hoarse-sounding cough. It is due to the recurrent laryngeal nerve affected by tumor. The tumor can disrupt normal peristalsis and leads to nausea and vomiting, regurgitation, coughing and aspiration pneumonia. The tumor surface is fragile and easy bleeding, which may cause hematemesis. In advanced disease, compression of local structures leads to an upper airway obstruction and superior vena cava syndrome.
When esophageal cancer is suspected clinically, some examinations will be arranged for further evaluation:
1. Chest X-ray
2. Upper gastrointestinal barium film: X-ray films of abdomen are taken after oral administration of contrast medium. This can outlines the lesion that can’t be seen in the plain films.
3. Endoscope examination: the endoscope was put into esophagus via mouth or nostril. It can inspect the lesion directly and do biopsy.
4. Biopsy: This is performed by endoscope. The biopsy specimen can be stained and evaluated under microscope.
Some factors affecting treatment effect and patient prognosis: cancer stage, size of cancer, health condition of the patient. The prognosis will be better if the esophageal cancer is in its early stage. But most esophageal cancer is diagnosed at late stage and the chance to cure is low. Some examinations are arranged to determine the stage of an esophageal cancer. For example, bronchoscope can determine cancer invasion into trachea or main bronchus. Computed tomography can evaluate extension of the esophageal cancer and possible distal metastasis. Endoscopic ultrasound (EUS) is an ultrasound probe was place at the tip of the endoscope. This tool is used to exam the depth of cancer invasion and possible involvement of the adjacent organ and regional lymph nodes.
Stages of Esophageal Cancer
Stage 0: cancer limited under basement membrane of the mucosa
Stage I: cancer limited to mucosa and submucosa
Stage II: further divided into IIa and IIb
IIa: cancer involves muscular layer or adventitia, but no lymph node metastasis
IIb: with lymph node metastasis, but cancer is limited to mucosa, submucosa, or muscular layer
Stage III: cancer invades adjacent organ or cancer invades adventitia with lymph node metastasis.
Stage IV: cancer with distal organ metastasis
There are standard treatments for esophageal cancer at present:
1. Surgery: Esophagectomy is the most common treatment for esophageal cancer. Surgeon will use stomach or colon for reconstruction. So the patient can still have oral intake after operation. The regional lymph will be dissected during operation for possible cancer metastasis.
2. Stent: A stent can provide patency of the esophagus.
3. Radiotherapy: Radiotherapy uses the high energy X-ray or other radiation to kill cancer cell.
4. Chemotherapy: Using specific chemical medication to stop caner growing and kill cancer cell.
5. Nutrition: The nutrition status should be monitored and maintained during treatment. Most of the esophageal cancer patients can not have adequate nutrition by mouth due stenosis or adverse effect of treatment. These patients should receive temporal intravenous nutrition supplement, or tube feeding via mouth or nostril, until patient can have oral intake.
Nowadays, radiation therapy may be combined with chemotherapy, used as a component of induction therapy, used in the adjuvant setting, or used for palliation of advanced disease [1]. The treatment for esophageal is multimodal and the proper treatment needs to be tailored from patient to patient [2].
Stomach
Stomach connects esophagus above and connects duodenum below and is one of the important digestive organ. Gastric cancer can happen at any parts of the stomach. It contains many different histological types, but the most common one is the adenocarcinoma derived from epithelium of the gastric mucosa. It accounts for 90-95% of all gastric cancer. A gastric cancer often refers to gastric adenocarcinoma. The incidence of gastric cancer is declining and it is also the same in Taiwan. But until now, gastric cancer is still a common cancer in Taiwan.
The actual etiology of gastric cancer is not very clear. Many factors are considered associated with gastric cancer. These factors include:
1. Infection of Helicobactor Pylori (HP): Infection of HP will cause chronic inflammation and atrophy of gastric mucosa and therefore leads to some precancerous change.
2. Consumption of too much smoked, Salted or marinated food: Salted and marinated food will be changed into a carcinogenic substance call Nitrite amine in stomach. Smoked foods contain large amounts of polycyclic hydrocarbons. People who eat these two kinds of food for long term have higher incidence of gastric cancer. Japanese people and people of Iceland are best examples.
3. Smoking and alcohol use
4. Too much red meat consumption: Some researches reveal that frequent red meat consumption may increase incidence of gastric cancer.
5. Genetic factors: People with blood type A are more likely to have gastric cancer. People who have family history of gastric cancer also carry higher incidence.
6. Pernicious anemia: These patients have a certain antibody that will attack the epithelium of gastric mucosa, causing mucosa destruction and atrophy. This change increases cancer risk.
7. Received partial gastrectomy: Patients who had received partial gastrectomy due to peptic ulcer or other reason, their inner environment of stomach had a dramatic change after operation. The gastric mucosa sustained long term irritation by the regurgitated bile, pancreatic juice and bowel secretion. After chronic stimulation, gastric mucosa had cancer change and resulted in stump cancer. In general, stump cancer usually happens in about 15 years after partial gastrectomy.
8. Adenomatous polyp: the adenomatous polpy of stomach has high risk for cancer change.
The metastasis of gastric cancer includes 4 types:
1. Lymphatic metastasis: the cancer cell will invade into lymphatic system. It usually invades perigastric lymph node first, and then other intraabdominal lymph node. In advance stage patient; even left supraclavicle lymph node can be involved.
2. Hematogenous metastasis: If the cancer cell enters blood stream, it will be transferred into liver by portalcirculation later. Other locations such as lung, bone, and brain are all possible for metastasis.
3. Direct invasion: Gastric cancer can direct invades adjacent organ, most commonly includes liver, diaphragm, colon and pancreas.
4. Peritoneal metastasis: The stomach cancer cell leaves stomach and form tumors inside peritoneum, as known as peritoneal seeding.
Gastric cancer is divided into 4 stages, by the TMN method. It includes: the depth of invasion of cancer in stomach; status of lymph node metastasis and status of distal metastasis. The clinical stage of a gastric cancer is determined by the 3 factors mentioned above. In general, cancer stage is the key point for treatment plan and prognosis.
The clinical presentations of a gastric cancer are related to its location and degree of invasion. In early stage, gastric cancer is difficult to differentiate from gastritis or peptic ulcer by symptoms. Patient may have dyspepsia or epigastric bloating, and other non specific symptoms such as epigastric discomfort, mild dull pain, nausea or poor appetite. With the progression of cancer; patient may develop some severe symptoms such as dysphagia, vomiting, epigastric pain, hematochezia and body weight loss. Not the entire symptoms list above is cancer related. Most of the minor symptoms are caused by benign disease. However, if the symptoms last for a period too long, a possible gastric cancer should be considered.
At present, there is no large scale screening plan for gastric cancer in Taiwan. Patient will receive endoscopy if gastric cancer is clinically suspected. If the doctor find suspect lesion during endoscopy, a biopsy of the lesion will be done. And the specimen will be sent for pathological exam. A diagnosis of gastric cancer is usually confirmed by endoscopic biopsy. Upper GI series can provide cancer location and extend of invasion in another way. Abdominal ultrasound or computed tomography can be used for evaluation of liver metastasis, regional lymph node metastasis, other peritoneal metastasis or invasion of adjacent organs (Figure 1). Endoscopic ultrasound is a type of endoscope with an ultrasound probe. It can determine the depth of invasion by gastric cancer, enlargement of regional lymph node and involvement of adjacent organs. This is very helpful for preoperative stage of gastric cancer. The chest X-ray can detect lung metastasis or not. Other laboratory exams such as blood cell count, liver or renal function, are also necessary as part of complete study.
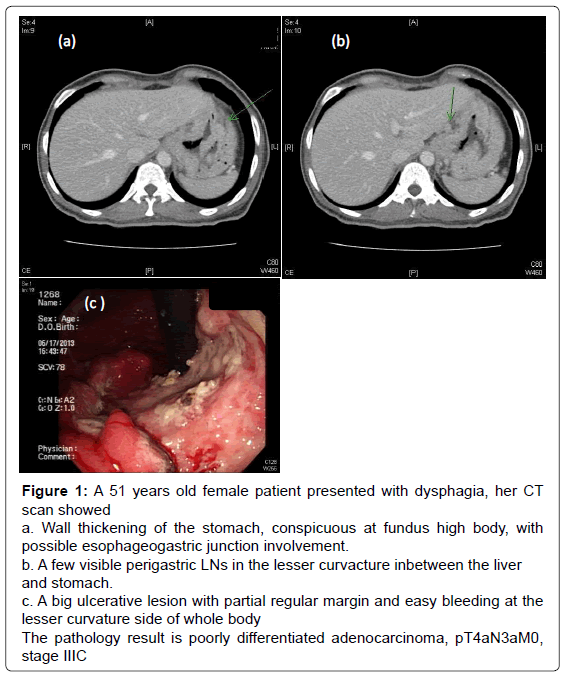
Figure 1: A 51 years old female patient presented with dysphagia, her CT
scan showed
a. Wall thickening of the stomach, conspicuous at fundus high body, with
possible esophageogastric junction involvement.
b. A few visible perigastric LNs in the lesser curvacture inbetween the liver
and stomach.
c. A big ulcerative lesion with partial regular margin and easy bleeding at the
lesser curvature side of whole body
The pathology result is poorly differentiated adenocarcinoma, pT4aN3aM0,
stage IIIC
Treatment of gastric cancer includes mainly operation, chemotherapy and radiotherapy. Surgical resection is still the main treatment. If the cancer is limited to mucosa epithelium and indicated, endoscopic submucosa resection is an option. In general, most gastric cancer patients need to receive traditional stomach resection including:
1. Subtotal Gastrectomy: This operation is indicated for gastric cancer located more distally, or near duodenum. The surgeon will remove about 2/3 to 3/4 of the stomach including the cancer. And reconstruction was done by using anastamosis between residual stomach and duodenum or jejunum.
2. Total Gastrectomy: It is indicated for gastric cancer at proximal part, more near esophagus or diffuses type gastric cancer. The operation includes resection of whole stomach and reconstructed with anastamosis between esophagus and jejunum. If the invaded adjacent organ is resectable, the invaded organ should be removed in the same operation.
Recently, minimal invasive surgery is getting more and more widely use in gastric cancer surgery [3].
Another important aspect of gastric cancer operation is lymph node dissection. This means resection of regional lymph node inside the abdomen because the lymph node may contain metastatic cancer. It is still controversial now for the extent of lymph node dissection. A generally accepted concept is the D2 dissection.
After operation, surgeon will arrange further chemotherapy or radiotherapy according to various factors such as age, health status, cancer stage , residual cancer noted during operation, resection margin, present of distal metastasis or not. Besides diet adjust after operation for gastric cancer, regular outpatient department follow us is also very important. Regular physical examination, along with blood chemistry, X ray and endoscope is essential for follow up and detection of cancer recurrence. The prognosis of gastric cancer is tightly associated with stage. According to the result of studies in Japan, after proper treatment; the five year survival rate of stage I patient is about 90% (IA: 93%, IB: 87%); in stage II, it is about 68%; it is only 50% for stage III; and as low as 17% in stage IV. Therefore it requires early detection and early treatment to provide cure of gastric cancer and long term survival.
There are some different in treatment of gastric cancer between the Eastern and Western countries. In western countries, preoperative chemotherapy or adjuvant chemo-radiation is preferred; however in Asia, surgery followed by adjuvant chemotherapy is favored. The extent of the lymph node dissection also varies by region. D2 gastrectomy is difficult to implement in most western countries while it is standardized and is a routine in Asia [4].
Small Bowel
Small bowel cancer is relative rare. According to the results of one of the largest studies of small-bowel cancer to date, a substantial racial and ethnic variation in the incidence of histological subtypes of small-bowel malignancy was noted. This also suggests possible etiologic diversity and/or disparities in detection [5].
Small bowel cancer usually presents with one or more of the symptoms as abdominal pain, intestinal bleeding, anemia, bowel obstruction, intra-abdominal mass, bowel perforation, fever, body weight loss. The clinical presentations of small bowel cancer are usually atypical. The presentations are related to cancer type, location, size, characters of the tumor. Abdominal pain is common but unspecific. Sometimes this is caused by bowel obstruction. Besides, abdominal pain is related to tumor traction and its related abnormal peristalsis, inflammation change after central necrosis, ulcer, or perforation. The pain can be dull, distension pain, persistent sharp pain or intermittent cramping pain. An intermittent sharp pain usually associates with bowel obstruction. It also accompanies nausea and vomiting. Persistent severe pain often happened with central necrosis of tumor, peritonitis due to ulcer or perforation Intestinal bleeding and anemia usually occurred after tumor had ulcer or surface erosion. About 1/3 of small bowel tumor have bleeding. Most of them are leiomyoma or angioma. Bleeding from adenoma is less. Tarry stool is noted in about 1/4 of small bowel cancer patients. Leiomyoma is the most likely to have bleeding. In general, bleeding in small bowel tumor is obscure. Most of them present with intermittent small amount tarry stool. Massive bleeding is uncommon. Persistent bleeding can cause anemia. Small bowel cancer and malignant lymphoma patient usually have severe anemia [6].
Obstruction is a more common presentation in small bowel tumor. It is usually caused by tumor related intussusception, bowel spasm, stricture or rotation. Besides, bulky small bowel tumor can also compress bowel lumen and resulted in chronic obstruction. Acute obstruction due to intussusception usually is caused by benign tumor. Malignant small bowel tumor usually causes chronic obstruction. About 2/3 of the small bowel cancer patients had symptoms of bowel obstruction. It is also common for bowel obstruction in malignant lymphoma.
Exophytic tumor usually presented with palpable abdominal mass. The induration of tumor varies from soft to firm. Sometimes it is cystic. In general, benign tumor is soft and malignant tumor is firm. The surface can be smooth, uneven or lobulated. The motility is high and the location is unfixed. Clinical palpation sometimes reveals palpable mass but sometimes not palpable. This is an important clinical picture and repeat palpation is necessary. Unable to palpate a mass does not exclude the possibility of small bowel tumor. Most of the benign lesion is impalpable. A palpable mass often indicates leiomyoma, fibroma, large lymphatic duct or tumor related intussusceptions. About 1/3 of small bowel cancer, malignant lymphoma, leiomyosarcoma presents with palpable mass. When the small bowel cancer invades adjacent organ with fistula formation, the margin became blunt and the motility decreased.
Perforation can happen in both benign and malignant tumor, but is more common in malignant tumor. It often happens in ulcerative cancer or leiomyoma. Perforation can be acute with diffuse peritonitis or it can be chronic resulting in limited abscess or fistula.
Sometimes small bowel cancer can cause epigastric discomforts and pain resembling peptic ulcer disease, accompanying nausea, abdominal distension and poor appetite. About half of them have nausea and vomiting. Constipation is also common. However, many patients can have diarrhea. Digestive symptoms happens most in malignant lymphoma. The main cause is that small bowel cancer associates with chronic ulcerative colitis, limited colitis, or steatorrhea. An impaired fat absorption and steatorrhea happened due to thickening of mesentery due to tumor infiltration. Impaired nutrition absorption also resulted from villi atrophy in jejunum.
Fever is often the presentation of malignant lymphoma of small bowel. The second most common one is leiomyoma. Small bowel cancer is less common. The fever pattern is irregular. The cause of fever includes central necrosis of tumor, ulcer infection or peritonitis, abscess after perforation.
Loss of body weight is more common in malignant tumor patients. Loss of body weight usually associated with poor appetite, poor digestion, diarrhea, obstruction, chronic anemia or fever. Cancer cachexia is often seen in advance stage patient. Sometimes low leg edema can be noted due to cancer compression over lymph node at mesenteric root. Ascites is common due to peritoneal seeding or malnutrition. Massive bleeding due to tumor can cause hemorrhagic shock. When the tumor locates at duodenum or periampula region, it may cause obstructive jaundice or bile duct infection.
Treatment of small bowel cancer is determined by histological type. Surgical resection is the choice for adenocarcinoma, or other cancers arising from muscle. For malignant lymphoma, the main treatment is chemotherapy.
Colon and Rectum
In general, colon/rectum cancer refers to adenocarcinoma of colon or rectum. This usually derived from uncontrolled growing of mucosa cell. Colon and rectal cancer occurs more in the elderly people. It usually happened at age about 40-45. But the young case is increasing recently. The incident is relatively equal in male and female. The colon/ rectum cancer is highly associated with diet habit. People who consume more meat, animal fat, protein, unpolished grains and less fiber are more likely to have colon-rectum cancer. The incidence of colon/ rectum cancer is also higher in the developed, industrialized countries.
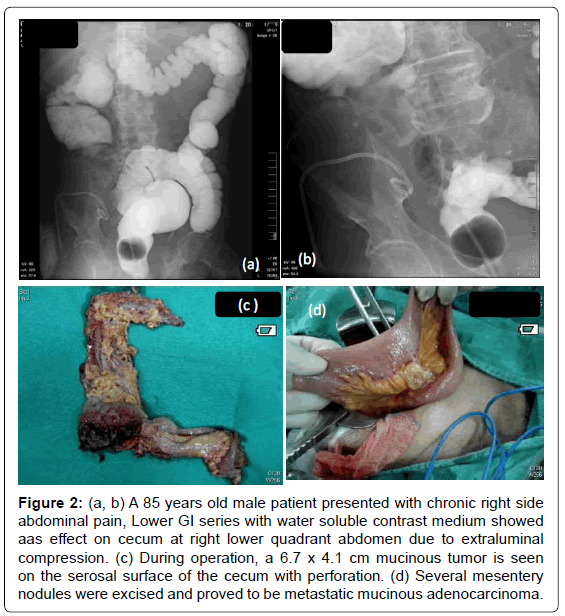
The location distribution of colon/rectum cancer is 40-50% at rectum or rectum-sigmoid junction; sigmoid colon is about 16- 20%; and about 60-70% is below sigmoid colon. Symptoms such as hematemesis, tenesmus, bowel habits change and small caliber of stool happen more early in rectum cancer due to closer location to anus. Obstruction is more common for descending colon cancer due to small lumen, more formed stool and cancer here are more frequent infiltrative type. Cancer in cecum and ascending colon is often polypoid type and usually causes anemia, abdominal floating, abdominal pain and body weight loss. In Taiwan, it is not uncommon that colon/rectum cancer was diagnosed after obvious obstruction (Figure 2). Right side colon cancer can be misdiagnosed as gall stone or peptic ulcer. Some cancer at transverse colon can invade stomach and causes fistula. Cancer at rectum or sigmoid colon can invade urinary bladder or vagina causing fistula with different presentations.
Figure 2: (a, b) A 85 years old male patient presented with chronic right side abdominal pain, Lower GI series with water soluble contrast medium showed aas effect on cecum at right lower quadrant abdomen due to extraluminal compression. (c) During operation, a 6.7 x 4.1 cm mucinous tumor is seen on the serosal surface of the cecum with perforation. (d) Several mesentery nodules were excised and proved to be metastatic mucinous adenocarcinoma.
Digital rectal examination is essential. A finger length can detect cancer within 10 cm from anus; it accounts for about 10% for all colon/ rectum cancer. A 25 cm rigid sigmoid scope can detect about 60-70% of all colon/rectum cancer. It requires colon fiberscope or barium x-ray study to evaluate whole colon. Besides examination, colon fiberscope can also do lesion biopsy, polyp resection and even laser therapy. The drawback is that colon fiberscope can’t pass the lumen if the cancer is too bulky. The barium study usually does double contrast study. The “apple core lesion” is typical for colon cancer. If obstruction is obvious, it is contraindicated to do double contrast study. The barium should be stopped if the lesion at obstruction level is seen. Abdominal sonography is usually used to evaluate liver or lymph node metastasis. Abdominal computed tomography is for evaluation of extend of invasion and status of metastasis.
It is noticed that stage is usually early for screening-detected colon/ rectum cancer. For high risk people, such as old age, with family history of colon-rectum cancer or other cancer family history, or has history of colon polyp, they should receive colon fiberscope regularly after 40 years old. It is still inconclusive for screening in asymptomatic people. The screen methods for colon-rectum cancer include digital rectal examination; occult blood test for stool; rigid sigmoid scope; colon fiberscope and barium study. The occult blood test for to detect blood mixed in stool, not for present of colon cancer. People with positive occult blood test should receive further colon fiberscope or barium study.
Commonly used tumor marker for colon rectum cancer includes CEA and CA199. CEA is the glycoprotein located at the surface of the cell membrane of cancer cell. Elevated CEA can be noticed in many digestive cancer, pancreatic cancer, non small cell cancer of lung and breast cancer. About 70% of colon rectum cancer patient have elevated CEA but it is only 20-40% in early stage. CEA is not used for screening or diagnosis of early colon-rectum cancer but used as indicator in following up. Perioperative CEA level is used for detection of cancer metastasis or recurrence. However, only 25% of recurrent rectum cancer has elevated CEA. With the recent advances in the field of molecular genetics, it has led to the identification of specific biomarkers involved in colorectal cancer progression, and the gene expression microarray technology has led to the identification of molecular profiles to predict recurrence or benefit from adjuvant chemotherapy. Nevertheless, none of these has been validated in large prospective clinical trials [7].
Polyp is the bulging outgrowth soft tissue on the surface of colon mucosa. There are many types of colon polyp and the adenomatous polyp is associated with cancer.
By the histological appearance, the adenomatous polyp is further divided into tubular, tubovillous or mixed type. Adenomatous polyps usually have no specific symptoms and were noticed during health exam colon fiberscope. The risk of cancer increased with size of the polyp. The excised polyp should receive pathological exam to determine cancer or not. Regular follow up is essential after excision of colon polyp. Recurrence of polyp is common and is tightly associated with colon cancer. Adenomatous polyp happened most at age 50-60; therefore, people older than 50 years old should receive screening.
Staging of colon/rectum cancer is important for treatment and prognosis.
Stage I: cancer limited to colon mucosa without any invasion to muscle layer, no lymph node metastasis
Stage II: cancer invades into muscle layer, but no lymph node metastasis
Stage III: cancer with lymph node metastasis
Stage IV: cancer with distal (liver, lung) metastasis
Operation is the main treatment of colon/rectum cancer. Only operation can provide chance for cure. A successful operation includes: adequate safety margin, adequate resection of lymphatic tissue, vessel, fat, soft tissue and even adjacent organ, avoiding cancer spreading or seeding during operation, and preserving a well functioning anus after radical resection for rectum cancer. The extent of resection and the preservation of anus are planned according to preoperative examination. Extensive resection of cancer and associated lymphatic tissue, vessel is the main principle of colon cancer surgery. For rectum cancer operation, the safety margin and preservation of anus should be considered simultaneously. To preserve anus or not is affect by location, cancer size, depth of invasion , lymph node status and the shape of patient’s pelvis. In general, cancer locates at upper 1/3 of rectum can preserve anus, most of the cancer at middle 1/3 rectum can try anus preservation. Cancer locates at lower 1/3 (within 6cm of anus) usually requires abdominoperineal resection and permanent colostomy.
Chemotherapy has evolved from a traditional palliative treatment to an adjuvant therapy for early, non metastasis patients. Adjuvant chemotherapy usually applied in stage II, III colon cancer after operation and there is no gross cancer visible. For lymph node positive patient, a half year to one year adjuvant chemotherapy after operation can improve 5 year disease free rate from 44% to 61%. For cancer invades through colon wall without lymph node metastasis, chemotherapy can increase 7 year disease free rate from 71% to 79%.
Radiotherapy is widely used in rectum cancer. It includes isolated radical radiotherapy, perioperative radiotherapy, salvage radiotherapy and palliative radiotherapy. Radiotherapy combines with chemotherapy can improve local control rate and alleviated symptoms. Preoperative radiotherapy can reduce tumor volume and made operation feasible. Other benefit includes decreased local recurrence rate.
The major drawback is that operation will be postponed for 4-6 weeks after completion of radiotherapy. The purpose of postoperative radiotherapy in rectum cancer is to eradicate residual lesion therefore reduce recurrent rate. The postoperative radiotherapy can reduce about 20% recurrence in stage II and stage III rectal cancer. It is very helpful for local control and overall survival rate. Because the patient of colon cancer is increasing, the treatment can consume much resource. There are discussions about the balance between resource use and the efficacy of treatment [8].
Conclusion
Gastrointestinal cancer is common and complex. A proper treatment should consider many aspects including patient factors, tumor factors, doctor factors and the facility of the hospital. As a whole, early detection and early treatment brings better outcome. An effective screening is important for early detection. However, the invasive nature and high-cost associated with these screening tools hinders implementation of GI cancer screening programs. Moreover, only a small fraction of general population is truly predisposed to developing gastrointestinal malignancies, and requires surveillance. To spare the average-risk individuals from invasive procedures and achieve effective cancer prevention, it’s important to identify cohorts in general population that are at high risk of developing GI malignancies (riskstratification), and select suitable screening program [9].
References
- Cooper SL, Russo JK, Chin S (2012) Definitive chemoradiotherapy for esophageal carcinoma. Surg Clin North Am 92: 1213-1248.
- Hölscher AH, Bollschweiler E (2012) Choosing the best treatment for esophageal cancer: criteria for selecting the best multimodal therapy. Recent Results Cancer Res 196: 169-177.
- Phillips JD, Nagle AP, Soper NJ (2013) Laparoscopic gastrectomy for cancer. Surg Oncol Clin N Am 22: 39-57, v-vi.
- Blum MA, Takashi T, Suzuki A, Ajani JA (2013) Management of localized gastric cancer. J Surg Oncol 107: 265-270.
- Goodman MT, Matsuno RK, Shvetsov YB (2013) Racial and ethnic variation in the incidence of small-bowel cancer subtypes in the United States, 1995-2008. Dis Colon Rectum 56: 441-448.
- Poddar N, Raza S, Sharma B, Liu M, Gohari A, et al. (2011) Small bowel adenocarcinoma presenting with refractory iron deficiency anemia - case report and review of literature. Case Rep Oncol 4: 458-463.
- Akiyoshi T, Kobunai T, Watanabe T (2012) Recent approaches to identifying biomarkers for high-risk stage II colon cancer. Surg Today 42: 1037-1045.
- Ku G, Tan IB, Yau T, Boku N, Laohavinij S, et al. (2012) Management of colon cancer: resource-stratified guidelines from the Asian Oncology Summit 2012. Lancet Oncol 13: e470-481.
- Tiwari AK, Laird-Fick HS, Wali RK, Roy HK (2012) Surveillance for gastrointestinal malignancies. World J Gastroenterol 18: 4507-4516.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 15068
- [From(publication date):
July-2013 - Apr 10, 2025] - Breakdown by view type
- HTML page views : 10513
- PDF downloads : 4555