Review Article Open Access
Galanin Receptors as Pharmacological Targets in the Treatment of Addiction,Drug Rehabilitation, Drug Addiction Treatment,Morphine Addiction, Drug Addiction Treatment, Cocaine-Related Disorders, Cocaine Addiction, Opioid-Related Disorders, Substance-Related Disorders
Belinda L. Ash and Elvan Djouma*School of Public Health and Human Biosciences, Department of Human Biosciences, La Trobe University, Bundoora, Victoria, Australia
- *Corresponding Author:
- Elvan Djouma
School of Public Health and Human Biosciences
Department of Human Biosciences
La Trobe University, Bundoora, Victoria, 3086, Australia
Tel: +61 3 9479 5005
Fax: +61 3 9479 5784
E-mail: e.djouma@latrobe.edu.au
Received November 14, 2011; Accepted December 16, 2011; Published December 20, 2011
Citation: Ash BL, Djouma E (2011) Galanin Receptors as Pharmacological Targets in the Treatment of Addiction. J Addict Res Ther S4:003. doi:10.4172/2155-6105.S4-003
Copyright: © 2011 Ash BL, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Drug and alcohol abuse present an ongoing problem from both a financial and psychosocial perspective. As the worldwide prevalence of drug-abuse grows, research into the use of novel pharmacotherapies continues. Recently, the neuropeptide galanin has been implicated in the rewarding effects of addictive substances and drug-seeking behaviour. Galanin acts by binding to three receptor subtypes, which are localised within many brain regions that play a primary role in addiction. Consequently, this paper sought to review the most recent literature with particular interest in the role of galanin and its receptors in alcoholism, drug-abuse and associated mood disorders. Further, we compile the experimental findings that suggest a potential role for galanin and its three receptor subtypes in the treatment of addiction and drug-seeking behaviour. Of particular focus in this review is the large amount of experimental evidence that supports an association between the galanin-3 receptor, alcoholism and mood disorders. Ultimately, further investigation of galanin receptors as potential drug targets may contribute to the creation of new pharmacotherapies for drug dependence.
Keywords
Addiction; Alcohol; Anxiety; Depression; Galanin; GALR1; GALR2; GALR3; Nicotine; Opiates; DMN: Hypothalamic Dorsomedial Nucleus
Abbreviations
5-HT: Serotonin; Ach: Acetylcholine; ADH: Antidiuretic Hormone; AMG: Amygdala; Arc: Arcuate Hypothalamic Nucleus; BST: Bed Nucleus Of The Stria Terminalis; Cb: Cerebellum; CeA: Central Amygdaloid Nucleus; CNS: Central Nervous System; CPu: Caudate Putamen (striatum); DA: Dopamine; DRN: Dorsal Raphe Nucleus; GABA: Gamma-AminoButyric Acid; GAL-KO: Galanin Knockout; GAL-OE: Galanin Over-Expressing; GALR1: Galanin-1 Receptor; GALR2: Galanin-2 Receptor; GALR3: Galanin-3 Receptor; HIP: Hippocampus; HYP: Hypothalamus; IPSP: Inhibitory Post-Synaptic Potential; LC: Locus Coeruleus; LH: Lateral Hypothalamus; MDS: Mesolimbic Dopamine System; NA: Noradrenaline; NAc: Nucleus Accumbens; PAG: Periaqueductal Gray; PFC: Pre-Frontal Cortex; PNS: Peripheral Nervous System; PVN: Paraventricular Hypothalamic Nucleus; SN: Substantia Nigra; TH: Thalamus; VTA: Ventral Tegmental Area
Introduction
Alcohol has historically been consumed in ceremonies and celebrations, as an addition to meals to enhance enjoyment of food [1], and has been used for its analgesic and sedative properties [2]. For recreational purposes, alcohol is the most widely used drug of choice in Australia, with an average of 7.2 litres consumed annually per person [3]. While the majority of drinkers are not considered to be dependent on alcohol, the prevalence of alcohol abuse is increasing at an alarming rate.
Approximately 10% of Australians self-report that they drink at levels considered to be of risk to their health [4] and a similar percentage consume alcohol on a daily basis [3]. In addition, the abuse of other illicit substances in Australia is on the rise with the number of cannabis- and amphetamine-related hospital admissions increasing each year [5]. The social and economic burden of drug abuse in Australia was estimated to be around $55.2 billion in 2004/05 [6] with alcohol accounting for a large $15.3 billion portion of this figure [6]. What this figure does not represent is the incalculable and devastating human cost in terms of lost lives and drug-associated illness.
The prevalence and associated costs of drug use in Australia is certainly not isolated but is comparable to that of the rest of the world. The World Health Organisation reported that 5.4% of the world’s annual burden of disease in 2010 was attributable to the use of illicit drugs and alcohol [7]. Alcohol-use disorders are more common worldwide than drug-use disorders, and in accordance, there is a greater demand for medical treatment of alcohol-related conditions [7]. Despite a wide range of prescription medications being available to treat alcoholism and drug abuse, the problem of alcoholism still persists due to such profound effects on the brain and the wide range of symptoms that must be concurrently treated. Chronic use of a drug promotes progressive and persistent neural adaptation in the brain [8-11], which leads to changes in behaviours that positively reinforce drug seeking, ultimately leading to addiction.
Addiction is characterised by its stubborn persistence and compulsive nature that drives the user to constantly secure and use a drug, despite adverse consequences [12]. Common characteristics of addiction include drug craving and a very high tendency to relapse in response to stress or environmental drug-related cues [8,11,13-16] for weeks or even years after withdrawal from the drug [8,12,17]. The issues of relapse and drug-craving represent a major obstacle to treating addictions and developing new therapeutic medicines to successfully treat drug-dependence is challenging. To date, the available treatments for drug-addiction remain somewhat inadequate for most individuals [18].
Over three decades of research has looked at how addictive drugs interact with the brain and lead to changes in the circuitry involved in reward [for reviews see 13,15]. As our understanding of the mechanisms behind drug abuse increase, new pharmacotherapies targeted toward disrupting these mechanisms are being developed. In recent years, the neuropeptide galanin and its receptors have been identified as promising drug targets for the treatment of addiction [19]. A large amount of pre-clinical evidence highlights a potential therapeutic role for galanin in the treatment of alcoholism. In terms of new pharmacological approaches to the treatment of alcoholism, interventions should be targeted at: (1) preventing or reducing consumption, (2) reducing the symptoms experienced as a result of drug withdrawal, (3) preventing relapse or (4) a combination of the above.
This review aims to evaluate the role of the neuropeptide galanin and its receptors in the treatment of addiction and drug-seeking behaviour with a focus on alcoholism. Thus, a greater understanding of the actions of galanin in the brain may contribute to the development of novel and effective pharmacotherapies for addiction.
Galanin
Introduction to galanin
Galanin is a neuropeptide of 30 amino-acids in humans and 29 amino-acids in other species [20] which was first isolated from the porcine intestine in 1980 [21,22]. Galanin has since been found in many species including humans [23], primates [24], rodents [25,26] and fish [27], suggesting that the protein has been well conserved throughout the evolution of species. There have been many important physiological functions identified for galanin, but of particular relevance to this review are the higher functions of the brain that contribute to addictive behaviour. Galaninergic mechanisms are recognised as playing an important role in cognition, learning, memory [21], anxiety and depression [28], as well as reward and drug-seeking behaviour [29,30]. Brain regions that contribute to these functions highly express the galanin peptide and have a high density of galanin binding sites, which provides an explanation as to how galanin modifies these functions and behaviours.
Galanin is expressed in a wide range of tissues including the brain, spinal cord and gut [20]. Centrally, the galanin peptide is highly concentrated within particular brain regions, especially the forebrain, hypothalamus (HYP), amygdala (AMG) and locus coeruleus (LC) [25,31]; areas which contribute to learning, feeding and emotion. Galanin is synthesised at very high levels in the dorsal raphe nucleus (DRN) and the LC [32,33], as well as many hypothalamic nuclei [34], again supporting a role for galanin in feeding and emotion. In addition, the density of galanin binding sites in these brain areas is high, which further validates galanin receptors as a target for pharmacological modification of these functions.
Distribution of galanin binding sites
Early studies by Kohler and colleagues using post-mortem human brain and monkey brain revealed that 125I-galanin binding sites were widely spread in a similar pattern throughout the brain in both species [35,36]. High densities of receptors were widely spread throughout the human forebrain, neocortex, anterior hypothalamus, lateral hypothalamus (LH), bed nucleus of stria terminalis (BST), preoptic area, perifornical area [35,36], periventricular nucleus and medial hypothalamus [37], with a medium density of galanin receptors found in the central and medial amygdala nuclei [35] and regions of the hippocampus (HIP) [36]. Similarly, a high density of galanin binding sites were found in hippocampal regions and the neocortex of the monkey brain [36]. Galanin binding sites, characterised by125Igalanin in the mouse brain, were found to be highest in the nucleus accumbens (NAc), caudate putamen (CPu), AMG, BST, ventral tegmental area (VTA), HYP, LC, and DRN and widely throughout other brain regions at a lower density [38]. The distribution of galanin receptors in the human brain closely resembles that of the monkey brain [35,36] and even the brains of rodents [25,39], which is important, due to the very limited number of galanin mapping studies that have used human brain tissue. Although brains of primates and rodents are much smaller, the galanin receptor distribution pattern closely resembles much of the distribution pattern found in the more complex and larger human brain.
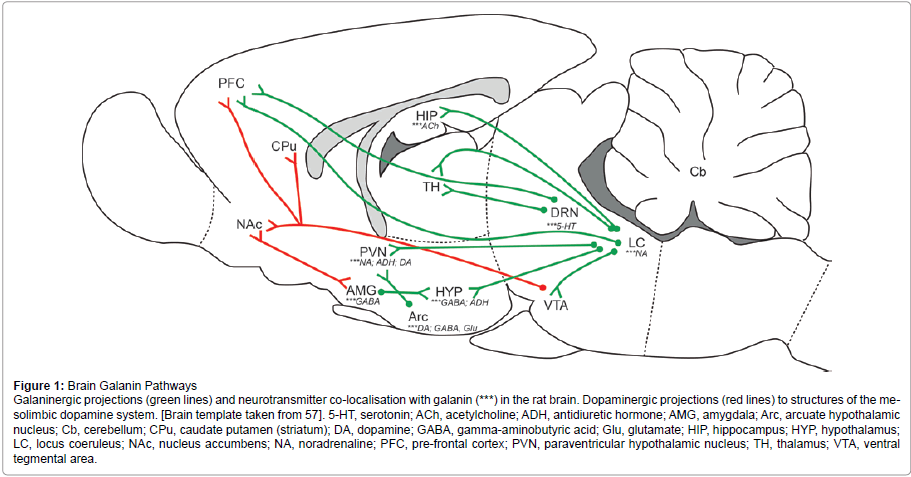
Figure 1:Brain Galanin Pathways
Galaninergic projections (green lines) and neurotransmitter co-localisation with galanin (***) in the rat brain. Dopaminergic projections (red lines) to structures of the mesolimbic
dopamine system. [Brain template taken from 57]. 5-HT, serotonin; ACh, acetylcholine; ADH, antidiuretic hormone; AMG, amygdala; Arc, arcuate hypothalamic
nucleus; Cb, cerebellum; CPu, caudate putamen (striatum); DA, dopamine; GABA, gamma-aminobutyric acid; Glu, glutamate; HIP, hippocampus; HYP, hypothalamus;
LC, locus coeruleus; NAc, nucleus accumbens; NA, noradrenaline; PFC, pre-frontal cortex; PVN, paraventricular hypothalamic nucleus; TH, thalamus; VTA, ventral
tegmental area
The aforementioned brain areas have been shown to have a role in processes related to drug addiction such as feeding, reward, reinforcement, emotion, stress, anxiety, learning and memory consolidation [40-56]. Regions of the HYP that have previously been shown to have a role in promoting feeding behaviour include the preoptic area [50], perifornical area [41], LH [52,55] and PVN (paraventricular hypothalamic nucleus) [41,48,55], while the ventromedial HYP functions to decrease feeding behaviour as a signal of satiety [40]. Both the BST and CeA (central amygdaloid nucleus) have been shown to have a role in the reinforcement of drug dependence [54,56]; with the medial nucleus of the AMG [42], central nucleus of the AMG and BST of the AMG having an involvement in stress and emotional responses [43,46,53]. Neurons projecting from the BST to the VTA contribute to functions of reward [46,51] and motivation [46]. The LH plays a role in mediating learning and reward-seeking behaviours [44,45] and also stimulates food intake [52]. The HIP is predominantly involved with the transformation and consolidation of memories [47,49].
Due to the high density of galanin binding sites in areas of the brain involved in learning, memory, reward and emotion along with the high density of receptors in hypothalamic regions that control feeding [55], it is to be expected that galanin would play a role in feeding behaviour, with an extension to the consumption of alcohol. In addition, the co-localisation of galanin with classical neurotransmitters [32] in brain regions that mediate these functions further supports a role for galanin in drug-abuse.
Galaninergic projections and neurotransmitter coexpression within the brain
Figure 1 provides a schematic representation of galaninergic pathways that interconnect regions of the brain discussed below in relation to a role in addictive behaviours and drug dependence. In addition, galanin is co-localised in many cases with commonly known neurotransmitters, which are indicated in Figure 1
The hypothalamus: Within the HYP, galaninergic projections exist from the Arc (arcuate nucleus) to the PVN [58], two regions that play a role in appetite and feeding behaviour. In the Arc, galanin is co-expressed with two neurotransmitters well known to mediate the physiological effects of alcohol, GABA (gamma-aminobutyric acid) [32,59] and glutamate [60]. It is widely accepted that one of alcohol’s many actions is to reduce excitatory transmission at the glutamate receptor subtype NMDA (N-methyl-d-aspartate) [reviewed by 61], an effect which galanin may potentiate through an inhibitory effect on glutamate release in the Arc [60]. A small proportion of neurons in the Arc co-express galanin with DA (dopamine) [32,62], where it is suggested that galanin may stimulate DA release [63]. DA is also co-expressed with galanin in the PVN [64] and galanin injection (300 pmol) into the PVN of Sprague-Dawley rats has previously been shown to increase the release of DA, ultimately leading to an increase in feeding behaviour [65]. Galanin is also positively related to NA (noradrenaline) in the PVN, as shown by increased local levels of NA following galanin infusion (0.5 μg/min) into the PVN of Wistar rats [48]. Increased levels of NA in the PVN are known to stimulate feeding behaviour, with or without food available [66-68]. NA release in the PVN is increased by galanin [48], which may ultimately stimulate the consumption of alcohol due to its similarity with food in terms of the energy content. In addition, galanin in the HYP has been reported to increase DA release into the NAc [69], which contributes to the pleasurable and rewarding effects that follow drug consumption. The co-localisation of ADH (anti-diuretic hormone) with galanin in hypothalamic neurons [37,70] is suggestive of a role in water balance.
The co-existence of galanin with opioids in many hypothalamic neurons [71] is of importance in establishing a link between galanin, feeding behaviour and ethanol consumption. The mu-opioid receptor antagonist, beta-funaltrexamine (20 nmol), when centrally co-injected with galanin (0.03 nmol) is known to decrease Galanin initiated feeding behaviour in chicks, which suggests that galanin may act indirectly via the mu-opioid receptor to stimulate food intake [72]. Likewise, it has been confirmed that ethanol intake can be stimulated through microinfusion of the opioid agonist, DAMGO (D-Ala2, NMe-Phe4, Glyol5-enkephalin; 0.25 μg), into the NAc of Sprague-Dawley rats [73].
The locus coeruleus: Galanin is synthesised in very high levels within the LC [74,75] and is highly co-expressed with NA in around 80% of neurons [25,32]. The activation of LC neurons is responsible for providing a major source of noradrenergic innervation to many forebrain structures, including some limbic regions [76]. Galaninergic neurons of the LC project mostly to the HYP [77] and subregions such as the PVN [58], medial and lateral thalamus [78], HIP [33,77] and to a lesser degree from the LC to the cerebral cortex [77] and the VTA [76]. Galanin acts to hyperpolarise neurons in the LC [79,80], most likely through binding to GALR1 or GALR3 [80], therefore inhibiting the release of NA to regions of the forebrain [81]. This effect has been demonstrated using brain slices in vitro [79,80] and also through an electrophysiological study that reported the GALR1 to mediate an inhibitory effect on the polarisation of LC neurons in vitro [82]. In addition to modulating NA release to regions of the forebrain, galanin also acts locally within the LC to inhibit the release of NA within the LC [74,81,83].
The dorsal raphe nucleus: Galanin is synthesised in the DRN [32] and co-expressed with 5-HT (serotonin) in around 40-60% of DRN neurons [84,85]. Both the GALR1 [86] and GALR3 [87,88] subtypes are present in the DRN and are known to mediate an inhibitory relationship between galanin and 5-HT in which hyperpolarisation of DRN neurons decreases the release of 5-HT [48,89]. Galaninergic neurons project from the DRN to the medial and lateral thalamus [78] and regions of the cortex [77,90].
The amygdala: Galaninergic neurons project from the AMG to the HYP via the stria terminalis [91] and research has shown that galanin microinfusion (0.3 μl) into both the AMG and PVN increased food intake in Sprague-Dawley rats [92]. In this study, microinfusion of two galanin receptor antagonists, C7 and M40 (0.3 μl), ceased galanin-induced feeding, thereby confirming a role for galanin in feeding behaviour via its action in the AMG [92]. The neurotransmitter GABA also co-exists with galanin in the CeA [93], which suggests a possible role for galanin in response to fear or stress. Neurons of the BST have also been identified as sites that release galanin in response to stress [94].
The hippocampus: Galanin and the neurotransmitter ACh (acetylcholine) are co-expressed in the rat ventral hippocampus [32], where galanin acts to inhibit ACh release [95]. High levels of ACh in the HIP contribute to learning and memory consolidation [96,97]. So it is conceivable that galanin may interfere with memory processes in drug addiction such as associative learning [for review see 98].
The ventral tegmental area: It is speculated that galaninergic projections exist from the LC to the VTA to modulate NA release in the VTA [76]. This connection provides an important link between galanin and the MDS (mesolimbic dopamine system), which plays a crucial role in mediating addictive behaviours and reward following consumption of drugs of abuse. NA in the VTA stimulates firing of dopaminergic neurons that project to regions of the forebrain and limbic structures [99] and it has been shown that galanin injection (1 nmol) into the VTA increases DA accumulation in regions of the CPu and NAc in Wistar rats [69].
The Mesolimbic Dopamine System
Galanin and the mesolimbic dopamine system
As previously mentioned, galanin modulates DA activity within limbic regions. The MDS is often called the ‘reward circuit’ of the brain because of the pivotal role it plays in the reinforcement of pleasurable experiences. Both endogenous mediators and exogenous substances introduced into the body including foods, alcohol and other drugs of abuse are capable of activating the MDS, and it is widely accepted that this system plays a role in the habit-forming actions of all drugs of abuse [12,100,101].
Figure 1 illustrates the regions of the brain that are involved in reward function including the VTA, NAc and PFC (pre-frontal cortex) [101]. Neuronal cell bodies located in the VTA produce DA and project into the PFC, CPu and the NAc, with reciprocal projections from the NAc to the AMG and other regions of the limbic system [12,100,102]. The NAc is a critical site for reinforcing the pleasurable sensations associated with alcohol and other drugs of abuse [102,103], while the AMG plays a role in learning and conditioned reward [15,104]. Projections from the LC to the VTA provide a direct link for galaninergic innervation of the MDS via the release of NA.
The MDS in food and alcohol consumption
| Treatment / condition | Dose / concentration | Species | Route of administration | Physiological or behavioural effect | Primary target | Reference |
|---|---|---|---|---|---|---|
| Galanin | 1 and 3 nmol | Sprague-Dawley rat | Microinjection into the third ventricle | Increase in voluntary ethanol consumption | [116] | |
| M40 (non-selective galanin receptor antagonist) | 1 nmol | Sprague-Dawley rat | Microinjection into the third ventricle | Blocked galanin-induced increased in voluntary ethanol consumption | [116] | |
| Galanin | 1 nmol | Sprague-Dawley rat | Microinjection into the PVN | Increase in voluntary ethanol consumption | [126] | |
| M40 (non-selective galanin receptor antagonist) | 1 nmol | Sprague-Dawley rat | Microinjection into the PVN | Reversal of galanin-induced increase in voluntary ethanol consumption | [126] | |
| Ethanol | 1.0 g/kg | Sprague-Dawley rat | Intraperitoneal injection | Increased galanin peptide mRNA expression | Hypothalamic PVN | [127] |
| Ethanol | 0.8 g/kg | Sprague-Dawley rat | Intraperitoneal injection | Increased galanin peptide mRNA expression | Hypothalamic DMN, PVN, PLH | [125] |
| SNAP 37889 (GALR3 antagonist) | 30 mg/kg | iP rat | Intraperitoneal injection | Decreased operant responding for ethanol | GALR3 | [133] |
| Co-superfusion of galanin with ethanol | 1 µM | C57Bl/6J mouse | Brain slices in vitro | Increased IPSPs in CeA neurons | GALR3 | [132] |
| Co-superfusion of galanin with ethanol | 1 µM | C57Bl/6J mouse | Brain slices in vitro | Decreased IPSPs in CeA neurons | GALR2 | [132] |
| Co-superfusion of galanin with ethanol and SNAP 37889 | 1 µM; 200nM | C57Bl/6J mouse | Brain slices in vitro | Prevented a galanin-induced increase in IPSPs in CeA neurons | GALR3 | [132] |
| Voluntary ethanol consumption | 9% v/v (12 hour limited access schedule for 28-30 days) | Sprague-Dawley rat | Oral | Increased galanin peptide expression | Hypothalamic PVN | [127] |
| Voluntary ethanol consumption | 2% w/w (12 hour limited access for 5 days) | Sprague-Dawley rat | Oral | Increased galanin peptide expression | Hypothalamic PVN | [128] |
| Voluntary ethanol consumption | 9% w/w (12 hour limited access for 20 days) | Sprague-Dawley rat | Oral | Increased galanin peptide mRNA expression and increased galanin immunoreactivity | Hypothalamic PVN, DMN | [125] |
| Voluntary ethanol consumption | 15% w/w (ad libitum access; concentration incrementally increased over 16 days) | Male GAL-OE mouse | Oral | Increased voluntary ethanol intake and increased preference for ethanol | [131] | |
| Voluntary ethanol consumption | 15% w/w (ad libitum access; concentration incrementally increased over 16 days) | Male and female GAL-KO mouse | Oral | Decreased voluntary ethanol intake and decreased preference for ethanol | [130] |
Abbreviations: GAL-KO, Galanin knockout; GAL-OE, Galanin over-expressing.
Table 1: Experimental interactions between galanin, galanin-receptor regulators and alcohol.
The overlap in the role of circuits involving the HYP and the MDS and their contribution to excess food and alcohol consumption has recently been reviewed in detail [105]. Neuronal activity in mesocorticolimbic structures including the PFC, CPu and VTA was reported to be greater in brains of heavy drinkers following exposure to the taste of alcohol [106]. In addition, this study also reported activation of the same MDS structures following the taste of pleasant tasting non-alcoholic drinks such as fruit juice [106]. This further provides evidence of a link between the motivation to drink and brain structures that control drug-seeking, which overlap with structures that control food intake and nutrition. Repeated exposure to highly palatable food is thought to be similar to repeated drug exposure in that both actions are driven by the rewarding consequences that follow. In both instances, neural circuits become sensitised and the reinforcing effects of both food and drug intake increase DA in limbic regions [reviewed by 107].
The reinforcing effects of dopamine
DA (dopamine) is one of the most important neurotransmitters involved in the brain reward pathway and increased levels of DA in the NAc are associated with the pleasurable effects experienced following drug self-administration [12,101]. Another positive link between food, alcohol and galanin, is that all three act to stimulate the release of DA into the NAc [for review see 108]. Microinjection of galanin (300 pmol) into the NAc specifically stimulates the release of DA in the NAc and decreases ACh release in the NAc in Sprague- Dawley rats [65]. Increases in both DA and NA concentrations in the VTA have been observed following alcohol consumption in mice [109] and given that the main noradrenergic input to the VTA comes from the LC, galanin may contribute indirectly to an increase in alcohol consumption via this pathway. Previous research has indicated a strong relationship between galanin and DA, as injection of galanin directly into the PVN increases DA release into the NAc [65]. DA is released by neurons projecting from the VTA into the NAc, causing increased levels of DA in the NAc [103,104]. DA antagonists injected directly into the NAc reduce ethanol consumption in animal models [for reviews see 110,111]. Indeed, evidence supports a possible modulatory effect of galanin on DA release within limbic regions, which may contribute to altered alcohol consumption.
Pre-Clinical Research into Galanin and Addiction
Table 1 summarises the most important findings to date from pre-clinical studies that have confirmed a link between galanin, galanin receptors and alcohol consumption. Interactions between other galanin, galanin receptors and other drugs of abuse, including nicotine, opiates, cocaine and amphetamine are summarised in Table2.
Galanin and alcohol
Research into a proposed link between galanin and alcohol began more than 20 years ago [112] but the first published study to report a positive link between galanin and alcohol was in 2001 by Hauge and colleagues [113]. An increased density of galaninergic nerves (along with other neuropeptides) was reported in biopsies of small intestine taken from chronic alcoholics, indicative of an increase in galanin expression in response to heavy alcohol consumption [113]. More recently, research has looked at the effect of alcohol on galaninserum levels in alcohol-dependent humans [114] and it was reported that galanin serum levels were significantly lower during the early stages of alcohol withdrawal with the cause of this decrease remaining unexplained [114].
| Treatment / condition | Dose / concentration | Species | Route of administration | Physiological or behavioural effect | Primary target | Reference |
|---|---|---|---|---|---|---|
| Galnon | 0.5 mg/kg | BXD mouse | Intraperitoneal injection | Reversed nicotine withdrawal signs | [139] | |
| Nicotine withdrawal (precipitated by mecamylamine-hydrochloride) | 2 mg/kg | BXD mouse | Subcutaneous injection | Increased GALR1 expression | NAc | [139] |
| Nicotine | 0.01 ml/g | GAL-KO mouse | Intraperitoneal injection | Decreased sensitivity to nicotine | [138] | |
| Morphine | 20 – 100 mg/kg | Galanin-LacZ mouse | Subcutaneous injection | Increased galanin expression | LC | [141] |
| Opiate withdrawal (precipitated by naloxone) | 1 mg/kg | GALR1-KO mouse | Subcutaneous injection | More severe withdrawal signs than wild-type | GALR1 | [141] |
| Opiate withdrawal (precipitated by naltrexone) | 1 mg/kg | C57Bl/6 mouse | Subcutaneous injection | Upregulation of galanin binding and GALR1 mRNA | LC | [142] |
| Galnon | 2 mg/kg | C57Bl/6 mouse | Intraperitoneal injection | Decreased withdrawal symptoms and decreased preference for morphine | [143] | |
| Opiate withdrawal (precipitated by naloxone) | 1 mg/kg | GAL-OE mouse | Subcutaneous injection | Increased withdrawal signs of opiate withdrawal | [143] | |
| Opiate withdrawal (precipitated by naloxone) | 1 mg/kg | GAL-KO mouse | Subcutaneous injection | Decreased withdrawal signs of opiate withdrawal | [143] | |
| Galnon | 2 mg/kg | GAL-KO mouse | Intraperitoneal injection | Blocked cocaine-induced place preference | [145] | |
| Cocaine | 3 or 10 mg/kg | GAL-KO mouse | Intraperitoneal injection | Greater cocaine-induced increased place preference than wild-type | [145] | |
| Amphetamine | 3 mg/kg | GAL-KO mouse | Injection | Smaller increase in amphetamine induced locomotor activity than galanin wild-type | [147] |
Abbreviations: GALR1-KO, galanin-1 receptor knockout; GAL-KO, Galanin knockout; GAL-OE, Galanin over-expressing.
Table 2: Experimental interactions between galanin, galanin-receptor regulators and other drugs of abuse.
Galanin is known to have an orexigenic effect, more specifically in fat intake and alcohol consumption [115]. A similarity between these two macronutrients is their high kilojoule content but one notable difference is that alcohol is also a drug of abuse. Unlike other drugs of abuse, alcohol has a rich caloric content and is therefore postulated to interact with regions of the brain involved in the control of appetite and feeding [for reviews see 108,111,116]. Interestingly, the brain mechanisms that stimulate the intake of fat and ethanol are similar, and somewhat cross-over in their roles. The mesocorticolimbic system, which is known to regulate the consumption of drugs of abuse [117] is also implicated in feeding behaviour [118,119], and likewise, the HYP which controls the intake of food [120] is also involved in alcohol consumption [52]. Furthermore, galanin is known to modulate both general feeding behaviour [65,121-123] and ethanol intake [116,124,125] via pathways involving many sub-regions of the HYP. In accordance with these findings, injection of galanin directly into particular hypothalamic regions produced a stimulatory effect on voluntary ethanol intake in Sprague-Dawley rats [116,126]. More specifically, microinjection of galanin (1 nmol) directly into the third ventricle [116] and the PVN [126] increased voluntary alcohol consumption in Sprague-Dawley rats, an effect that was reversed following administration of the non-selective galanin antagonist M40 (1 nmol) [126].
Galanin peptide expression in the PVN [127,128] and the LH [125,128] of Sprague-Dawley rats has been shown to increase following voluntary ethanol consumption. Voluntary consumption of 2% w/w ethanol by Sprague-Dawley rats has also been shown to increase galanin peptide expression in the PVN [128]. In humans, it has been reported that alcohol-dependent individuals often obtain 30-50% of their daily energy intake from alcohol [129]. Galanin may contribute to this through the development of a positive feedback loop, by which alcohol consumption triggers an increase in galanin production, which in turn induces an increase in alcohol intake. The promotion of excessive drinking as a result of this mechanism is suggested to be a factor that may contribute to a physical dependence on alcohol [108]. Further support of a stimulatory relationship between galanin and alcohol comes from studies that have used transgenic mice that over-express galanin, or alternatively have a deletion of the galanin gene. Previous research has shown that galanin knockout mice show a significant decrease in voluntary ethanol intake and preference for ethanol when compared with wild-type mice [130]. In contrast, galanin over-expressing mice have been found to have an increased intake and preference for ethanol when compared with wild-types, further suggesting that galanin plays a role in stimulating ethanol consumption [131].
Electrophysiological studies in C57Bl/6J mice have shown that galanin, when superfused with ethanol increases IPSPs (inhibitory post-synaptic potentials) in a subpopulation of neurons within the CeA, and decreases IPSPs within another subpopulation of CeA neurons [132]. The differential effects are speculated to be due to activation of two different receptor subtypes. It was proposed that galanin may act post-synaptically in neurons of the CeA via GALR2 to decrease the size of IPSPs and also via GALR3 to enhance IPSPs [132]. As previously noted, galanin is co-expressed with GABA in the AMG, so this may ultimately lead to increased GABAergic transmission in the CeA, which highlights a role for GALR3 antagonism and GALR2 agonism in the CeA to reduce the synergistic interaction between galanin and ethanol [132].
Despite the large amount of evidence to support a role for galanin in the modulation of ethanol consumption, the relationship between alcohol and specific galanin-receptor subtypes has not been studied in great detail. Our laboratory has shown that administration of the selective GALR3 antagonist, SNAP 37889 (30 mg/kg, i.p.), decreased preference for ethanol in rats that had been chronically drinking as part of a fixed-ratio operant model [133]. More recently, we have shown that the same GALR3 antagonist reduced the breakpoint for alcohol under a progressive ratio schedule (Ash, et al. 2011; unpublished data). This finding validates the GALR3 as a potential target in the treatment of alcohol dependence and warrants further research to elucidate the exact mechanism by which GALR3 antagonism alters alcohol-seeking behaviour.
A recent genomic study looked at two polymorphisms in the galanin transcriptional start site; of which the weaker and less active allele is suggested to alter food and alcohol preference [134]. A single-nucleotide-polymorphism in the GALR3 gene has been strongly associated with alcoholism, while there was no correlation found between alcoholism and the GALR1 or GALR2 gene [135]. This provides further evidence that the GALR3 mediates actions of galanin that contribute to alcohol dependence. The GALR3 gene has also been implicated in susceptibility to alcoholism in various populations of very different backgrounds in terms of ethnicity and country of origin [136].
Galanin and nicotine
A correlation between a single-nucleotide-polymorphism of the GALR1 gene in humans and self-reported tobacco cravings during a recent smoking cessation study suggested a link between the GALR1 genotype and nicotine dependence [137]. Another recent study used mice that lacked the galanin peptide gene to investigate the relationship between galanin and nicotine-seeking behaviour in a conditioned place preference paradigm [138]. These mice were found to require administration of a higher dose of nicotine than galanin wild-type mice to induce conditioned place preference [138], indicating that galanin decreases sensitivity to the rewarding effects of nicotine and possibly also promotes nicotine-seeking behaviour. Contradictory findings were reported in a different study where nicotine withdrawal signs in BXD mice, which had been induced by the nicotinic receptor antagonist mecamylamine (2mg/kg, s.c.), were reversed following administration of galnon (0.5mgl/kg, i.p.), a non-specific galanin receptor agonist [139]. This study reported an increase in GALR1 expression in the NAc in conjunction with decreased galanin expression in the ventral midbrain of mice that had displayed increased withdrawal symptoms [139]. Collectively, this data supports a role for galanin signalling in protecting against nicotine dependence, which is possibly mediated via GALR1. The role of galanin in nicotine dependence and withdrawal has not been extensively studied and differences in the findings of the abovementioned studies could evidently be due to variation in mice strains and the possible involvement of GALR2 and/or GALR3. Indeed, there is evidence of a role for galanin in nicotine dependence and further research will provide insight into the use of galanin receptors as potential drug targets for nicotine dependence.
Galanin and opiates
A well established mechanism of addiction is the upregulation of the cAMP messenger pathway following chronic administration of drugs of abuse [9]. Upregulation of cAMP can indirectly contribute to physical dependence, drug tolerance and withdrawal symptoms when the site of cAMP upregulation projects to particular brain regions that mediate physical dependence and withdrawal such as the LC and VTA [9]. Chronic morphine administration in 129OlaHsd mice increased cAMP activity and CREB phosphorylation [140]. Studies have shown that galanin administration inhibits cAMP and decreases CREB phosphorylation in the LC, which supports a role for galanin agonists in opiate withdrawal [140]. Another study reported that both morphine administration and withdrawal increased the expression of galanin within the LC of mice [141]. In addition, a positive correlation was identified between increased galanin in the LC and a reduction in withdrawal signs exhibited by the mice [141]. This effect was suggested to be mediated through GALR1, as GALR1 knockout mice showed severe signs of withdrawal compared to wild-type mice, while GALR2 knockout mice did not show a difference in withdrawal signs when compared with their wild-type counterparts [141]. Similarly, another study reported that opiate withdrawal in C57Bl/6 mice resulted in an upregulation of both galanin binding and GALR1 mRNA in the LC [142], further suggesting a role for GALR1 in opiate withdrawal.
In further support of a relationship between galanin and opiates, another study reported that administration of galnon (2 mg/kg, i.p.), decreased preference for morphine and decreased withdrawal symptoms from morphine in C57Bl/6 mice [143]. In agreement with this finding, galanin over-expressing mice were found to display increased withdrawal signs, while galanin knockout mice displayed decreased signs of opiate withdrawal [143]. This indicates that galanin acts to decrease behaviours relating to opiate-dependence. In support of this finding, Wu and colleagues found that there was a significant decrease in galanin-induced, anti-nociceptive effects in morphinetolerant rats, which further implicates a role for galanin in morphine tolerance [144].
Galanin and cocaine
Only a small number of studies that have looked at a role for galanin in cocaine addiction. Cocaine has been shown to increase conditioned place preference in galanin knockout mice compared with galanin wild-type and this preference was reduced following the administration of galnon [145]. In another study, cocaine-induced hyperactivity was observed in both galanin wild-type and knockout mice, however, administration of galnon failed to alter general activity, which does not support a role for galanin in cocaine-induced hyperactivity [146]. Furthermore, a different experiment in the same study found that both wild-type and galanin knockout mice acquired cocaine self-administration tasks, again suggesting that galanin does not play a major role in cocaine-seeking behaviour [146].
Galanin and amphetamine
One study looking at the role of galanin in relation to amphetamine found that galanin over-expressing mice displayed a smaller increase in locomotor activity that was induced by amphetamine administration [147]. The connection between amphetamine and galanin remains underexplored. Amphetamine and cocaine are both classed as stimulants, so the lack of evidence of a relationship with galanin would suggest that stimulant abuse may be more strongly linked to other neuropeptides.
Roles for Individual Galanin Receptors in Addiction
Receptor functionality
Galanin exerts its effects via binding to one or more receptor subtypes. In terms of receptor functionality, all galanin-receptor subtypes are membrane bound G-protein coupled receptors but have very different pharmacological profiles [20,148]. Galanin typically acts as a neuromodulator at the pre-synaptic level, to inhibit the post-synaptic release of neurotransmitters via the opening of ATP-sensitive K+ (potassium) channels or closing of the Ca2+ (calcium) channels [95], however, some effects of galanin via GALR2 are of a stimulatory nature [88,132].
The GALR1 and GALR3 share similar signalling pathways in that they are both coupled to Gi /Go-proteins to mediate an inhibitory effect on neurotransmitter release via the inhibition of cAMP turnover [20,149]. Activation of the GALR2 is associated with coupling to Go/Gq which stimulates the turnover of inositol phospholipid and increases intracellular levels of Ca2+ to mediate a stimulatory effect on neurotransmitter release [88].
General patterns of receptor distribution
The unique distribution of galanin receptor subtypes (GALR1, GALR2 and GALR3) through the central nervous system and periphery, allows galanin to mediate a variety of physiological actions, with some crossover due to localisation of multiple receptor subtypes within the same regions [28]. Given that mRNA expression for the subtypes is not necessarily reflective of the presence of the receptor, the exact patterns of receptor distribution in the rat brain remains undetermined. The lack of selective radioligands and selective antibodies [150,151] for galanin receptors has meant that mRNA expression has been relied upon in terms of speculating about receptor distribution patterns; however, these reported patterns of mRNA distribution have been fairly consistent with presumptions made in the literature [38,150], along with confirmed immunoreactivity in the mouse brain [38].
GALR1 distribution
GALR1 mRNA is found primarily in regions of the rat brain involved with the processes of feeding, addiction, nociception and memory [20]. According to multiple studies that have looked at GALR1 mRNA expression in the rat brain, the general consensus is that the PFC [152], some hippocampal regions, AMG [39,153], some hypothalamic nuclei [39,86,152,153] and thalamic nuclei [152,153] exhibit the highest levels of GALR1 mRNA expression, followed by moderate levels within the LC [86,153] and DRN [86]. The pattern of GALR1 immunoreactivity in the mouse brain, was largely reflective of rat GALR1 mRNA distribution with the highest levels in the VTA, SN (substantia nigra), LC, some hippocampal regions [38] and moderate levels observed in the NAc, CPu, BST, AMG and hypothalamic regions [38]. There is limited information available on GALR1 mRNA distribution in the human brain, but what has been reported is high GALR1 mRNA distribution in the PFC, AMG and SN [86]. GALR1 mRNA in the human cortex [86] and ventral hippocampus [153] is supportive of a role for the GALR1 in memory and learning processes. The high distribution in the NAc highlights a possible role for GALR1 in DA function. Early pre-clinical evidence exists for GALR1 mediation of opiate withdrawal [reviewed by 154]. GALR1 immunoreactivity in the NAc, VTA and SN was comparatively higher than GALR2 and GALR3 immunoreactivity [38] indicating a stronger role for drug-reward mediated through GALR1 in these regions.
Early evidence exists for a stimulatory role of GALR1 in the intake of food, as shown by increased consumption of high-fat milk following administration of a selective GALR1 agonist in Sprague-Dawley rats [155]. This opens the possibility of GALR1 antagonists for potential use in the treatment of alcoholism, given the high energy content of alcohol and the previously described regulatory role of the HYP on alcohol consumption. In addition, the high density of GALR1 in the HYP validates further research into the effect of GALR1 antagonism on ethanol consumption.
GALR2 distribution
GALR2 is the most abundantly distributed galanin receptor within both the CNS (central nervous system) and PNS (peripheral nervous system) [20]. In the rat brain, GALR2 mRNA is expressed at the highest levels within regions of the HIP [156], followed by moderate to high expression in the HYP [156] and the AMG [156-158], and moderate expression in the PAG (periaqueductal gray) and olfactory bulbs [159]. GALR2 immunoreactivity in the mouse brain is very similar to that of rat, with high expression in hippocampal regions, moderate expression in the PFC, HYP, AMG and PAG [38] and lower levels reported in the LC, VTA and NAc [38]. Borowsky and colleagues reported human GALR2 mRNA to be abundantly expressed in the HYP and HIP [160] which is in agreement with GALR2 mRNA in the rat and mouse brain [88]; however, the overall distribution pattern differs greatly between rodents and humans. GALR2 seems to have more importance in peripheral neuron functions according to the high distribution of GALR2 in the PNS, relative to the CNS. Dense expression of GALR2 is found within the heart, kidney, small intestine, liver [160], injured bone [161], prostate, uterus, ovary, pancreas, stomach and large intestine [156] indicating a crucial role for the GALR2 in the regulation of cardiovascular, neuroendocrine, bone remodelling, reproductive and digestive functions.
Despite the large number of studies that have identified GALR2 mRNA within brain regions involved in addiction, it appears that a possible relationship between GALR2 and addictive behaviours it yet to be studied. Localisation of GALR2 within the HYP and HIP supports a possible role in the modulation of feeding, learning and memory, however we can only speculate about GALR2 involvement in the modulation of addictive behaviours as a recent search of the literature failed to find any evidence of a relationship between the GALR2 and any drug of dependence.
GALR3 distribution
GALR3 mapping studies in the brain are limited in comparison to GALR1 and GALR2 until recent years, due to a lack of GALR3 selective antibodies. GALR3 mRNA is highest in the rat brain within the HYP, specifically detected in the ventromedial, dorsomedial, paraventricular [87,162], Arc and supraoptic nuclei [162] which strongly supports a major role for GALR3 in feeding behaviours. High GALR3 mRNA expression has also been reported in the DRN, LC, PFC [87,88] preoptic area, BST [87], olfactory bulb [149] various hippocampal regions [162], and moderate expression in the CPu [149,162], NAc, AMG, SN [162]. Mouse brain patterns of GALR3 immunoreactivity reflect that of rat GALR3 mRNA distribution with high levels of expression reported in the SN, VTA, PAG, NAc, some thalamic nuclei and moderate levels in the NAc [38]. Overall, the distribution pattern of GALR3 in the brain suggests that major physiological effects would be highly likely to include feeding, reward, memory and emotion.
Galanin receptors make a good site for potential therapeutic intervention in addiction as the normal inhibitory action of galanin can be somewhat manipulated via GALR1 and GALR3; where the role of these receptors in this context is supported by the literature. Immunoreactivity for both the GALR1 and GALR3 in the LC are very high in comparison to GALR2 [38], indicating a role in noradrenergic transmission from the LC to regions of the forebrain. The location of these two receptor subtypes shows that they are well situated for inclusion in processes of motivation and reward.
A Role for the Galanin-3 Receptor in Mood Disorders
In addition to new therapies for addiction, many studies have highlighted the potential for mood disorders, which often occur co-morbidly with drug abuse, to be treated via the galanin system [reviewed by 163], most likely via modulation of GALR3 function [28,164]. The occurrence of mood-disorders in drug users is of major concern, particularly depression [165,166] and anxiety [167], for which the prevalence is reported to be significantly higher in drug-dependent patients than the general population [168,169]. The pattern of GALR3 distribution in areas involved with emotion such as the LC [87,95,169] and AMG [162], along with the co-localisation of galaninergic neurons with 5-HT in the DRN [32] suggests a role for galanin via the GALR3 in aspects of mood disorders.
Early experimental animal models indicate that GALR3 may be an effective target for reducing depressive and anxiety-like symptoms [170,171]. In 2005, the first specific GALR3 antagonist that was able to cross the blood brain barrier was synthesised [172] and administered by Swanson et al. to examine the role of GALR3 in behaviours related to anxiety and depression [167]. Two selective GALR3 antagonists, SNAP 37889 and SNAP 398299, reduced anxiety and depressivelike symptoms across a broad range of species [171]. Both of these GALR3 antagonists were found to have a very high binding affinity for GALR3 and were highly selective for GALR3 over GALR1 and GALR2 [171]. A different study using another novel GALR3 selective antagonist, 3-(3,4-dichlorophenylimino)-1-(6-methoxypyridin-3-yl) indolin-2-one, also reported antidepressant-like effects in mice and rats in a variety of behavioural paradigms [170]. Our laboratory has shown that administration of the GALR3 antagonist, SNAP 37889 (30 mg/kg, i.p.), did not alter anxiety-like behaviour in the elevated plus maze and light-dark paradigms [133]. However, this finding should not exclude the possibility of GALR3 as a target in the treatment of anxiety and depression. Our study used a single dose to primarily investigate the effect of GALR3 antagonism on alcohol consumption, so it is feasible that alternative doses may have altered anxiety and depressive-like behaviours. Overall, GALR3 antagonists appear to be a promising target in the treatment of affective disorders that are often present in conjunction with drug-abuse.
In terms of the role of GALR1 or GALR2 in anxiety and depression, there is very little information to suggest a strong role for these receptors in the mediation of mood-disorders. One study reported that GALR1 did not play a role in mediating depression in GALR1 receptor knockout mice as antidepressant responses were unchanged in the tail suspension test [170]. A literature search for the specific involvement of GALR2 did not disclose any published findings to indicate a role for this receptor in mood regulation.
Conclusions
The distribution of galanin receptors and coexistence of galanin with classical neurotransmitters in brain regions involved in addiction make the galanin system and its receptors viable targets for addiction pharmacotherapy. Galanin appears to modulate a drug-induced neurochemical response for a wide range of drugs of abuse; however, the effect of galanin is not uniform for each class of drug. Pre-clinical research indicates that galanin stimulates alcohol-seeking behaviour, whereas galanin counteracts dependence and withdrawal signs from opiates. Conflicting evidence exists as to whether galanin has an inhibitory or stimulatory effect on nicotine dependence. Furthermore, the treatment of co-morbid affective disorders often associated with drug dependence may be modulated via the galanin system.
The varied effects of galanin are suggested to be via the activation of different galanin-receptor subtypes, in particular, GALR1 and GALR3. Pre-clinical research indicates that the modulation of nicotine dependence and opiate withdrawal most likely occur via GALR1 and alterations in alcohol-seeking behaviour and emotional states via GALR3. In accordance with these findings, it is appropriate to suggest that GALR1 agonists and GALR3 antagonists may be favourable as novel treatments. Further understanding of the relationship between galanin receptors and drugs of abuse will provide the framework for developing effective therapies for addiction.
References
- Christmon K (1995) Historical Overview of Alcohol in the African American Community. JBS 25: 318-330.
- Roth T, Roehrs T, Zorick F Conway W (1985) Pharmacological effects of sedative-hypnotics, narcotic analgesics, and alcohol during sleep. Med Clin North Am 69: 1281-1288.
- Australian Institute of Health and Welfare (2007) Statistics on drug use in Australia 2006. Canberra: AIHW.
- Australian Institute of Health and Welfare (2008) 2007 National Drug Strategy Household Survey: detailed findings. Canberra: AIHW.
- Roxburgh A Degenhardt L (2008) Characteristics of drug-related hospital separations in Australia. Drug Alcohol Depend 92: 149-155.
- Collins D, Lapsley H (2008) The costs of tobacco, alcohol and illicit drug abuse to Australian society in 2004/05. Australian Government Department of Health and Ageing, Canberra.
- Rehm J, Patra J (2010) Chapter 1: Psychoactive substance use: epidemiology and burden of disease. ATLAS on substance use (2010) - Resources for the prevention and treatment of substance use disorders. Geneva, Switzerland: World Health Organization, 2010.
- Hyman SE (2005) Addiction: a disease of learning and memory. Am J Psychiatry 162: 1414-1422.
- Nestler EJ (2001) Molecular neurobiology of addiction. Am J Addict 10: 201- 217.
- Nestler EJ, Aghajanian GK (1997) Molecular and cellular basis of addiction. Science 278: 58-63.
- Nestler EJ, Malenka RC (2004) The addicted brain. Sci Am 290: 78-85.
- Stahl SM (2000) Essential Psychopharmacology. 2. Cambridge University Press, Cambridge.
- Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481-1489.
- Hyman SE, Malenka RC (2001) Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci 2: 695-703.
- Kalivas PW, O'Brien C (2008) Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33: 166-180.
- Liu X, Weiss F (2002) Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci 22: 7856-7861.
- Berke JD, Hyman SE (2000) Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25: 515-532.
- Nestler EJ (2002) From neurobiology to treatment: progress against addiction. Nat Neurosci 5: 1076-1079.
- Picciotto MR (2008) Galanin and addiction. Cell Mol Life Sci 65: 1872-1879.
- Branchek TA, Smith KE, Gerald C, Walker MW (2000) Galanin receptor subtypes. Trends Pharmacol Sci 21: 109-117.
- Hokfelt T (2005) Galanin and its receptors: introduction to the Third International Symposium, San Diego, California, USA, 21-22 October 2004. Neuropeptides 39: 125-142.
- Tatemoto K, Rokaeus A, Jornvall H, McDonald TJ, Mutt V (1983) Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett 164: 124- 128.
- Evans HF, Shine J (1991) Human galanin: molecular cloning reveals a unique structure. Endocrinology 129: 1682-1684.
- Evans HF, Huntley GW, Morrison JH, Shine J, Paxinos G (1992) Localization of preprogalanin mRNA in the monkey hippocampal formation. Neurosci Lett 146: 171-175.
- Melander T, Hokfelt T, Rokaeus A (1986) Distribution of galaninlike immunoreactivity in the rat central nervous system. J Comp Neurol 248: 475-517.
- Kaplan LM, Spindel ER, Isselbacher KJ, Chin WW (1988) Tissue-specific expression of the rat galanin gene. Proc Natl Acad Sci U S A 85: 1065-1069.
- Mensah ET, Volkoff H, Unniappan S (2011) Galanin systems in nonmammalian vertebrates with special focus on fishes. EXS 102: 243-262.
- RM, Holmes A (2006) Galanin as a modulator of anxiety and depression and a therapeutic target for affective disease. Amino Acids 31: 231-239.
- Robinson JK, Brewer A (2008) Galanin: a potential role in mesolimbic dopaminemediated instrumental behavior. Neurosci Biobehav Rev 32: 1485-1493.
- Brewer A, Echevarria DJ, Langel U, Robinson JK (2005) Assessment of new functional roles for galanin in the CNS. Neuropeptides 39: 323-326.
- Skofitsch G, Sills MA, Jacobowitz DM (1986) Autoradiographic distribution of 125I-galanin binding sites in the rat central nervous system. Peptides 7: 1029-1042.
- Melander T, Hokfelt T, Rokaeus A, Cuello AC, Oertel WH, et al. (1986) Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci 6: 3640-3654.
- Melander T, Staines WA, Rokaeus A (1986) Galanin-like immunoreactivity in hippocampal afferents in the rat, with special reference to cholinergic and noradrenergic inputs. Neuroscience 19: 223-240.
- Sweerts BW, Jarrott B, Lawrence AJ (1999) Expression of preprogalanin mRNA following acute and chronic restraint stress in brains of normotensive and hypertensive rats. Brain Res Mol Brain Res 69: 113-123.
- Kohler C, Chan-Palay V (1990) Galanin receptors in the post-mortem human brain. Regional distribution of 125I-galanin binding sites using the method of in vitro receptor autoradiography. Neurosci Lett 120: 179-182.
- Kohler C, Persson A, Melander T, Theodorsson E, Sedvall G, et al. (1989) Distribution of galanin-binding sites in the monkey and human telencephalon: preliminary observations. Exp Brain Res 75: 375-380.
- Gai WP, Geffen LB, Blessing WW (1990) Galanin immunoreactive neurons in the human hypothalamus: colocalization with vasopressin-containing neurons. J Comp Neurol 298: 265-280.
- Hawes JJ, Picciotto MR (2004) Characterization of GalR1, GalR2, and GalR3 immunoreactivity in catecholaminergic nuclei of the mouse brain. J Comp Neurol 479: 410-423.
- Jungnickel SR, Gundlach AL (2005) [125I]-Galanin binding in brain of wildtype, and galanin- and GalR1-knockout mice: strain and species differences in GalR1 density and distribution. Neuroscience 131: 407-421.
- Becker EE, Kissileff HR (1974) Inhibitory controls of feeding by the ventromedial hypothalamus. Am J Physiol 226: 383-396.
- Dailey MJ, Bartness TJ (2009) Appetitive and consummatory ingestive behaviors stimulated by PVH and perifornical area NPY injections. Am J Physiol Regul Integr Comp Physiol 296: R877-R892.
- Dayas CV, Buller KM, Day TA (1999) Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci 11: 2312-2322.
- Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, et al. (2009) Social defeat stress activates medial amygdala cells that express type 2 corticotropinreleasing factor receptor mRNA. Neuroscience 162: 5-13.
- Harris GC, Wimmer M, Aston-Jones G (2005) A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437: 556-559.
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G (2007) Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res 183: 43-51.
- M, Aston-Jones G, Herzog E, Manzoni O, Georges F (2009) Role of the bed nucleus of the stria terminalis in the control of ventral tegmental area dopamine neurons. Prog Neuropsychopharmacol Biol Psychiatry 33: 1336- 1346.
- Kim SM, Frank LM (2009) Hippocampal lesions impair rapid learning of a continuous spatial alternation task. PLoS One 4: e5494.
- Kyrkouli SE, Strubbe JH, Scheurink AJ (2006) Galanin in the PVN increases nutrient intake and changes peripheral hormone levels in the rat. Physiol Behav 89: 103-109.
- Nadel L, Moscovitch M (1997) Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol 7: 217-227.
- Patterson M, Murphy KG, Thompson EL, Smith KL, Meeran K, et al. (2006) Microinjection of galanin-like peptide into the medial preoptic area stimulates food intake in adult male rats. J Neuroendocrinol 18: 742-747.
- Puente N, Elezgarai I, Lafourcade M, Reguero L, Marsicano G, et al. (2010) Localization and function of the cannabinoid CB1 receptor in the anterolateral bed nucleus of the stria terminalis. PLoS One 5: e8869.
- Schick RR, Samsami S, Zimmermann JP, Eberl T, Endres C, et al. (1993) Effect of galanin on food intake in rats: involvement of lateral and ventromedial hypothalamic sites. Am J Physiol 264: R355-R361.
- Walker DL, Toufexis DJ, Davis M (2003) Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol 463: 199-216.
- Walker JR, Ahmed SH, Gracy KN, Koob GF (2000) Microinjections of an opiate receptor antagonist into the bed nucleus of the stria terminalis suppress heroin self-administration in dependent rats. Brain Res 854: 85-92.
- Wang J, Akabayashi A, Yu HJ, Dourmashkin J, Alexander JT, et al. (1998) Hypothalamic galanin: control by signals of fat metabolism. Brain Res 804: 7-20.
- Zhu W, Bie B, Pan ZZ (2007) Involvement of non-NMDA glutamate receptors in central amygdala in synaptic actions of ethanol and ethanol-induced reward behavior. J Neurosci 27: 289-298.
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates. Elsevier Academic Press,
- Levin MC, Sawchenko PE, Howe PR, Bloom SR, Polak JM (1987) Organization of galanin-immunoreactive inputs to the paraventricular nucleus with special reference to their relationship to catecholaminergic afferents. J Comp Neurol 261: 562-582.
- Meister B, Hokfelt T (1988) Peptide- and transmitter-containing neurons in the mediobasal hypothalamus and their relation to GABAergic systems: possible roles in control of prolactin and growth hormone secretion. Synapse 2: 585-605.
- Kinney GA, Emmerson PJ, Miller RJ (1998) Galanin receptor-mediated inhibition of glutamate release in the arcuate nucleus of the hypothalamus. J Neurosci 18: 3489-3500.
- Tsai G, Coyle JT (1998) The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med 49: 173-184.
- Chaillou E, Tramu G, Thibault J, Tillet Y (1998) Presence of galanin in dopaminergic neurons of the sheep infundibular nucleus: a double staining immunohistochemical study. J Chem Neuroanat 15: 251-259.
- Sun YG, Gu XL, Lundeberg T, Yu LC (2003) An antinociceptive role of galanin in the arcuate nucleus of hypothalamus in intact rats and rats with inflammation. Pain 106: 143-150.
- Meister B, Villar MJ, Ceccatelli S, Hokfelt T (1990) Localization of chemical messengers in magnocellular neurons of the hypothalamic supraoptic and paraventricular nuclei: an immunohistochemical study using experimental manipulations. Neuroscience 37: 603-633.
- Rada P, Mark GP, Hoebel BG (1998) Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Res 798: 1-6.
- Hagemann LF, Costa CV, Zeni LZ, Freitas CG, Marino-Neto J, et al. (1998) Food intake after adrenaline and noradrenaline injections into the hypothalamic paraventricular nucleus in pigeons. Physiol Behav 64: 645-652.
- Leibowitz SF, Roossin P, Rosenn M (1984) Chronic norepinephrine injection into the hypothalamic paraventricular nucleus produces hyperphagia and increased body weight in the rat. Pharmacol Biochem Behav 21: 801-808.
- Swiergiel AH, Peters G (1987) Injection of noradrenaline into the hypothalamic paraventricular nucleus produces vigorous gnawing in satiated rats. Life Sci 41: 2251-2254.
- Ericson E, Ahlenius S (1999) Suggestive evidence for inhibitory effects of galanin on mesolimbic dopaminergic neurotransmission. Brain res 822: 200- 209.
- Landry M, Roche D, Vila-Porcile E, Calas A (2000) Effects of centrally administered galanin (1-16) on galanin expression in the rat hypothalamus. Peptides 21: 1725-1733.
- Kalra SP, Kalra PS (1996) Nutritional infertility: the role of the interconnected hypothalamic neuropeptide Y-galanin-opioid network. Front Neuroendocrinol 17: 371-401.
- Tachibana T, Mori M, Khan MS, Ueda H, Sugahara K, et al. (2008) Central administration of galanin stimulates feeding behavior in chicks. Comp Biochem Physiol A Mol Integr Physiol 151: 637-640.
- M, Kelley AE (2002) Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 159: 415-423.
- Vila-Porcile E, Xu ZQ, Mailly P, Nagy F, Calas A, et al. (2009) Dendritic synthesis and release of the neuropeptide galanin: morphological evidence from studies on rat locus coeruleus neurons. J Comp Neurol 516: 199-212.
- Hokfelt T, Xu ZQ, Shi TJ, Holmberg K, Zhang X (1998) Galanin in ascending systems. Focus on coexistence with 5-hydroxytryptamine and noradrenaline. Ann N Y Acad Sci 863: 252-263.
- Grenhoff J, Nisell M, Ferre S, Aston-Jones G, Svensson TH (1993) Noradrenergic modulation of midbrain dopamine cell firing elicited by stimulation of the locus coeruleus in the rat. J Neural Transm Gen Sect 93: 11-25.
- Holets VR, Hokfelt T, Rokaeus A, Terenius L, Goldstein M (1988) Locus coeruleus neurons in the rat containing neuropeptide Y, tyrosine hydroxylase or galanin and their efferent projections to the spinal cord, cerebral cortex and hypothalamus. Neuroscience 24: 893-906.
- Lechner J, Leah JD, Zimmermann M (1993) Brainstem peptidergic neurons projecting to the medial and lateral thalamus and zona incerta in the rat. Brain Research 603: 47-56.
- Seutin V, Verbanck P, Massotte L, Dresse A (1989) Galanin decreases the activity of locus coeruleus neurons in vitro. Eur J Pharmacol 164: 373-376.
- Sevcik J, Finta EP, Illes P (1993) Galanin receptors inhibit the spontaneous firing of locus coeruleus neurones and interact with mu-opioid receptors. Eur J Pharmacol 230: 223-230.
- Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, et al. (1995) Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience 64: 861-874.
- Lundstrom L, Sollenberg U, Brewer A, Kouya PF, Zheng K, et al. (2005) A Galanin Receptor Subtype 1 Specific Agonist. International Journal of Peptide Research and Therapeutics 11: 17-27.
- Tsuda K, Tsuda S, Nishio I, Masuyama Y, Goldstein M (1992) Modulation of norepinephrine release by galanin in rat medulla oblongata. Hypertension 20: 361-366.
- Xu ZQ, Hokfelt T (1997) Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J Chem Neuroanat 13: 169-187.
- Sharkey LM, Madamba SG, Siggins GR, Bartfai T (2008) Galanin alters GABAergic neurotransmission in the dorsal raphe nucleus. Neurochem Res 33: 285-291.
- Sullivan KA, Shiao LL, Cascieri MA (1997) Pharmacological characterization and tissue distribution of the human and rat GALR1 receptors. Biochem Biophys Res Commun 233: 823-828.
- Mennicken F, Hoffert C, Pelletier M, Ahmad S, O'Donnell D (2002) Restricted distribution of galanin receptor 3 (GalR3) mRNA in the adult rat central nervous system. J Chem Neuroanat 24: 257-268.
- Smith KE, Forray C, Walker MW, Jones KA, Tamm JA, et al. (1997) Expression cloning of a rat hypothalamic galanin receptor coupled to phosphoinositide turnover. J Biol Chem 272: 24612-24616.
- Xu ZQ, Zhang X, Pieribone VA, Grillner S, Hokfelt T (1998) Galanin-5- hydroxytryptamine interactions: electrophysiological, immunohistochemical and in situ hybridization studies on rat dorsal raphe neurons with a note on galanin R1 and R2 receptors. Neuroscience 87: 79-94.
- Cortes R, Villar MJ, Verhofstad A, Hokfelt T (1990) Effects of central nervous system lesions on the expression of galanin: a comparative in situ hybridization and immunohistochemical study. Proc Natl Acad Sci U S A 87: 7742-7746.
- Gray TS, Magnuson DJ (1987) Galanin-like immunoreactivity within amygdaloid and hypothalamic neurons that project to the midbrain central grey in rat. Neurosci Lett 83: 264-268.
- Corwin RL, Robinson JK, Crawley JN (1993) Galanin antagonists block galanin-induced feeding in the hypothalamus and amygdala of the rat. Eur J Neurosci 5: 1528-1533.
- Cassell MD, Freedman LJ, Shi, C (1999) The intrinsic organization of the central extended amygdala. Ann N Y Acad Sci 877: 217-241.
- Barrera G, Echevarria DJ, Poulin JF, Laforest S, Drolet G, et al. (2005) One for all or one for one: does co-transmission unify the concept of a brain galanin "system" or clarify any consistent role in anxiety? Neuropeptides 39: 289-292.
- Kask K, Langel U, Bartfai T (1995) Galanin--a neuropeptide with inhibitory actions. Cell Mol Neurobiol 15: 653-673.
- Pych JC, Chang Q, Colon-Rivera C, Haag R, Gold PE (2005) Acetylcholine release in the hippocampus and striatum during place and response training. Learn Mem 12: 564-572.
- Pych, JC, Chang Q, Colon-Rivera C, Gold PE (2005) Acetylcholine release in hippocampus and striatum during testing on a rewarded spontaneous alternation task. Neurobiol Learn Mem 84: 93-101.
- Davis JA, Gould TJ (2008) Associative learning, the hippocampus, and nicotine addiction. Curr Drug Abuse Rev 1: 9-19.
- Anden N, Grabowska M (1976) Pharmacological evidence for a stimulation of dopamine neurons by noradrenaline neurons in the brain. Eur J Pharmacol 39: 275-282.
- Anton RF (2001) Pharmacologic approaches to the management of alcoholism. J Clin Psychiatry 62: 11-17.
- Wise RA (1998) Drug-activation of brain reward pathways. Drug Alcohol Depend 51: 13-22.
- Appel SB, McBride WJ, Diana M, Diamond I, Bonci A, et al. (2004) Ethanol Effects on Dopaminergic "Reward" Neurons in the Ventral Tegmental Area and the Mesolimbic Pathway. Alcohol Clin Exp Res 28: 1768-1778.
- Weiss F, Porrino LJ (2002) Behavioral neurobiology of alcohol addiction: recent advances and challenges. J Neurosci 22: 3332-3337.
- Tomkins DM, Sellers EM (2001) Addiction and the brain: the role of neurotransmitters in the cause and treatment of drug dependence. CMAJ 164: 817-821.
- Barson JR, Morganstern I, Leibowitz SF (2011) Similarities in hypothalamic and mesocorticolimbic circuits regulating the overconsumption of food and alcohol. Physiol Behav 104: 128-137.
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, et al. (2008) Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology 33: 1391-1401.
- Volkow ND, Wang GJ, Fowler JS, Telang F (2008) Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Philos Trans R Soc Lond B Biol Sci 363: 3191-3200.
- Lewis MJ, Rada P, Johnson DF, Avena NM, Leibowitz SF, et al. (2005) Galanin and alcohol dependence: neurobehavioral research. Neuropeptides 39: 317-321.
- Bailey CP, Andrews N, McKnight AT, Hughes J, Little HJ (2000) Prolonged changes in neurochemistry of dopamine neurones after chronic ethanol consumption. Pharmacol Biochem Behav 66: 153-161.
- Brodie MS (2002) Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res 26: 1024-1030.
- Koob GF, Sanna PP Bloom FE (1998) Neuroscience of addiction. Neuron 21: 467-476.
- Roy A, Berrettini W, Adinoff B Linnoila M (1990) CSF galanin in alcoholics, pathological gamblers, and normal controls: a negative report. Biol Psychiatry 27: 923-926.
- Hauge T, Persson J Sjolund K (2001) Neuropeptides in the duodenal mucosa of chronic alcoholic heavy drinkers. Alcohol Alcohol 36: 213-218.
- Heberlein A, Muschler M, Frieling H, Lenz B, Wilhelm J, et al. (2010) Decreased galanin serum levels are associated with alcohol-craving during withdrawal. Prog Neuropsychopharmacol Biol Psychiatry 35: 568-572.
- Barson JR, Morganstern I, Leibowitz SF (2010) Galanin and Consummatory Behavior: Special Relationship with Dietary Fat, Alcohol and Circulating Lipids. EXS 102: 87-111.
- Lewis MJ, Johnson DF, Waldman D, Leibowitz SF, Hoebel BG (2004) Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcohol Clin Exp Res 28: 1822-1828.
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35: 217-238.
- Bassareo V, Di Chiara, G (1999) Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci 11: 4389-4397.
- Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, et al. (2009) Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 10: 89-98.
- Williams KW, Elmquist JK (2011) Lighting up the hypothalamus: coordinated control of feeding behavior. Nat Neurosci 14: 277-278.
- Crawley JN, Robinson JK, Langel U, Bartfai T (1993) Galanin receptor antagonists M40 and C7 block galanin- induced feeding. Brain Res 600: 268-272.
- Koegler FH, York DA, Bray GA (1999) The effects on feeding of galanin and M40 when injected into the nucleus of the solitary tract, the lateral parabrachial nucleus, and the third ventricle. Physiol Behav 67: 259-267.
- Leibowitz SF, Akabayashi A, Wang J (1998) Obesity on a high-fat diet: role of hypothalamic galanin in neurons of the anterior paraventricular nucleus projecting to the median eminence. J Neurosci 18: 2709-2719.
- Leibowitz SF (2005) Regulation and effects of hypothalamic galanin: relation to dietary fat, alcohol ingestion, circulating lipids and energy homeostasis. Neuropeptides 39: 327-332.
- Leibowitz SF, Avena NM, Chang GQ, Karatayev O, Chau DT, et al. (2003) Ethanol intake increases galanin mRNA in the hypothalamus and withdrawal decreases it. Physiol Behav 79: 103-111.
- Rada P, Avena NM, Leibowitz SF, Hoebel BG (2004) Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol 33: 91-97.
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, et al. (2007) Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res 31: 249-259.
- Karatayev O, Barson JR, Carr AJ, Baylan J, Chen YW, et al. (2010) Predictors of ethanol consumption in adult Sprague-Dawley rats: relation to hypothalamic peptides that stimulate ethanol intake. Alcohol 44: 323-334.
- Lieber CS (1988) The influence of alcohol on nutritional status. Nutr Rev 46: 241-254.
- Karatayev O, Baylan J, Weed V, Chan, S, Wynick D, et al. (2010) Galanin Knockout Mice Show Disturbances in Ethanol Consumption and Expression of Hypothalamic Peptides that stimulate ethanol intake. Alcohol Clin Exp Res 34: 72-80.
- Karatayev O, Baylan J, Leibowitz SF (2009) Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol 43: 571-580.
- Bajo M, Madamba SG, Lu X, Sharkey LM, Bartfai T, et al. (2011) Receptor subtype-dependent galanin actions on gamma-aminobutyric acidergic neurotransmission and ethanol responses in the central amygdala. Addict Biol, 10.1111/j.1369-1600.2011.00360.x.
- Ash BL, Zanatta SD, Williams SJ, Lawrence AJ, Djouma E (2011) The galanin-3 receptor antagonist, SNAP 37889, reduces operant responding for ethanol in alcohol-preferring rats. Regul Pept 166: 59-67.
- Davidson S, Lear M, Shanley L, Hing B, Baizan-Edge A, et al. (2011) Differential Activity by Polymorphic Variants of a Remote Enhancer that Supports Galanin Expression in the Hypothalamus and Amygdala: Implications for Obesity, Depression and Alcoholism. Neuropsychopharmacology 36: 2211-2221.
- Belfer I, Hipp H, Bollettino A, McKnight C, Evans C, et al. (2007) Alcoholism is associated with GALR3 but not two other galanin receptor genes. Genes Brain Behav 6: 473-481.
- Belfer I, Hipp H, McKnight C, Evans C, Buzas B, et al. (2006) Association of galanin haplotypes with alcoholism and anxiety in two ethnically distinct populations. Mol Psychiatry 11: 301-311.
- Lori A, Tang Y, O'Malley S, Picciotto MR, Wu R, et al. (2011) The Galanin Receptor 1 Gene Associates with Tobacco Craving in Smokers Seeking Cessation Treatment. Neuropsychopharmacology 36: 1412-1420.
- Neugebauer NM, Henehan RM, Hales CA, Picciotto MR (2011) Mice lacking the galanin gene show decreased sensitivity to nicotine conditioned place preference. Pharmacol Biochem Behav 98: 87-93.
- Jackson KJ, Chen X, Miles MF, Harenza J, Damaj MI (2011) The Neuropeptide Galanin and Variants in the GalR1 Gene are Associated with Nicotine Dependence. Neuropsychopharmacology 36: 2339-2348.
- Hawes JJ, Narasimhaiah R, Picciotto MR (2006) Galanin attenuates cyclic AMP regulatory element-binding protein (CREB) phosphorylation induced by chronic morphine and naloxone challenge in Cath.a cells and primary striatal cultures. J Neurochem 96: 1160-1168.
- Holmes FE, Armenaki A, Iismaa TP, Einstein EB, Shine J, et al. (2011) Galanin negatively modulates opiate withdrawal via galanin receptor 1. Psychopharmacology (Berl), 10.1007/s00213-011-2515-x.
- Zachariou V, Thome J, Parikh K, Picciotto MR (2000) Upregulation of galanin binding sites and GalR1 mRNA levels in the mouse locus coeruleus following chronic morphine treatments and precipitated morphine withdrawal. Neuropsychopharmacology 23: 127-137.
- Zachariou V, Brunzell DH, Hawes J, Stedman DR, Bartfai T, et al. (2003) The neuropeptide galanin modulates behavioral and neurochemical signs of opiate withdrawal. Proc Natl Acad Sci U S A 100: 9028-9033.
- Wu X, Yu LC (2006) Alternation of galanin in nociceptive modulation in the central nervous system of rats during morphine tolerance: a behavioral and immunohistochemical study. Brain Res 1086: 85-91.
- Narasimhaiah R, Kamens HM, Picciotto MR (2009) Effects of galanin on cocaine-mediated conditioned place preference and ERK signaling in mice. Psychopharmacology 204: 95-102.
- Brabant C, Kuschpel AS, Picciotto MR (2010) Locomotion and selfadministration induced by cocaine in 129/OlaHsd mice lacking galanin. Behav Neurosci 124: 828-838.
- Kuteeva E, Hokfelt T, Ogren SO (2005) Behavioural characterisation of young adult transgenic mice overexpressing galanin under the PDGF-B promoter. Regul Pept 125: 67-78.
- Walton KM, Chin JE, Duplantier AJ, Mather RJ (2006) Galanin function in the central nervous system. Curr Opin Drug Discov Devel 9: 560-570.
- Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, et al. (1998) Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem 273: 23321-23326.
- Lu X, Bartfai T (2009) Analyzing the validity of GalR1 and GalR2 antibodies using knockout mice. Naunyn Schmiedebergs Arch Pharmacol 379: 417- 420.
- Michel MC, Wieland T, Tsujimoto G (2009) How reliable are G-proteincoupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol 379: 385-388.
- Lu X, Mazarati A, Sanna P, Shinmei S, Bartfai T (2005) Distribution and differential regulation of galanin receptor subtypes in rat brain: effects of seizure activity. Neuropeptides 39: 147-152.
- Parker EM, Izzarelli DG, Nowak HP, Mahle CD, Iben LG, et al. (1995) Cloning and characterization of the rat GALR1 galanin receptor from Rin14B insulinoma cells. Brain Res Mol Brain Res 34: 179-189.
- Picciotto MR, Hawes JJ, Brunzell DH, Zachariou V (2005) Galanin can attenuate opiate reinforcement and withdrawal. Neuropeptides 39: 313-315.
- Saar I, Runesson J, McNamara I, Jarv J, Robinson JK, et al. (2011) Novel galanin receptor subtype specific ligands in feeding regulation. Neurochem Int 58: 714-720.
- Waters SM, Krause JE (2000) Distribution of galanin-1, -2 and -3 receptor messenger RNAs in central and peripheral rat tissues. Neuroscience 95: 265-271.
- Fathi Z, Cunningham AM, Iben LG, Battaglino PB, Ward SA, et al. (1997) Cloning, pharmacological characterization and distribution of a novel galanin receptor. Brain Res Mol Brain Res 51: 49-59.
- Depczynski B, Nichol K, Fathi Z, Iismaa T, Shine J, et al. (1998) Distribution and characterization of the cell types expressing GALR2 mRNA in brain and pituitary gland. Ann N Y Acad Sci 863: 120-128.
- O'Donnell D, Ahmad S, Wahlestedt C, Walker P (1999) Expression of the novel galanin receptor subtype GALR2 in the adult rat CNS: distinct distribution from GALR1. J Comp Neurol 409: 469-481.
- Borowsky B, Walker MW, Huang LY, Jones KA, Smith KE, et al. (1998) Cloning and characterization of the human galanin GALR2 receptor. Peptides 19: 1771-1781.
- McDonald AC, Schuijers JA, Gundlach AL, Grills BL (2007) Galanin treatment offsets the inhibition of bone formation and downregulates the increase in mouse calvarial expression of TNFalpha and GalR2 mRNA induced by chronic daily injections of an injurious vehicle. Bone 40: 895- 903.
- Kolakowski LF, Jr.O'Neill GP, Howard AD, Broussard SR, Sullivan KA, et al. (1998) Molecular characterization and expression of cloned human galanin receptors GALR2 and GALR3. J Neurochem 71: 2239-2251.
- Kask K, Berthold M, Bartfai T (1997) Galanin receptors: involvement in feeding, pain, depression and Alzheimer's disease. Life Sci 60: 1523-1533.
- Lu X, Barr AM, Bartfai T (2005) Galanin receptors as novel drug targets for the treatment of depression and anxiety. Drug Dev Res 65: 227-236.
- Rao U, Chen LA (2008) Neurobiological and psychosocial processes associated with depressive and substance-related disorders in adolescents. Curr Drug Abuse Rev 1: 68-80.
- Markou A, Kosten TR, Koob GF (1998) Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology 18: 135-174.
- Schuckit MA, Hesselbrock V (1994) Alcohol dependence and anxiety disorders: what is the relationship? Am J Psychiatry 151: 1723-1734.
- World Health Organisation (2004) Global Status Report on Alcohol 2004. Department of Mental Health and Substance Abuse, Geneva.
- Teesson M, Hall W, Slade T, Mills K, Grove R, et al. (2010) Prevalence and correlates of DSM-IV alcohol abuse and dependence in Australia: findings of the 2007 National Survey of Mental Health and Wellbeing. Addiction 105: 2085-2094.
- Barr AM, Kinney JW, Hill MN, Lu X, Biros S, et al. (2006) A novel, systemically active, selective galanin receptor type-3 ligand exhibits antidepressant-like activity in preclinical tests. Neurosci Lett 405: 111-115.
- Swanson CJ, Blackburn TP, Zhang X, Zheng K, Xu ZQ, et al. (2005) Anxiolytic and antidepressant-like profiles of the galanin-3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci U S A 102: 17489-17494.
- Konkel MJ, Lagu B, Boteju LW, Jimenez H, Noble S, et al. (2006) 3-arylimino-2-indolones are potent and selective galanin GAL3 receptor antagonists. J Med Chem 49: 3757-3758.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15734
- [From(publication date):
specialissue-2011 - Dec 27, 2024] - Breakdown by view type
- HTML page views : 11271
- PDF downloads : 4463